An Easy and Economical Way to Produce a Three-Dimensional Bone Phantom in a Dog with Antebrachial Deformities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
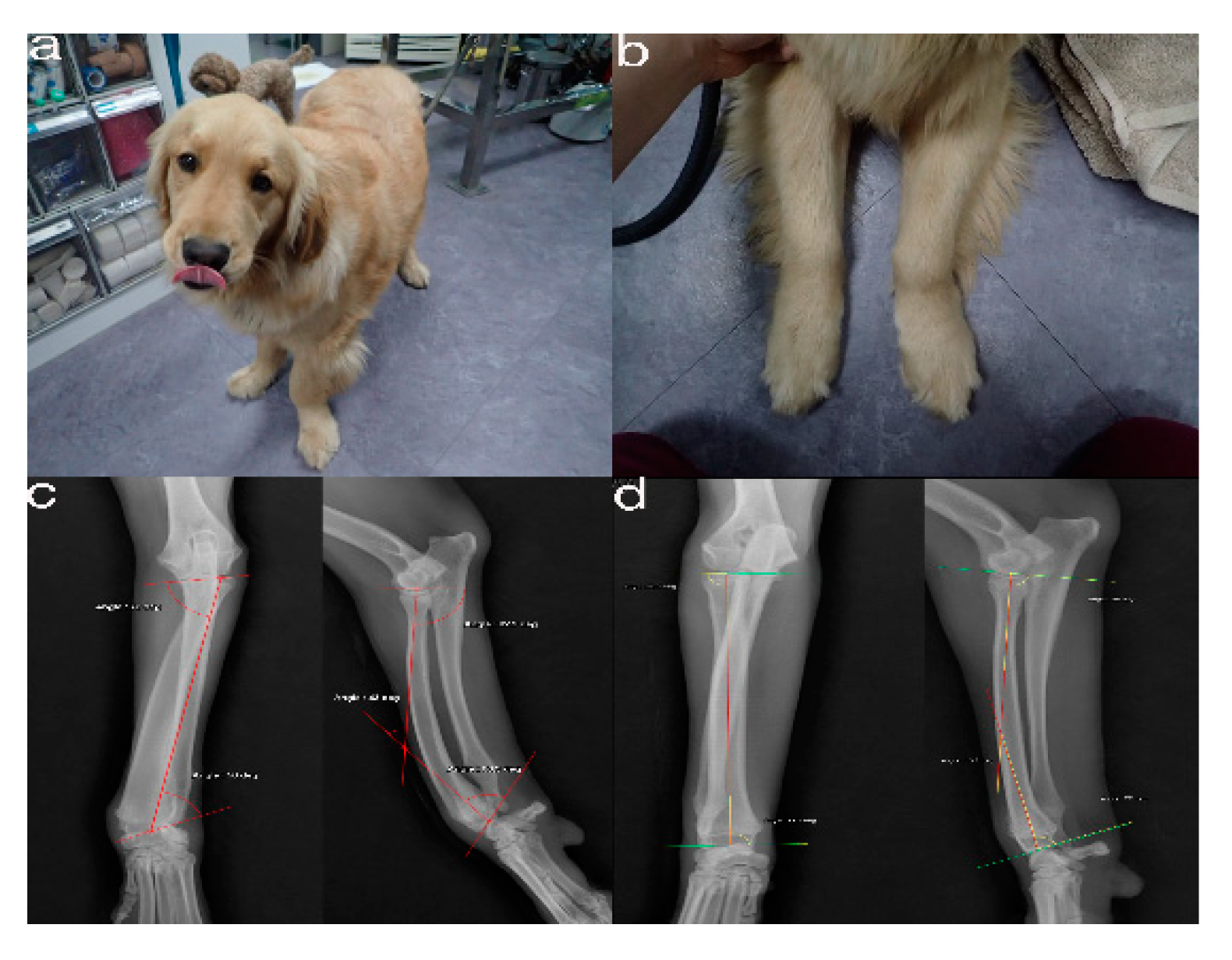
2.1. Case Presentation and Imaging
2.2. Virtual Corrective Osteotomy
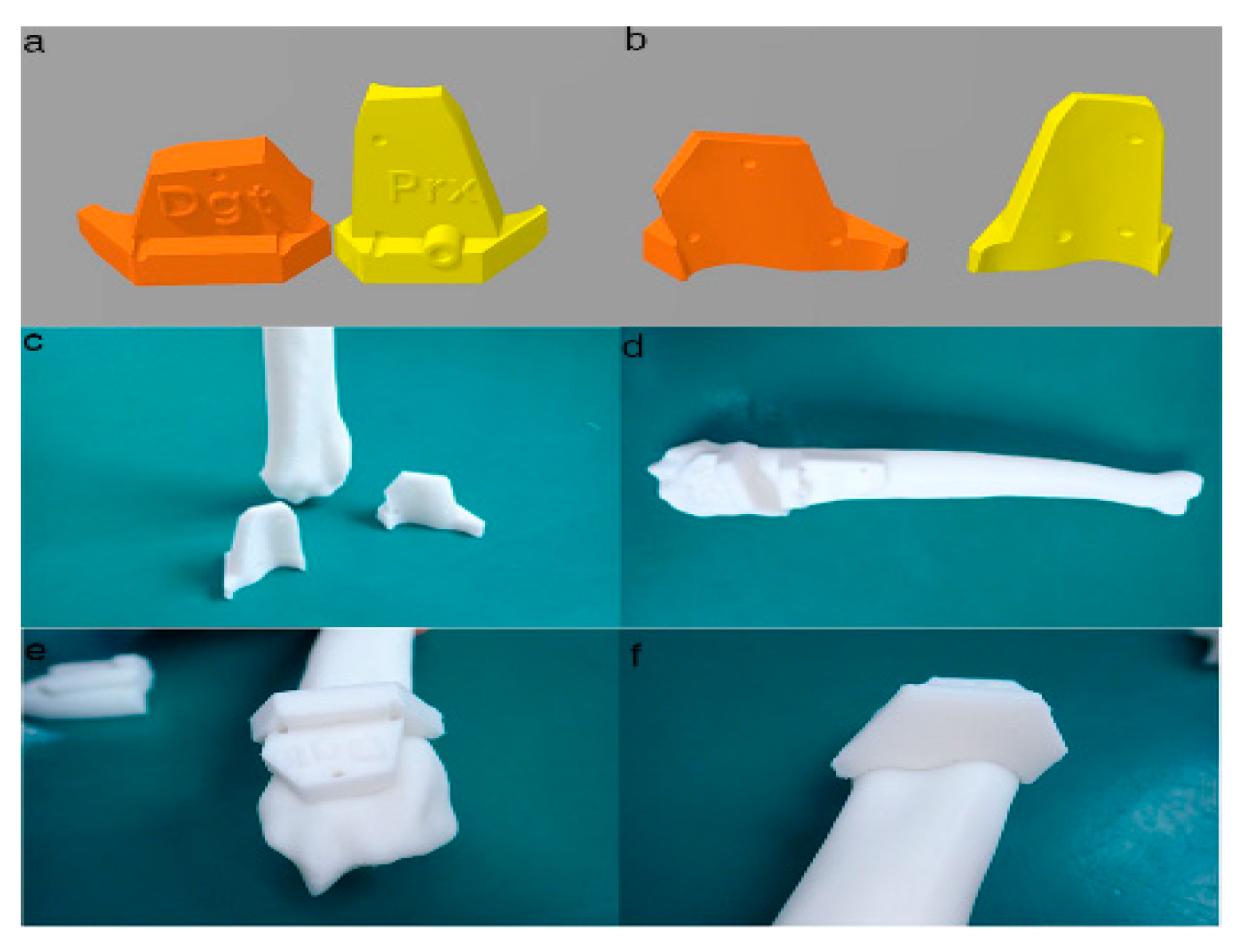
2.3. PSI Design for the Distal Radial Surface
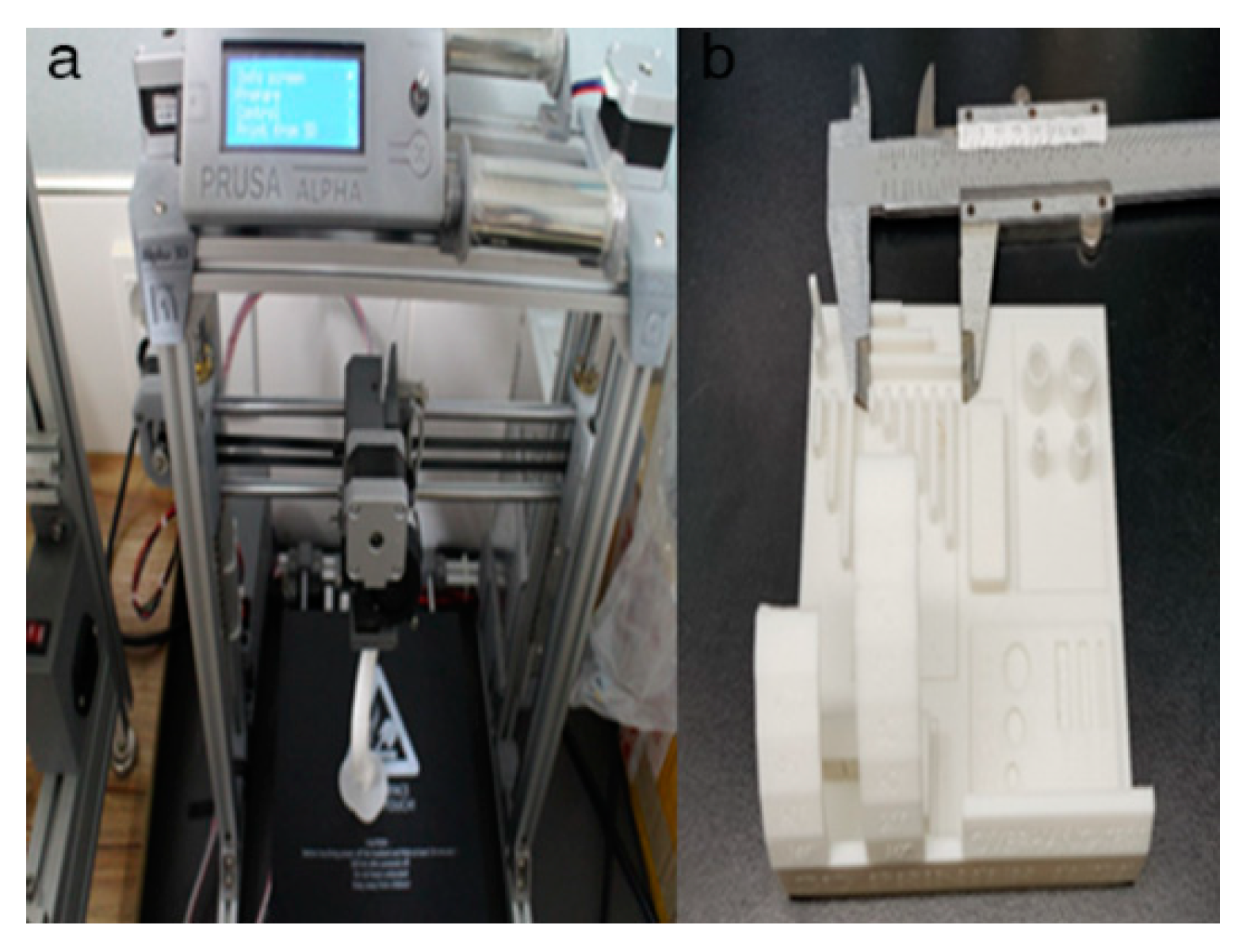
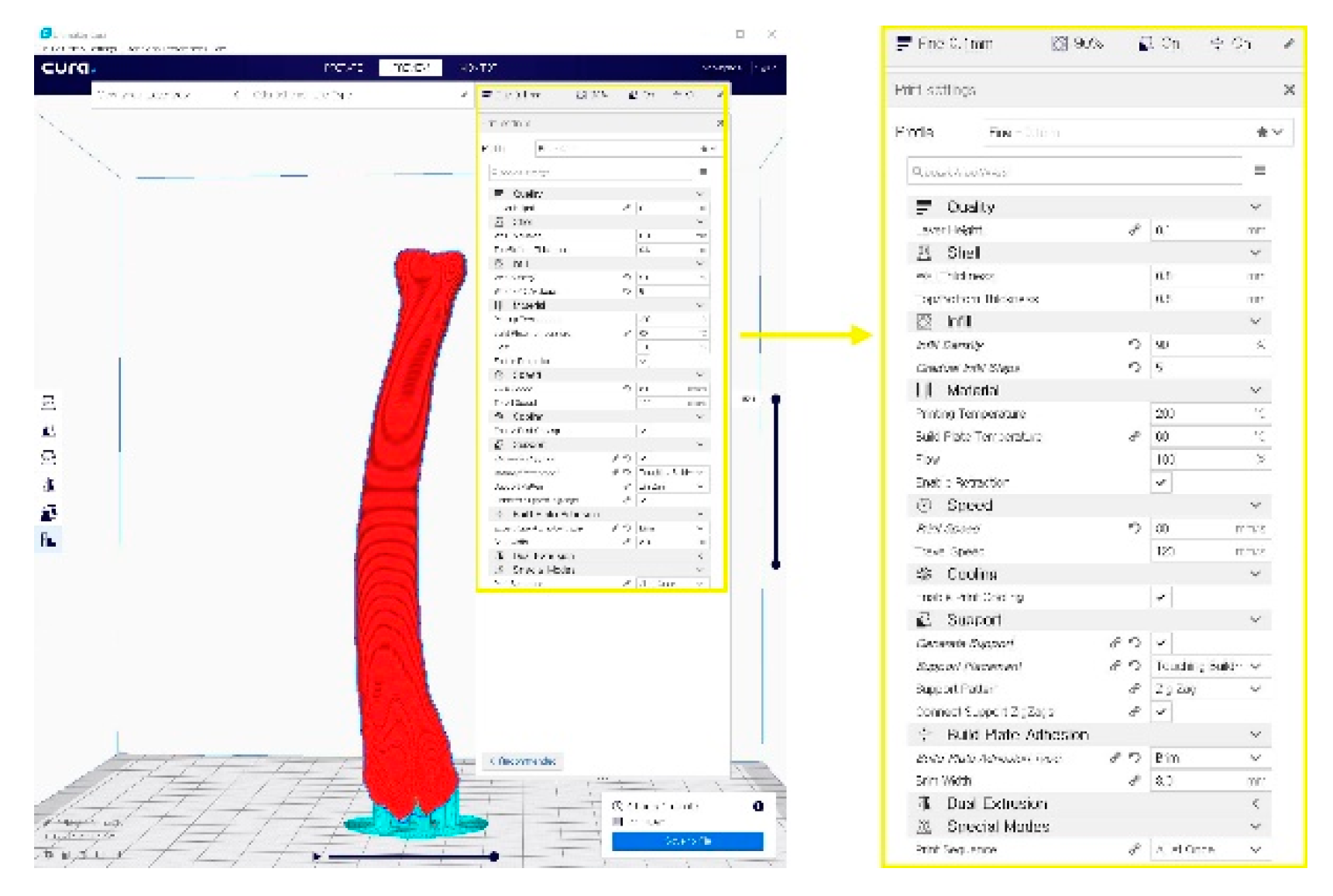
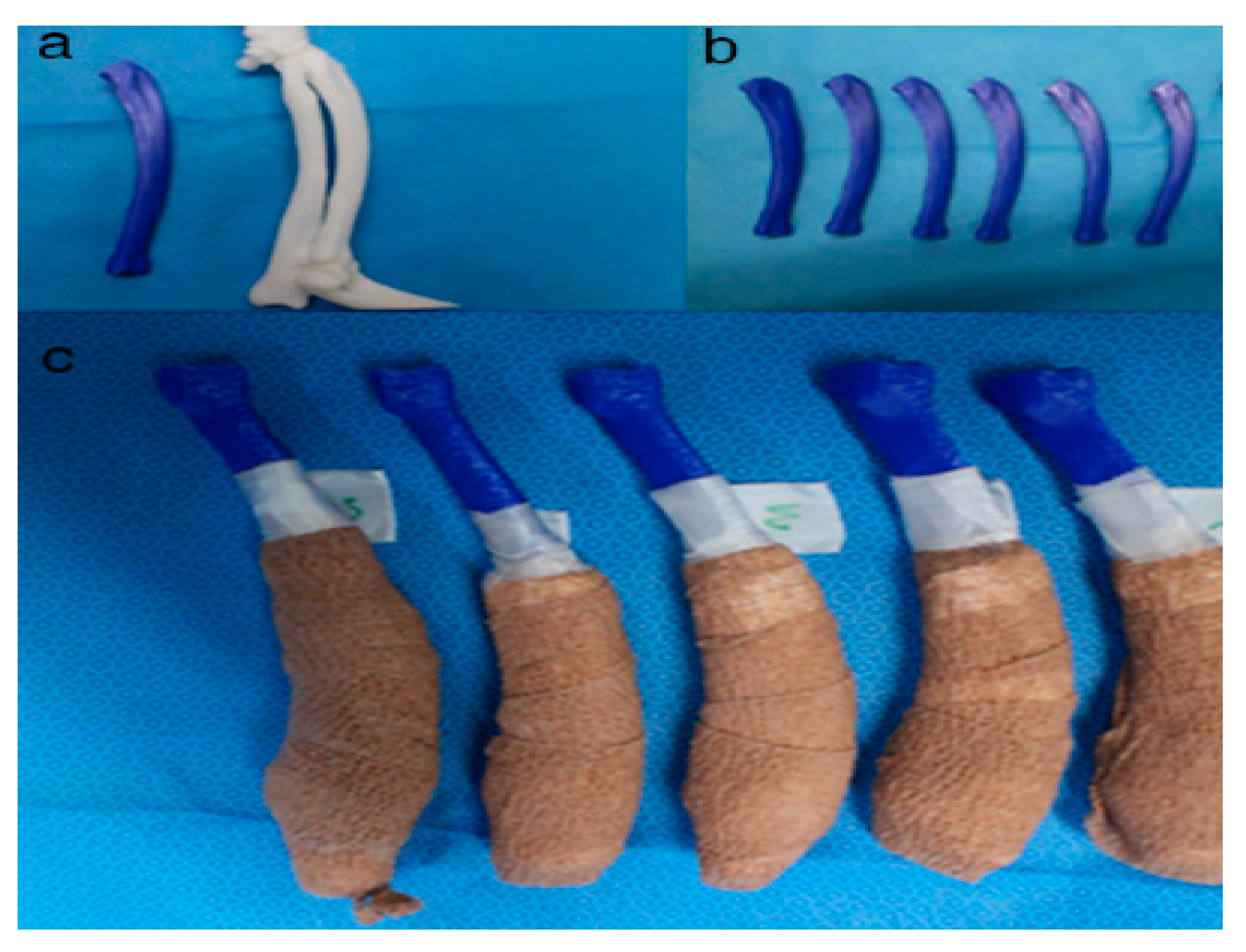
2.4. In-House Fabrication
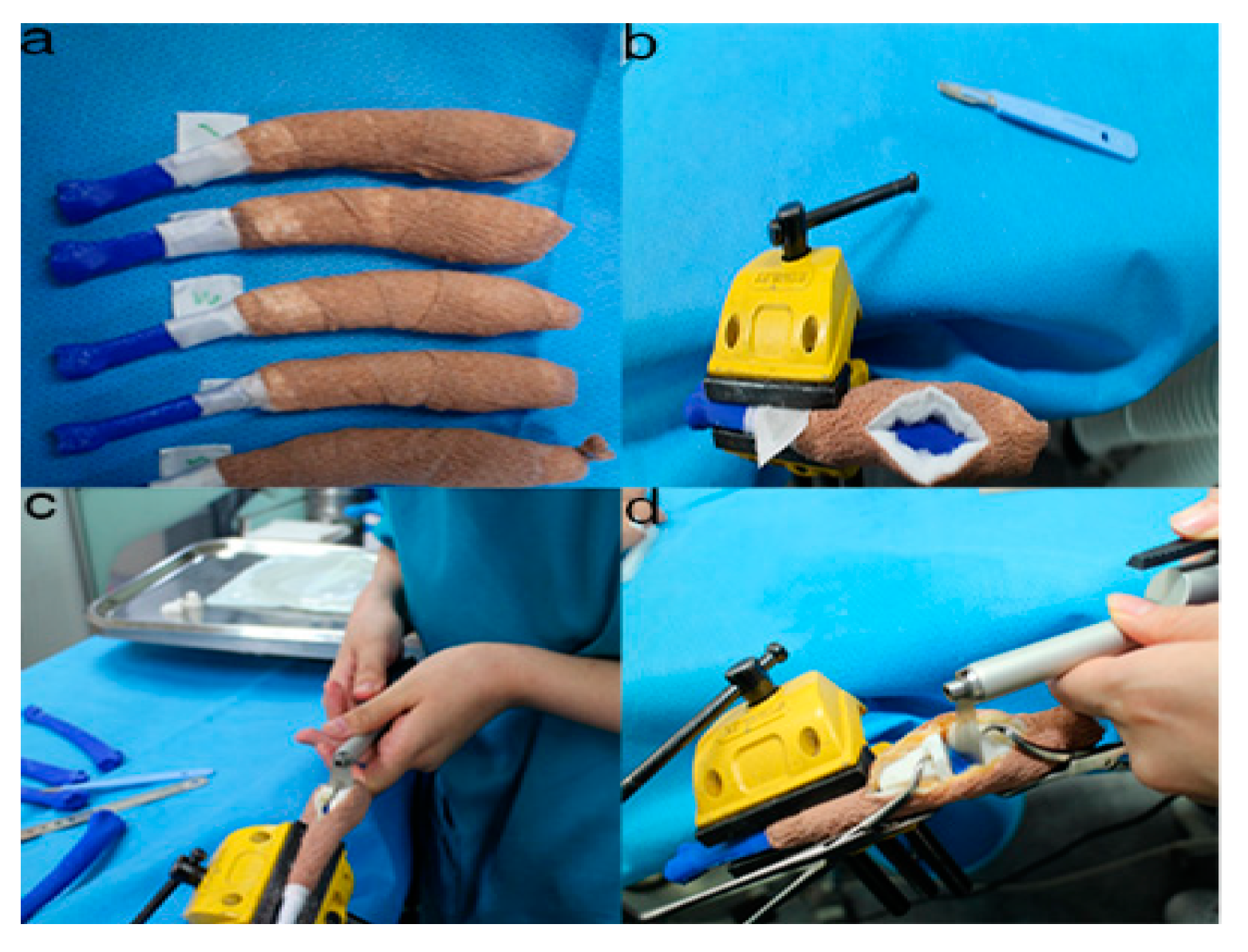
2.5. Rehearsal Surgery on the Phantom Bone Models with PSI
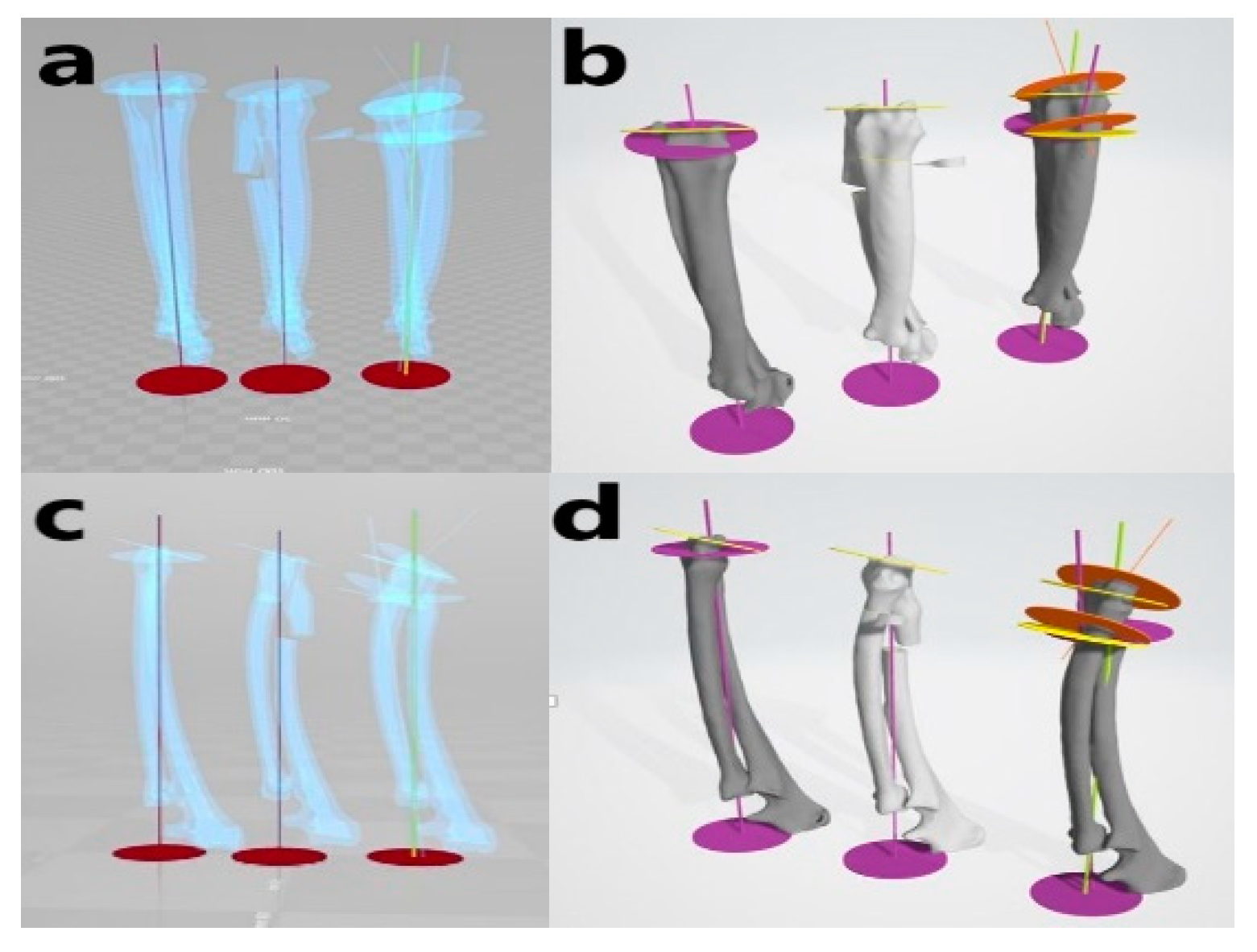
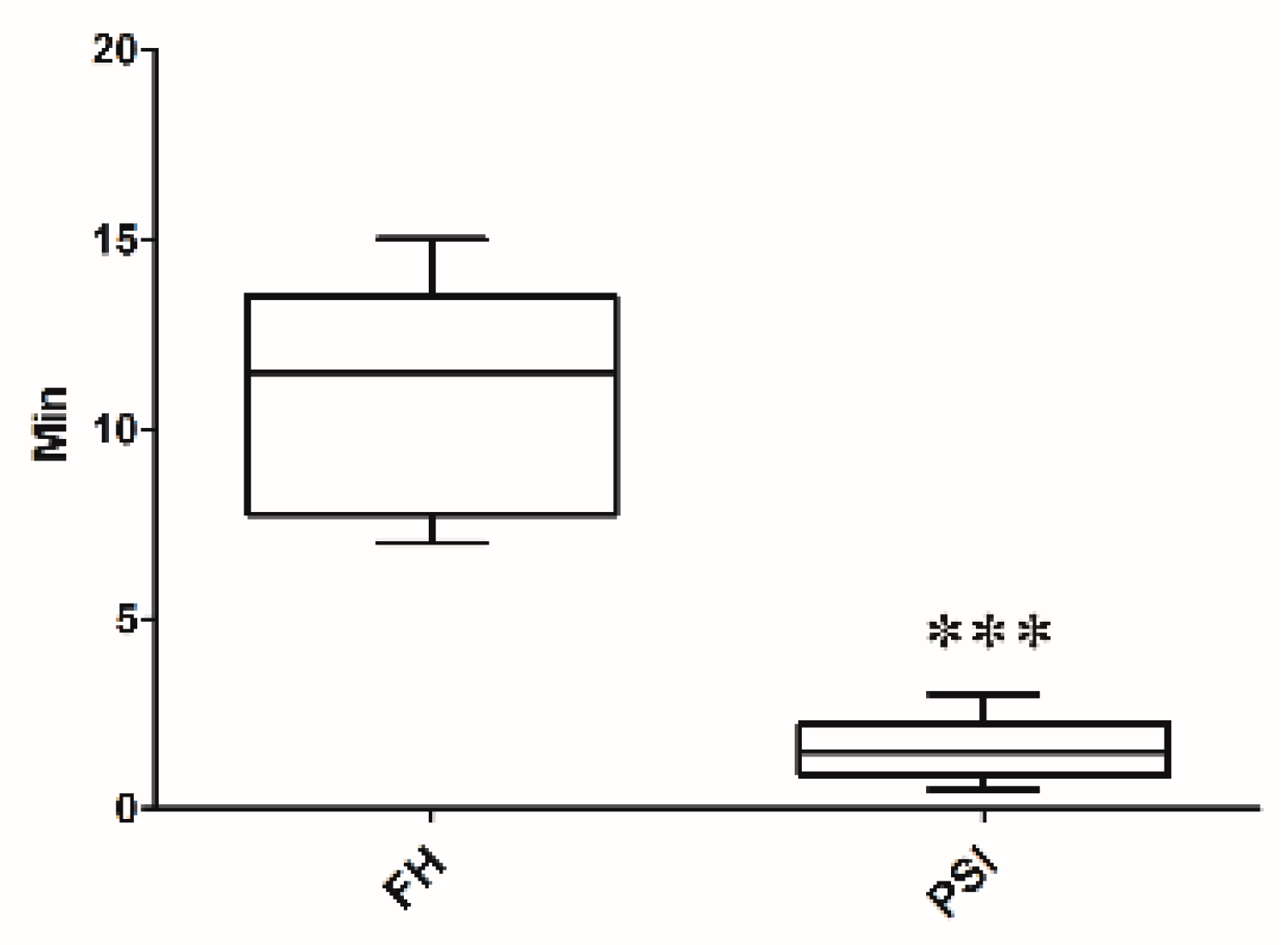
2.6. Accuracy Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kodama, H.A. Scheme for three-dimensional display by automatic fabrication of three-dimensional model. IEICE Trans. Electron. 1981, 64, 237–241. [Google Scholar]
- Kodama, H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Adepu, S.; Dhiman, N.; Laha, A.; Sharma, C.S.; Ramakrishna, S.; Khandelwal, M. Three-dimensional bioprinting for bone tissue regeneration. Curr. Opin. Biomed. Eng. 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Goto, M.; Katsuki, T.; Noguchi, N.; Hino, N. Surgical simulation for reconstruction of mandibular bone defects using photocurable plastic skull models: Report of three cases. J. Oral Maxillofac. Surg. 1997, 55, 772–780. [Google Scholar] [CrossRef]
- Iolascon, G.; Gimigliano, F.; Moretti, A.; De Sire, A.; Migliore, A.; Brandi, M.L.; Piscitelli, P. Early osteoarthritis: How to define, diagnose, and manage. A systematic review. Eur. Geriatr. Med. 2017, 8, 383–396. [Google Scholar] [CrossRef]
- Escott, B.G.; Kelley, S.P. Management of traumatic physeal growth arrest. Orthop. Trauma 2012, 26, 200–211. [Google Scholar] [CrossRef]
- Crosse, K.R.; Worth, A.J. Computer-assisted surgical correction of an antebrachial deformity in a dog. Vet. Comput. Orthop. Traumatol. 2010, 23, 354–361. [Google Scholar] [CrossRef]
- Knapp, J.L.; Tomlinson, J.L.; Fox, D.B. Classification of angular limb deformities affecting the canine radius and ulna using the center of rotation of angulation method. Vet. Surg. 2016, 45, 295–302. [Google Scholar] [CrossRef]
- Savio, G.; Baroni, T.; Concheri, G.; Baroni, E.; Meneghello, R.; Longo, F.; Isola, M. Computation of femoral canine morphometric parameters in three-dimensional geometrical models: 3d morphometric parameters in canine femur. Vet. Surg. 2016, 45, 987–995. [Google Scholar] [CrossRef]
- Murphy, S.B.; Kijewski, P.K.; Simon, S.R.; Chandler, H.P.; Griffin, P.P.; Reilly, D.T.; Penenberg, B.L.; Landy, M.M. Computer-aided simulation, analysis, and design in orthopedic surgery. Orthop. Clin. N. Am. 1986, 17, 637–649. [Google Scholar]
- Sangeorzan, B.J.; Sangeorzan, B.P.; Hansen, S.T.; Judd, R.P. Mathematically directed single-cut osteotomy for correction of tibial malunion. J. Orthop. Trauma 1989, 3, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Patralekh, M.K.; Vaish, A.; Agarwal, A.K.; Vijay, V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J. Clin. Orthop. Trauma 2018, 9, 194–201. [Google Scholar] [CrossRef]
- Ippolito, R.; Iuliano, L.; Gatto, A. Benchmarking of rapid prototyping techniques in terms of dimensional accuracy and surface finish. CIRP Ann. 1995, 44, 157–160. [Google Scholar] [CrossRef]
- Webb, P.A. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J. Med. Eng. Technol. 2000, 24, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.C.; Siebenrock, K.A.; Schiele, B.; Gerber, C. A new methodology for the planning of single-cut corrective osteotomies of mal-aligned long bones. Clin. Biomech. Bristol Avon 2005, 20, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, R.; Fox, R.; Comerford, E.; Newitt, A. Bilateral angular carpal deformity in a dog with craniomandibular osteopathy. Vet. Comput. Orthop. Traumatol. 2012, 25, 149–154. [Google Scholar]
- Arzi, B.; Cissell, D.D.; Pollard, R.E.; Verstraete, F.J.M. Regenerative approach to bilateral rostral mandibular reconstruction in a case series of dogs. Front. Vet. Sci. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Hespel, A.M.; Wilhite, R.; Hudson, J. Invited review—Applications for 3D printers in veterinary medicine. Vet. Radiol. Ultrasound 2014, 55, 347–358. [Google Scholar] [CrossRef]
- Castilho, M.; Rodrigues, J.; Vorndran, E.; Gbureck, U.; Quental, C.; Folgado, J.; Fernandes, P.R. Computational design and fabrication of a novel bioresorbable cage for tibial tuberosity advancement application. J. Mech. Behav. Biomed. Mater. 2017, 65, 344–355. [Google Scholar] [CrossRef] [PubMed]
- European Society of Articial Organs. Proceedings of the 46th ESAO Congress, Hannover, Germany, 3–7 September 2019: Abstracts. Int. J. Artif. Organs 2019, 42, 386–474. [Google Scholar] [CrossRef]
- Singhal, A.J.; Shetty, V.; Bhagavan, K.R.; Ragothaman, A.; Koneru, G.; Agarwala, M. Improved surgery planning using 3-D printing: A case study. Indian J. Surg. 2016, 78, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Worth, A.J.; Crosse, K.R.; Kersley, A. Computer-Assisted Surgery Using 3D Printed Saw Guides for Acute Correction of Antebrachial Angular Limb Deformities in Dogs. Vet. Comput. Orthop. Traumatol. 2019, 32, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Penelas, A.; Gutbrod, A.; Pozzi, A. Three-dimensional computer-assisted corrective osteotomy with a patient-specific surgical guide for an antebrachial limb deformity in two dogs. Schweiz. Arch. Tierheilkd. 2019, 161, 473–479. [Google Scholar] [CrossRef] [PubMed]
- João, B.; Dias, M.I.; Luís, C.; Requicha, J.F.; Viegas, C.A.A.; Jean, B. A 3D printed model for radius curvus surgical treatment planning in a dog. Pesqui. Vet. Bras. 2017, 38, 1178–1183. [Google Scholar]
- Dorbandt, D.M.; Joslyn, S.K.; Hamor, R.E. Three-dimensional printing of orbital and peri-orbital masses in three dogs and its potential applications in veterinary ophthalmology. Vet. Ophthalmol. 2017, 20, 58–64. [Google Scholar] [CrossRef]
- Jeong, B.; Jung, J.; Park, J.; Jeong, S.M.; Lee, H. 3D-printing bone model for surgical planning of corrective osteotomy for treatment of medial patellar luxation in a dog. J. Vet. Clin. 2016, 33, 385–388. [Google Scholar] [CrossRef]
- Fox, D.B.; Tomlinson, J.L.; Cook, J.L.; Breshears, L.M. Principles of uniapical and biapical radial deformity correction using dome osteotomies and the center of rotation of angulation methodology in dogs. Vet. Surg. 2006, 35, 67–77. [Google Scholar] [CrossRef]
- Rosseels, W.; Herteleer, M.; Sermon, A.; Nijs, S.; Hoekstra, H. Corrective osteotomies using patient-specific 3D-printed guides: A critical appraisal. Eur. J. Trauma Emerg. Surg. 2019, 45, 299–307. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef]
- Popescu, D.; Laptoiu, D. Rapid prototyping for patient-specific surgical orthopaedics guides: A systematic literature review. Proc. Inst. Mech. Eng. H 2016, 230, 495–515. [Google Scholar] [CrossRef]
- Schemitsch, E.; Richards, R. The effect of malunion on functional outcome after plate fixation of. J. Bone Jt. Surg. Am. 1992, 74, 1068–1078. [Google Scholar] [CrossRef]
- Athwal, G.S.; Ellis, R.E.; Small, C.F.; Pichora, D.R. Computer-assisted distal radius osteotomy. J. Hand Surg. 2003, 28, 951–958. [Google Scholar] [CrossRef]
- Croitoru, H.; Ellis, R.; Prihar, R.; Small, C.; Pichora, D. Fixation-based surgery: A new technique for distal radius osteotomy. Comput. Aided Surg. 2001, 6, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Kunz, M.; Gammon, B.; Ellis, R.E.; Pichora, D.R. A laboratory comparison of computer navigation and individualized guides for distal radius osteotomy. Int. J. Comput. Assist. Radiol. Surg. 2014, 9, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Meola, S.D.; Wheeler, J.L.; Rist, C.L. Validation of a technique to assess radial torsion in the presence of procurvatum and valgus deformity using computed tomography: A cadaveric study. Vet. Surg. 2008, 37, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bindra, R.R.; Cole, R.J.; Yamaguchi, K.; Evanoff, B.A.; Pilgram, T.K.; Gilula, L.A.; Gelberman, R.H. Quantification of the radial torsion angle with computerized tomography in cadaver specimens. J. Bone Jt. Surg. Am. 1997, 79, 833–837. [Google Scholar] [CrossRef]
- Mahaisavariya, B.; Sitthiseripratip, K.; Oris, P.; Tongdee, T. Rapid prototyping model for surgical planning of corrective osteotomy for cubitus varus: Report of two cases. Inj. Extra 2006, 37, 176–180. [Google Scholar] [CrossRef]
- Cartiaux, O.; Paul, L.; Docquier, P.L.; Francq, B.G.; Raucent, B.; Dombre, E.; Banse, X. Accuracy in planar cutting of bones: An ISO-based evaluation. Int. J. Med. Robot. 2009, 5, 77–84. [Google Scholar] [CrossRef]
- Oka, K.; Murase, T.; Moritomo, H.; Goto, A.; Nakao, R.; Yoshikawa, H.; Sugamoto, K. Accuracy of corrective osteotomy using a custom-designed device based on a novel computer simulation system. J. Orthop. Sci. 2011, 16, 85–92. [Google Scholar] [CrossRef]
- Bosma, S.E.; Wong, K.C.; Paul, L.; Gerbers, J.G.; Jutte, P.C. A cadaveric comparative study on the surgical accuracy of freehand, computer navigation, and patient-specific instruments in joint-preserving bone tumor resections. Sarcoma 2018, 2018, 4065846. [Google Scholar] [CrossRef]






| Cranial 1 | Caudal 1 | Medial 1 | Lateral 1 | Cranial 2 | Caudal 2 | Medial 2 | Lateral 2 | |
|---|---|---|---|---|---|---|---|---|
| Target | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FH 1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| FH 2 | 2 | 3 | 1 | 10 | 4 | 2 | 4 | 7 |
| FH 3 | 5 | 2 | 7 | 3 | 4 | 1 | 4 | 5 |
| FH 4 | 1 | 0 | 1 | 3 | 2 | 5 | 4 | 2 |
| FH 5 | 5 | 5 | 5 | 1 | 2 | 3 | 5 | 3 |
| PSI 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PSI 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PSI 3 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| PSI 4 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| PSI 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| t-test FH vs. PSI | 0.015 | 0.034 | 0.034 | 0.034 | 0.046 | 0.022 | 0.000 | 0.008 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-R.; Adam, G.O.; Yang, D.K.; Tungalag, T.; Lee, S.-J.; Kim, J.-S.; Kang, H.-S.; Kim, S.-J.; Kim, N.S. An Easy and Economical Way to Produce a Three-Dimensional Bone Phantom in a Dog with Antebrachial Deformities. Animals 2020, 10, 1445. https://doi.org/10.3390/ani10091445
Lee H-R, Adam GO, Yang DK, Tungalag T, Lee S-J, Kim J-S, Kang H-S, Kim S-J, Kim NS. An Easy and Economical Way to Produce a Three-Dimensional Bone Phantom in a Dog with Antebrachial Deformities. Animals. 2020; 10(9):1445. https://doi.org/10.3390/ani10091445
Chicago/Turabian StyleLee, Hee-Ryung, Gareeballah Osman Adam, Dong Kwon Yang, Tsendsuren Tungalag, Sei-Jin Lee, Jin-Shang Kim, Hyung-Sub Kang, Shang-Jin Kim, and Nam Soo Kim. 2020. "An Easy and Economical Way to Produce a Three-Dimensional Bone Phantom in a Dog with Antebrachial Deformities" Animals 10, no. 9: 1445. https://doi.org/10.3390/ani10091445
APA StyleLee, H.-R., Adam, G. O., Yang, D. K., Tungalag, T., Lee, S.-J., Kim, J.-S., Kang, H.-S., Kim, S.-J., & Kim, N. S. (2020). An Easy and Economical Way to Produce a Three-Dimensional Bone Phantom in a Dog with Antebrachial Deformities. Animals, 10(9), 1445. https://doi.org/10.3390/ani10091445







