Simple Summary
The presence of Salmonella on farms is a concern to the swine industry. Much of the focus of on-farm surveillance has been directed to the finishing stage because of food safety issues, but it is important to study Salmonella transmission during the nursery stage in order to develop control strategies. In this study, 50 cohorts of weaned pigs were monitored for Salmonella using blood samples taken at weaning and again near the end of the nursery stage and tested for antibodies. At the time of the second blood sampling, rectal swabs were obtained from the same pigs and cultured for Salmonella. A questionnaire regarding housing and management was also completed and used to evaluate risk factors for herds with active infection. If one pig out of the 20 tested in a cohort was found to be positive either based on the growth of Salmonella on culture or a rising antibody titre, then it was assumed that Salmonella was spreading among the pigs in that cohort. Active spread of Salmonella occurred in 80% of the nursery cohorts. Unfortunately, no risk factors were identified to explain the difference between positive and negative nurseries, including whether or not the farm used antibiotics.
Abstract
The objectives of this study were: to identify nursery cohorts with an active Salmonella infection using combined serological and bacteriological methods, and to try to identify risk factors associated with swine nurseries with active Salmonella spread. Twenty pigs from each of 50 cohorts of weaned pigs from 44 different nursery barns were sampled about the time of weaning and near the end of the nursery stage. Information regarding farm management and biosecurity practices were collected using a questionnaire. Blood samples were obtained at both visits, while rectal swabs were collected at the second visit. An enzyme-linked immunosorbent assay (ELISA) was used to test sera for Salmonella antibodies and rectal samples were cultured for Salmonella. A nursery cohort was identified as having an active Salmonella infection if Salmonella was cultured from one or more of the 20 pigs or if serological evidence suggested exposure to Salmonella. The association between farm-level management covariates and active Salmonella infection was assessed in 46 cohorts using a logistic regression model. Nine of 46 (20%) cohorts produced Salmonella-free pigs. The remaining 37 (80%) cohorts were classified as having an active infection. Examination of risk factors failed to identify how negative and positive nurseries differed.
1. Introduction
Salmonella infection is an important concern for the pig industry, primarily from a public health standpoint but also as a cause of economically important clinical disease in pigs [1,2,3]. The epidemiology of Salmonella in pork production has been widely studied [1,3,4,5,6,7,8,9,10,11,12,13,14]. However, the emphasis has generally been placed on understanding the prevalence at the finishing stage, because of food safety concerns [9,10,11,14]. However, as with many diseases, stopping the spread of Salmonella in the nursery might be an important strategy in reducing the prevalence of Salmonella in later stages of production. Identifying the spread of Salmonella in nursery pigs and assessing associated risk factors would be beneficial in developing control measures to prevent the circulation and maintenance of Salmonella in the later phases of production.
Newly weaned pigs are susceptible to disease because at this stage pigs are subjected to multiple stresses [15,16] and their immune system is not yet fully mature [17]. The control of endemic diseases in swine production often focuses on preventing newly weaned susceptible pigs from becoming infected with disease from older animals and thus perpetuating the disease within the herd. All-in and all-out management of nurseries combined with thorough cleaning between cohorts of weaned pigs is a common approach to prevent disease from cycling in this vulnerable population.
There are several challenges regarding the monitoring of Salmonella in the nursery to determine if weaned pigs are becoming actively infected with Salmonella. For example, most infected pigs become asymptomatic carriers, so the absence of clinical signs is not a good indication that Salmonella is not present [1]. In addition, infected pigs do not always shed Salmonella continuously or shed large numbers of bacteria and therefore negative results from bacterial culture may be misleading [1]. In addition, the best results for detecting Salmonella from an asymptomatic carrier pig is to use at least 25 g of fecal material [18,19] which is practical if the study design utilizes a pen sample, but it is more difficult to obtain a sufficient fecal sample from a specific individual pig. Serological testing methods to assess antibody response to Salmonella infection have been shown to be more effective in identifying the population of intermittent shedders than traditional bacteriological methods [7] but antibody titres in newly weaned pigs might be difficult to interpret because they may reflect either recent exposure or lingering passive immunity. Interpretation is clearer if antibodies from two time periods can be compared in order to verify that antibody levels are rising during the nursery stage, indicating exposure to Salmonella and not the presence of colostrum-derived immunity.
The objectives of this study were: (i) to identify Ontario swine nurseries with active Salmonella infections using serological and bacteriological testing methods and (ii) to determine risk factors associated with nursery cohorts with an active Salmonella infection.
2. Materials and Methods
The research was approved by the Animal Care Committee of the University of Guelph, in accordance with the guidelines set forward by the Canadian Council of Animal Care (Animal Utilization Protocol #3531).
2.1. Study Design and Sampling
Forty-four Ontario swine farms with nursery barns were included in this study which took place between 2014 and 2019. Farm selection, based on the producer’s willingness to participate in the study, was both purposive and convenient. Along with conventional farms, antibiotic-free systems (including both certified organic and “raised without antibiotics” farms) were purposively selected to help capture a wide spectrum of farming practices in Ontario. A second cohort of nursery pigs was studied on six of these 44 swine farms resulting in a total of 50 study cohorts. These six farms were selected for a second cohort because they had been the first farms included in the study and thus over one year had passed since the first cohort was investigated and the producers were willing to continue to participate. The study involved following a weaned cohort of pigs through the nursery stage of production and involved two farm visits per cohort with the first visit (V1) at weaning or soon after weaning and the second visit (V2) near the end of the nursery stage.
For each cohort, the nursery was visited (V1) within a few days after the pigs were moved into the facility. On six of the nursery farms, where a second cohort of pigs was studied, the initial visit (V1) was conducted at weaning, immediately prior to the pigs being transferred to the nursery barn. In each of the 50 cohorts of weaned pigs, 20 animals were selected, with an attempt to choose pigs representative of the rooms and/or pens in the nursery barn (for example, if the pigs in a cohort were housed in 10 pens, then 2 average-sized pigs per pen would be selected for the study). The choice of an average-sized pig was based on subjective observation. The 20 pigs in each cohort were ear-tagged to provide individual identification and blood samples were collected from either the jugular vein or suborbital sinus. The cohort was visited a second time (V2) within a few days of the pigs being moved from the nursery to a grower barn. At V2, blood samples were collected from the same 20 pigs. Blood samples were centrifuged for 20 min at 1500× g and the sera were separated and stored at −20 °C. Individual rectal swabs were also collected from pigs at V2 and stored at −20 °C.
2.2. Questionnaire
During the first farm visit, a questionnaire was administered to the farm owner or manager and information was recorded by the investigator. The cohort-level questionnaire focused on management and biosecurity practices. Farm management questions included; the type of farm (farrow-to-finish, farrow-to-feeder, wean-to-finish, nursery only), production flow (continuous flow of pigs into and out of the nursery or all-in and all-out by room, or by building, or by site), and antibiotic use (certified organic, “antibiotic-free” or conventional). Basic production data and farm details such as herd size, source of nursery pigs, weaning age, length of stay in the nursery barn, and stocking density were also collected. Biosecurity practice questions included; whether there was a hospital pen, controlled entry for human traffic, downtime for visitors, the disposal method of deadstock, and cleaning methods between batches.
2.3. Salmonella Antibody Detection
Serum samples were assessed for presence of antibodies to Salmonella serogroups B, C, D, and E (O-antigens 1, 3–7, 9, 10, and 12) using an indirect ELISA (pigtype® Salmonella Ab kit, QIAGEN, Leipzig, Germany). The ELISA was performed as described by the manufacturer. A sample-to-positive ratio (S/P) value was determined using the optical density (OD) values using the following equation:
S/P = (ODsample − ODnegative control)/(ODpositive control − ODnegative control)
The S/P ratio at V1 and V2 was identified as the antibody titre level at V1 and V2 for each pig. Based on the manufacturing guidelines, samples with a S/P ratio of ≥0.3 were identified as Salmonella seropositive and <0.3 were classified as Salmonella seronegative.
2.4. Salmonella Isolation
Rectal swabs were added to 9 mL of tetrathionate broth (TTB) (Becton Dickinson™, Franklin Lakes, NJ, USA) and incubated at 37 °C for 18 to 24 h. Then, 100 μL of TTB culture was transferred to 9.9 mL of Rappaport–Vassiliadis broth (RVB, Becton Dickinson™, Franklin Lakes, NJ, USA) and incubated at 41 °C for 18 to 24 h. Lastly, a loopful (~20 µL) of the RVB was plated onto xylose-lysine-tergoitol 4 (XLT4, Remel Thermo Fisher Scientific™, Lenexa, KS, USA) agar plates and incubated at 37 °C for 18 to 24 h. Plates with one or more Salmonella colonies were identified as Salmonella positive.
2.5. Data Analysis
Data were entered into Microsoft Excel for Mac 2019 Version 16.25 (Microsoft, Redmond, WA, USA). After cleaning, the data were imported into Stata (Stata/SE 14.2 for Mac; StataCorp, College Station, TX, USA) for further data management and descriptive analysis.
2.6. Salmonella Antibody Response Patterns
To explore the antibody response (based on the S/P ratio) in nursery pigs and to account for the possible presence of passive antibody titres at weaning, 8 possible patterns were established based on the pig’s Salmonella seropositivity status at V1 and V2, along with the change in direction of antibody response and whether the S/P ratio was ≥0.3 or not.
Specifically, pigs were considered serologically positive if the antibody titres from the two visits showed an increase in antibody titre (or the same antibody titre in both tests) resulting in a seropositive status at V2. All other patterns were considered serologically negative including a pattern with an initial high titre and a second lower S/P ratio even if it was ≥0.3. The assumption was that the decrease in antibody response reflects lingering passive immunity and not evidence of Salmonella exposure in the nursery.
A Python (Python v3.0.1, Fredericksberg, VA, USA) script was developed and performed to identify pigs into patterns (1–8). These results were presented and analyzed graphically. These pig-level data were also aggregated to the cohort-level.
2.7. Identifying Active Salmonella Infection in Nursery Cohorts
A cohort of nursery pigs was considered to have active Salmonella infection if one of the 20 pigs sampled tested positive by direct (culture) or by indirect (serological, antibody response patterns indicative of active infection) methods.
2.8. Agreement between Bacteriological and Serological Tests
The extent of agreement between the bacteriological and serological detection methods was determined using Cohen’s kappa (k) statistic. The kappa statistic was interpreted as follows: <0.2 slight agreement; 0.2–0.4 fair agreement; 0.4–0.6 moderate agreement; 0.6–0.8; substantial agreement; and >0.8 almost perfect agreement.
McNemar’s χ2 allowed assessment of the difference between the positive proportion of the bacteriology and serology testing methods. If the McNemar’s χ2 test was non-significant (p > 0.05), this would mean the proportions do not differ. Meanwhile, a significant McNemar’s χ2 test (p < 0.05) would mean there is a disagreement between the two testing methods and assessment of kappa would not be beneficial.
2.9. Risk Factors Associated with a Nursery Cohort with an Active Salmonella Infection
To assess risk factors associated with nursery barns with an active Salmonella infection, a logistic regression model was used. The active Salmonella infection (yes/no) of nursery barns was used as the dependent variable, while the second cohort conducted on six farms (farm cohort visit) was used as a fixed effect. Farm management and biosecurity practices, as explanatory variables, were initially screened using descriptive statistics and evaluated for collinearity using Spearman correlation coefficients. Continuous variables were assessed for linear relationships with the outcome graphically (lowess) and categorized if the relationship was not linear or quadratic. Explanatory variables were then independently assessed with the dependent variable using univariable analysis. The initial inclusion of variables in the model was based on a liberal p < 0.1. Using stepwise elimination, the full model was manually built excluding variables lacking statistical significance. The likelihood ratio test was used to assess the statistical significance (p < 0.05) of variables before removing them from the full model. Additionally, prior to exclusions, variables were tested for confounding to ensure there was less than 20% change in coefficients in main effects in the model. Since all explanatory variables were removed from the model, no further testing for interactions or fit of the model was required.
3. Results
3.1. Characteristics of the Nurseries
The 50 cohorts in this study included 19 (38%) farms that did not use antibiotics (including both certified organic farms and farms producing pigs for a program called “raised without antibiotics”) and the remaining cohorts were raised on conventional farms that used antibiotics (Table 1). Of 50 cohorts, 24 (48%) cohorts were located on a farrow-to-finish farm, while 17 (34%) cohorts were located on off-site nurseries (Table 1). The remaining 5 (10%) and 4 (8%) cohorts were nurseries associated with farrow-to-feeder pig and wean-to-finish operations, respectively. The flow of pigs varied as follows: 1/50 (2%) of the cohorts operated as an all-in/all-out (AIAO) flow by site, 25/50 (50%) of the cohorts operated as AIAO flow by building, and 13/50 (26%) cohorts AI/AO by room, and the remaining 11/50 (22%) cohorts used continuous pig flow in the nursery (Table 1).

Table 1.
Distribution of cohort-level risk factors (farm management and biosecurity) in 50 swine nursery cohorts (44 farms; 6 farms where a second cohort was conducted).
With regard to cohort size, the population of nursery pigs ranged from 120 to 6500 (median = 2125, mean = 2235). Forty-one of 43 (95%) cohorts sourced nursery pigs from within their own production system, with this information missing from 7 participating nurseries. On average, 837 pigs entered the nursery at the same time, based on data from 41 cohorts (median = 650, min = 15, max = 2800). Based on the producers’ survey responses, the average weaning age of pigs varied from 18 days to 38.5 days-old (median = 21, mean = 23.2). On average, pigs spent approximately 38.7 days in the barn (ranging from 16 to 55 days, median 41). At V1, individual pigs ranged in age from 17 days to 41.5 days (median = 22, mean = 24.5). While at V2, pigs ranged in age from 50 to 85 days old (median = 64, mean = 63.9).
3.2. Salmonella: Antibody Titres, Seropositivity, Shedding
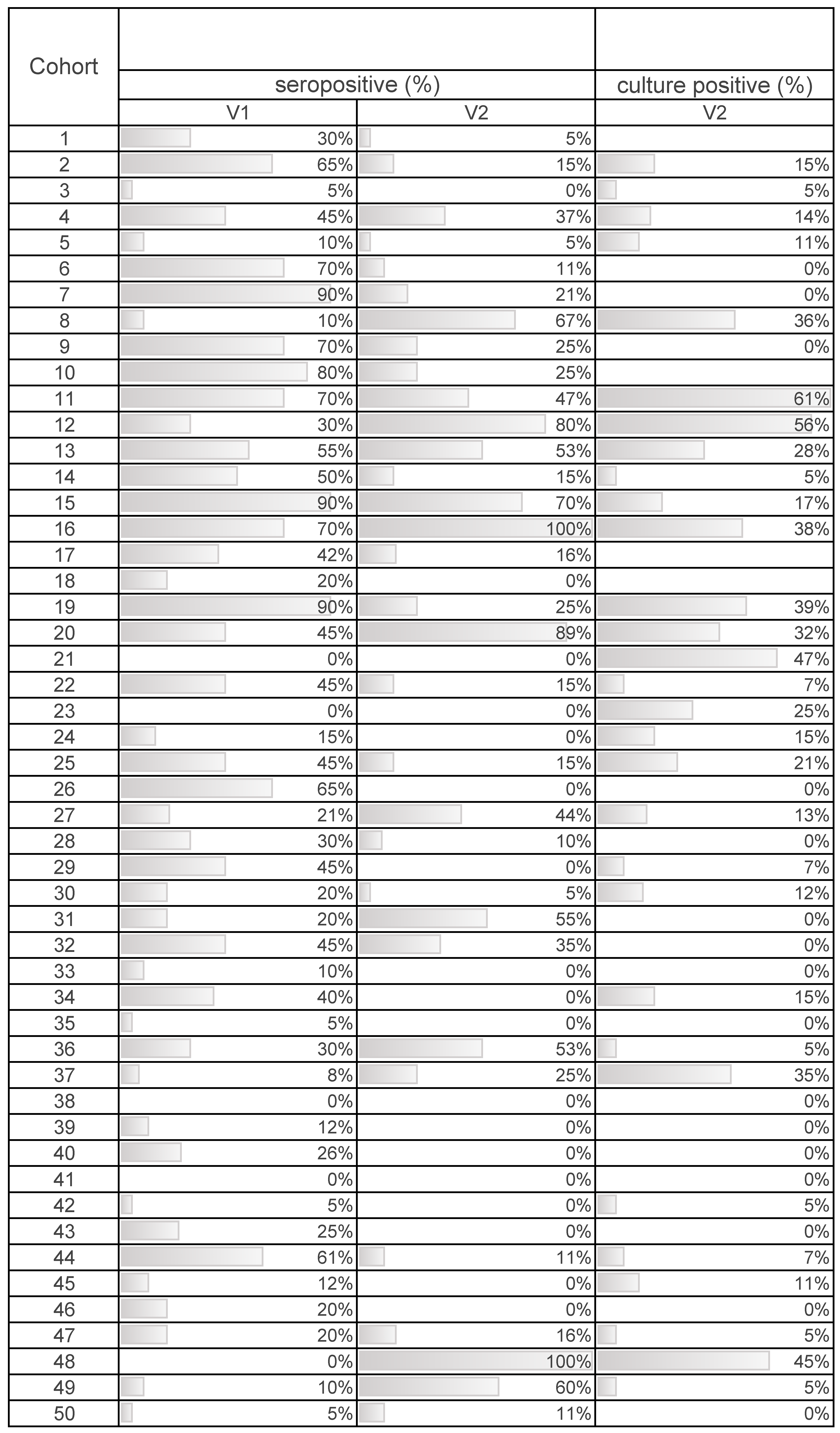
Salmonella antibody titres (based on the S/P ratio), seropositivity, and bacteriological results for nursery pigs at V1 and V2 are presented in Table 2. Overall, lower Salmonella antibody titres and number of pigs classified as Salmonella seropositive are observed in nursery pigs from V1 to V2. At the pig-level, 14.6% of pigs at the end of the nursery were found to be Salmonella-positive based on culture of a rectal swab.

Table 2.
Salmonella status (antibody titres, seropositivity, shedding) at visit 1 (V1; weaning) and visit 2 (V2; end of nursery) in nursery pigs in 50 cohorts (44 farms; 6 farms with a second cohort conducted).
Direct and indirect Salmonella testing results on a cohort basis are presented in Table 3. At the cohort level, 30 of 46 (65%) nursery cohorts had one or more pigs positive for Salmonella based on bacterial culture at V2 (Table 3). In terms of seropositivity, from weaning to the end of the nursery, 35 of 50 (70%) cohorts had a decrease in the number of seropositive pigs. Of the remaining cohorts 11 of 50 (22%) had an increase in the number of seropositive pigs, while 4 of 50 (8%) cohorts had no change in the percent of seropositive pigs (Table 3).

Table 3.
Proportion of pigs that were Salmonella culture positive and seropositive (identified using enzyme-linked immunosorbent assay (ELISA) testing, seropositivity based on S/P ratio ≥0.3) at weaning (V1) and at the end of the nursery stage (V2). Data were collected from 50 swine cohorts (44 farms; 6 farms with a second cohort included in the study).
3.3. Salmonella Antibody Response Patterns
At the individual level, 930 (69%) pigs had a decreasing Salmonella antibody response pattern (3, 4, 8) from V1 to V2 (Figure 1). Meanwhile, 31% of pigs were found to have an increase in antibody titre from V1 to V2 (patterns 1, 5, 6). No pigs were found to remain at baseline (patterns 2, 3). Based on antibody response patterns at the individual level, 18% of pigs were found to have an active Salmonella infection (patterns 1, 2, 5).
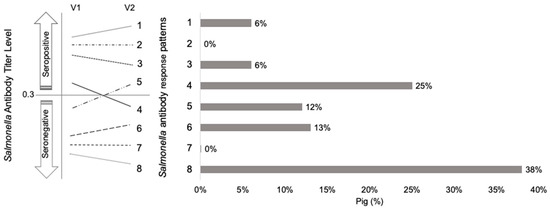
Figure 1.
The change in Salmonella antibody titres in nursery pigs (n = 930, from 50 swine cohorts) from weaning, at visit 1 (V1), to the end of the nursery, at visit 2 (V2), is characterized into eight possible Salmonella antibody response patterns (1–8) a. Salmonella seropositivity status (seropositive (Sp) if antibody titre level (based on S/P ratio) ≥0.3, seronegative (Sn) if antibody titre level <0.3).a Salmonella antibody response patterns (1 to 8) 1—Sp at V1, increases in antibody titre 2—remains Sp (no change) 3—Sp at V1, decreases in antibody titre but remains Sp 4—Sp at V1, Sn at V2 5—Sn at V1, Sp at V2 6—Sn at V1, increases in antibody titre but remains Sn 7—remains Sn (no change) 8—Sn at V1, decrease in antibody titre.
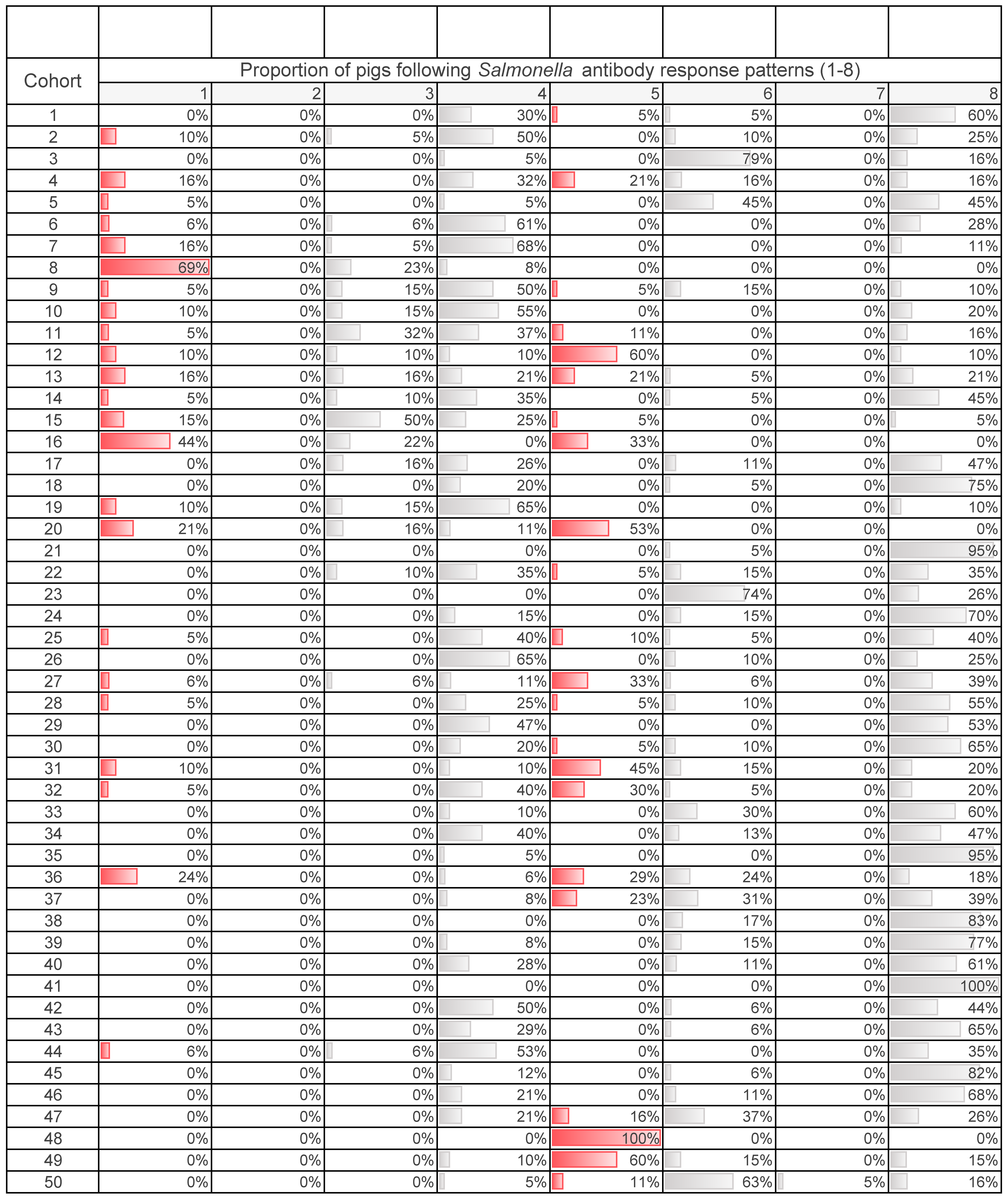
Salmonella antibody response patterns at the pig-level were aggregated to the cohort-level capturing the proportion of pigs following patterns 1 to 8 by cohort (Table 4). At the cohort level, 31/50 (62%) of nursery cohorts were identified as having an active Salmonella infection based on the serological criteria (Table 4).

Table 4.
The change in Salmonella antibody titres in nursery pigs (n = 930) from visit 1 (V1; weaning) to visit 2 (V2; end of nursery) was characterized into eight Salmonella antibody response patterns (1 to 8) a and aggregated to the cohort level (n = 50) b. This table captures the proportion of pigs following patterns 1 to 8 within each cohort.
3.4. Active Salmonella Infections
Although this study collected data from 50 Ontario nursery cohorts, complete bacteriological and serological testing information was only available on 46 nursery cohorts. A two-by-two table that identifies active Salmonella infections using bacteriological and serological detection methods is presented in Table 5. A non-significant McNemar’s χ2 indicated the positive proportions from the bacteriology and serological testing methods did not differ (McNemar’s χ2 = 0.07, p = 0.79). In addition, the k statistic revealed there was a fair agreement between the testing methods in identifying an active Salmonella infection on nursery cohorts (k = 0.29, p = 0.02).

Table 5.
Active Salmonella infection on 46 Ontario nursery cohorts a identified using bacteriological (at the end of nursery (V2)) and serological (based on pattern of antibody response from weaning (V1) to the end of the nursery) testing methods.
Only 9 of 46 (20%) nursery cohorts were found to be negative for Salmonella using both testing methods. The remaining 37 of 46 (80%) nurseries were found positive either using serological or bacteriological methods or both. Specifically, 22 (48%) nursery cohorts were identified with an active Salmonella infection using both identification methods, while 8 (17%) and 7 (15%) were only positive based on serology alone and bacteriology alone, respectively.
3.5. Risk Factors Associated with Swine Cohort Having an Active Salmonella Infection
The association between risk factors and nursery cohorts with an active Salmonella infection was evaluated using a logistic regression model. With univariable analysis, an antibiotic-free system, the use of a shower, and the days the pigs spent in the nursery barn were found significant with a liberal p-value and included in the initial full model (p < 0.1). In the final model, no explanatory variables were significant or required in the model. Thus, no risk factors for active Salmonella infection in nursery barns were found.
4. Discussion
In this study, the epidemiology of Salmonella in nursery pigs was investigated on 44 farms (50 nursery cohorts) using both direct (culture) and indirect (serology) techniques to establish whether pigs were becoming infected during the nursery stage or not. Many pigs entered the nursery with high antibody titres to Salmonella, presumably reflecting circulating antibodies obtained via colostrum [16,20]. Overall, there was evidence that passive immunity declined during the nursery stage. The number of Salmonella-seropositive pigs was higher at the beginning of the nursery compared to the end of the nursery. The presence of this passive immunity, as pigs enter the nursery stage, provides some immune protection if challenged with Salmonella shortly after weaning [16], but the presence of passively-acquired antibodies makes interpretation of serological testing to determine active Salmonella infection in the nursery challenging. During the nursery, the passive immunity wanes and pigs begin to develop acquired immunity if pathogens are encountered [21], and therefore by using two testing points it is possible to identify pigs within a cohort that exhibit a serological pattern indicative of active infection. If pigs encounter Salmonella during the nursery barn after passive immunity disappears, it is likely for pigs to experience an increase in antibody titres [8,21]. But if pigs do not encounter Salmonella in the nursery barn, the decline in antibody titres continues. However, if piglets entering the nursery barn are Salmonella carriers, they are likely to shed Salmonella due to stress associated from weaning, change in environment, diet and comingling with different litters [4,8,15,17]. These animals and their pen-mates are likely to exhibit an increase in Salmonella antibody response.
Although there is a decline in Salmonella seropositivity from weaning to the end of the nursery in pigs, Salmonella is still present and circulating in many of these nursery cohorts. By understanding the Salmonella status at the cohort level, it can help veterinarians and producers better target the control and prevention methods in the nursery barn. Previous studies have used different methods and classification schemes for identifying Salmonella status in pig herds or scoring them as low, moderate and high-risk, using serological (based on different OD values/S/P ratios) and/or bacteriological testing methods [7,22,23,24,25]. Various studies have solely relied on either bacteriology or serology to identify Salmonella status in pigs and on farms [11,25,26]. To improve sensitivity, the present study used both bacteriological and serological detection methods to identify active Salmonella infections on nursery cohorts. Using the specified S/P ratio cut-off provided by the ELISA kit manufacturers, a nursery cohort was identified serological Salmonella positive (active infection) given 1 or more pigs were positive for Salmonella antibody response patterns 1, 2, or 5. The bacteriological identification of a nursery cohort as positive (active infection), based on if 1 or more pigs were shedding Salmonella, was in line with previous studies [7,23].
Traditionally, seropositivity in pigs is assessed at one time and is subsequently tested to monitor Salmonella status at the farm level. However, by using the Salmonella antibody response patterns approach introduced in the present study, the change in antibody response for each individual pig on a farm is identified into categories. This provides more detailed information on the Salmonella antibody response during the nursery stage (i.e., how many pigs are seroconverting on a farm, or how many pigs are increasing in antibody titres but remaining seropositive vs. seronegative, how many pigs are decreasing in antibody titres and becoming seronegative or remaining seropositive). In addition, since this method explores seropositivity status at weaning and at the end of the nursery along with the change in direction of antibody response, it decreases the likelihood of misinterpretation of passive immunity as a Salmonella infection in nursery pigs. Although there are strengths to this method, limitations include its inability to account for the magnitude of change, the initial antibody titre baseline and age at weaning. For example, pigs with an increase in antibody titres from 0.3 to 0.4 and 1.5 to 3.0 were identified as Salmonella antibody response pattern 1. By categorizing these pigs into the same pattern, data is lost. By incorporating the degree of change along with the initial antibody titre baseline into the Salmonella antibody response patterns, there can be better identification of the severity of Salmonella in nursery cohorts. The wide range in age at weaning presents a limitation as animals that were weaned at an older age may have improved Salmonella antibody response compared to animals that were weaned at a younger age.
In the present study, pigs following pattern 6 also had an increase in antibody titre but did not surpass the S/P ratio cut-off to be identified as seropositive. Kranker et al. [4] found a 60-day delay in the peak of seroprevalence after the peak in Salmonella culture prevalence. Surveillance of nursery cohorts with a large number of pigs following pattern 6 is important as it is possible that pigs following this pattern may become seropositive a few weeks after the second sample was taken, or in other words, they were exposed but were falsely classified as negative because of the delay in immune response. On the other hand, pigs following patterns (3, 4, and 8) where titres decrease during the nursery are likely experiencing a decrease in passive immunity and no indication that they are beginning to produce active immunity. However, because of the delay from exposure to antibody response, there is still a possibility of a false negative classification as well. Nursery pigs following pattern 4 and 8 are seronegative at the end of the nursery and would have been classified as negative based on a single serological sample at the end of the nursery. However, pigs following pattern 3 demonstrate a decrease in antibody titre but remain seropositive at the end of the nursery and based on a single test at the end of the nursery would generally be classified as positive. However, our interpretation of pigs showing this pattern is that the results reflect passive immunity and these pigs are categorized as Salmonella negative.
Using this technique of two sampling time points and identifying serological patterns coupled with direct culture of rectal swabs, a large portion of nurseries were identified as having an active Salmonella infection. Some nursery cohorts were identified as having an active infection using serology but would have been considered negative based on culture. Similarly, a few cohorts would have been considered negative by serology, but culture showed that Salmonella was indeed present. Although research has drawn correlation between bacteriological and serological classification, both these testing methods for Salmonella have strengths and weaknesses [7]. Results are somewhat dependent on timing of the test relative to the exposure to Salmonella. Serological testing relies on detecting antibodies and a positive response requires a few weeks to develop, whereas Salmonella culture is more likely to provide positive results shortly after exposure and becomes less reliable after a few weeks [4,7]. In general, bacteriological testing identifies a current infection whereas serological testing identifies historical exposure [7,27,28]. However, an advantage of serological testing is that it allows for the detection of intermittent shedders [4] and is a more inexpensive method with possibly better sensitivity in comparison to bacteriological testing [24].
In the present study, there is evidence of the shortcomings of relying solely on culturing rectal swabs to identify herds with active Salmonella infection because there were herds deemed positive using serology that were negative using culture. This might have been partly due to the use of rectal swabs instead of using a fecal sample of greater than 25 g, which has been advocated for monitoring purposes in populations of animals not exhibiting clinical signs of disease [18]. Likewise, there were herds considered positive based on culture but were considered negative using only serology. The specific ELISA used for this study detected antibody for Salmonella serogroups B, C, D, and E (O-antigens 1, 3, 4, 5,6, 7,9, 10, and 12), commonly found in Ontario. Serotyping Salmonella isolates, found in the present study, would have been beneficial to capture Salmonella serogroups that may have been unidentified by ELISA testing. This presents a limitation, impacting the serology results, because there is a possibility that some Salmonella infections went undetected. In addition, false negatives are a possibility due to laboratory errors during bacteriology and serological testing. This reinforces the argument for using the two testing methods in combination in order to more accurately identify positive herds.
The active Salmonella infection identified in 37 of 46 Ontario nursery cohorts (with complete information on both testing methods) strengthens the point that Salmonella is commonly present on Ontario pig farms, but importantly emphasizes that the nursery stage is a time in production when pigs often become infected and can then carry and spread Salmonella to the grower-finisher barns and eventually to the abattoirs. This information is useful for the timing and implementing of control and prevention strategies. For example, if farmers know that pigs are becoming exposed to Salmonella in the early nursery stage, then the vaccination of sows [29] or young pigs [30] might be implemented. Although a large portion of swine cohorts in this study were identified as having an active Salmonella infection, nine cohorts appeared to be Salmonella-free at the end of the nursery period, based on both serological and bacteriological methods and using a sample population of 20 pigs to represent the cohort. These results suggest that it is possible to have a negative population of pigs to send to the grower-finisher barn.
Examination of risk factors (e.g., AIAO by room, AIAO by barn, continuous flow, use of disinfection etc.) didn’t identify why the nine negative farms were different from nurseries with an active Salmonella infection. It is possibly that no associations were found due to the low variability in risk factors amongst nurseries with an active Salmonella infection compared to nurseries without an active Salmonella infection. Other studies attempting to identify risk factors associated with the presence of Salmonella have been inconsistent [9,14,26,31]. Most of these studies involved testing of pigs close to market weight or testing in abattoirs so their findings may not be relevant to nursery pigs. With the ability for Salmonella to survive long periods of time in the environment [32], it is likely that cleaning, disinfection, and biosecurity are important in reducing Salmonella in the nursery but the farm to farm variation in the implementation of these protocols are hard to capture in a survey and this aspect of Salmonella control requires further study. Future research should explore additional risk factors (i.e., feed management, antibiotic usage) and should include a greater number of nursery cohorts within depth reports on clinical sign related to salmonellosis.
5. Conclusions
Active Salmonella infection can be identified in a high proportion of swine nurseries. Using both serological and bacteriological testing methods in parallel improves the likelihood of identifying a nursery with active transmission of Salmonella. This study identified that both detection methods have strengths and weaknesses and by combining the techniques, researchers can better monitor active Salmonella infections on farms. Although the present study did not identify any risk factors, further work is warranted to investigate how cohorts of pigs in some nurseries remain negative for Salmonella.
Author Contributions
Conceptualization, R.F. and A.F.; methodology, S.N. and A.F.; formal analysis, Z.P. and S.N.; investigation, S.N., A.F., R.F.; data curation, S.N.; writing—original draft preparation, S.N.; writing—review and editing, S.N., A.F., Z.P., R.F.; project administration, R.F.; funding acquisition, R.F. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ontario Pork, Ontario, grant number 02-16, and Agri-Food Innovation Alliance and the University of Guelph, grant number #2245.
Acknowledgments
Participating pork producers and Christopher Almond, Emily Hanna, and Karen De Bruyn for help in collecting samples and Emily Arndt for laboratory assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Griffith, R.W.; Carlson, S.A.; Krull, A.C. Salmonellosis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 912–925. [Google Scholar]
- Davies, P.R. Food safety and its impact on domestic and export markets. Swine Health Prod. 1997, 5, 13–20. [Google Scholar]
- Stevens, M.P.; Gray, J.T. Salmonella Infections in Pigs. In Salmonella in Domestic Animals, 2nd ed.; Barrow, P., Methner, U., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 263–294. [Google Scholar]
- Kranker, S.; Alban, L.; Boes, J.; Dahl, J. Longitudinal Study of Salmonella enterica Serotype Typhimurium Infection in Three Danish Farrow-to-Finish Swine Herds. J. Clin. Microbiol. 2003, 41, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, W.; Rajić, A.; Waldner, C.; McFall, M.; Chow, E.; Muckle, A.; Rosengren, L. Distribution of Salmonella serovars in breeding, nursery, and grow-to-finish pigs, and risk factors for shedding in ten farrow-to-finish swine farms in Alberta and Saskatchewan. Can. J. Vet. Res. 2010, 74, 81–90. [Google Scholar] [PubMed]
- Schut, C.H.; Farzan, A.; Ainslie-Garcia, M.H.; Friendship, R.M.; Lillie, B. Antibody Responses to Salmonella in Pigs from Weaning Up to Marketing and Presence of Salmonella at Slaughter. Foodborne Pathog. Dis. 2019, 16, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.L.F.; Dahl, J.; Wingstrand, A.; Van Der Wolf, P.J.; Von Altrock, A.; Thorberg, B.M. A European longitudinal study in Salmonella seronegative- and seropositive-classified finishing pig herds. Epidemiol. Infect. 2004, 132, 903–914. [Google Scholar] [CrossRef]
- Nollet, N.; Houf, K.; Dewulf, J.; Duchateau, L.; De Zutter, L.; De Kruif, A.; Maes, D. Distribution of Salmonella Strains in Farrow-to-Finish Pig Herds: A Longitudinal Study. J. Food Prot. 2005, 68, 2012–2021. [Google Scholar] [CrossRef]
- Belœil, P.-A.; Fravalo, P.; Fablet, C.; Jolly, J.-P.; Eveno, E.; Hascoet, Y.; Chauvin, C.; Salvat, G.; Madec, F. Risk factors for Salmonella enterica subsp. enterica shedding by market-age pigs in French farrow-to-finish herds. Prev. Vet. Med. 2004, 63, 103–120. [Google Scholar] [CrossRef]
- Davies, P.R.; Morrow, W.E.M.; Jones, F.T.; Deen, J.; Fedorka-Cray, P.T.; Harris, I.T. Prevalence of Salmonela in finishing swine raised in different production systems in North Carolina, USA. Epidemiol. Infect. 1997, 119, 237–244. [Google Scholar] [CrossRef]
- Pires, A.F.A.; Funk, J.A.; Bolin, C.A. Longitudinal study of Salmonella shedding in naturally infected finishing pigs. Epidemiol. Infect. 2012, 141, 1928–1936. [Google Scholar] [CrossRef]
- Rajić, A.; Keenliside, J.; McFall, M.E.; Deckert, A.E.; Muckle, A.C.; O’Connor, B.P.; Manninen, K.; Dewey, C.E.; McEwen, S.A. Longitudinal study of Salmonella species in 90 Alberta swine finishing farms. Vet. Microbiol. 2005, 105, 47–56. [Google Scholar] [CrossRef]
- Penmetchsa, T.V.; White, B.A.; Maddox, C.W.; Firkins, L.D.; Weigel, R.M. Molecular epidemiologic investigation of the role of gilts in the introduction and transmission of Salmonella in swine production systems. J. Swine Health Prod. 2009, 17, 81–89. [Google Scholar]
- Poljak, Z.; Dewey, C.E.; Friendship, R.M.; Martin, S.W.; Christensen, J. Multilevel analysis of risk factors for Salmonella shedding in Ontario finishing pigs. Epidemiol. Infect. 2007, 136, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Casanova-Higes, A.; Marín-Alcalá, C.M.; Andrés-Barranco, S.; Cebollada, A.; Alvarez, J.; Mainar-Jaime, R.C. Weaned piglets: Another factor to be considered for the control of Salmonella infection in breeding pig farms. Vet. Res. 2019, 50, 1–11. [Google Scholar] [CrossRef]
- Funk, J.A.; Davies, P.R.; Nichols, M.A. The Effect of Fecal Sample Weight on Detection ofSalmonella Entericain Swine Feces. J. Vet. Diagn. Investig. 2000, 12, 412–418. [Google Scholar] [CrossRef]
- Farzan, A.; Friendship, R.M. A clinical field trial to evaluate the efficacy of vaccination in controlling Salmonella infection and the association of Salmonella-shedding and weight gain in pigs. Can. J. Vet. Res. 2010, 74, 258–263. [Google Scholar]
- Matiasovic, J.; Kudlackova, H.; Babickova, K.; Stepanova, H.; Volf, J.; Rychlík, I.; Babak, V.; Faldyna, M. Impact of maternally-derived antibodies against Salmonella enterica serovar Typhimurium on the bacterial load in suckling piglets. Vet. J. 2013, 196, 114–115. [Google Scholar] [CrossRef]
- Nair, S.; Newman, J.; Farzan, A.; Friendship, R.M. Salmonella shedding and seropositivity and its association with in-feed flavophospholipol in nursery pigs. Can. J. Vet. Res. 2019, 83, 177–180. [Google Scholar]
- Alban, L.; Stege, H.; Dahl, J. The new classification system for slaughter-pig herds in the Danish Salmonella surveillance-and-control program. Prev. Vet. Med. 2002, 53, 133–146. [Google Scholar] [CrossRef]
- Korsak, N.; Degeye, J.-N.; Etienne, G.; Beduin, J.-M.; China, B.; Ghafir, Y.; Daube, G. Use of a serological approach for prediction of Salmonella status in an integrated pig production system. Int. J. Food Microbiol. 2006, 108, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Alban, L.; Baptista, F.M.; Møgelmose, V.; Sørensen, L.; Christensen, H.; Aabo, S.; Dahl, J. Salmonella surveillance and control for finisher pigs and pork in Denmark—A case study. Food Res. Int. 2012, 45, 656–665. [Google Scholar] [CrossRef]
- Vico, J.P.; Mainar-Jaime, R.C. Serological survey of Salmonella spp. infection in finishing pigs from northeastern Spain and associated risk factors. Span. J. Agric. Res. 2012, 10, 372. [Google Scholar] [CrossRef]
- Van Der Wolf, P.; Wolbers, W.; Elbers, A.; Van Der Heijden, H.; Koppen, J.; Hunneman, W.; Van Schie, F.; Tielen, M.; Van Der Wolf, P.J. Herd level husbandry factors associated with the serological Salmonella prevalence in finishing pig herds in The Netherlands. Vet. Microbiol. 2001, 78, 205–219. [Google Scholar] [CrossRef]
- Methner, U.; Rammler, N.; Fehlhaber, K.; Rösler, U. Salmonella status of pigs at slaughter—Bacteriological and serological analysis. Int. J. Food Microbiol. 2011, 151, 15–20. [Google Scholar] [CrossRef]
- Van Winsen, R.L.; Van Nes, A.; Keuzenkamp, D.; Urlings, H.; Lipman, L.; Biesterveld, S.; Snijders, J.; Verheijden, J.; Van Knapen, F. Monitoring of transmission of Salmonella enterica serovars in pigs using bacteriological and serological detection methods. Vet. Microbiol. 2001, 80, 267–274. [Google Scholar] [CrossRef]
- Smith, R.P.; Andres, V.; Martelli, F.; Gosling, B.; Marco-Jimenez, F.; Vaughan, K.; Tchorzewska, M.; Davies, R. Maternal vaccination as a Salmonella Typhimurium reduction strategy on pig farms. J. Appl. Microbiol. 2017, 124, 274–285. [Google Scholar] [CrossRef]
- Alborali, G.L.; Ruggeri, J.; Pesciaroli, M.; Martinelli, N.; Chirullo, B.; Ammendola, S.; Battistoni, A.; Ossiprandi, M.C.; Corradi, A.; Pasquali, P. Prime-boost vaccination with attenuated Salmonella Typhimurium (increment) znuABC and inactivated Salmonella Choleraesuis is protective against Salmonella Choleraesuis challenge infection in piglets. BMC Vet. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Pires, A.F.A.; Funk, J.; Bolin, C. Risk factors associated with persistence of Salmonella shedding in finishing pigs. Prev. Vet. Med. 2014, 116, 120–128. [Google Scholar] [CrossRef]
- Guan, T.Y.; Holley, R.A. Pathogen survival in swine manure environments and transmission of human enteric illness—A review. J. Environ. Qual. 2003, 32, 383–392. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).