C57BL/6J and B6129F1 Embryo Transfer: Unilateral and Bilateral Transfer, Embryo Number and Recipient Female Background Control for the Optimization of Embryo Survival and Litter Size
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
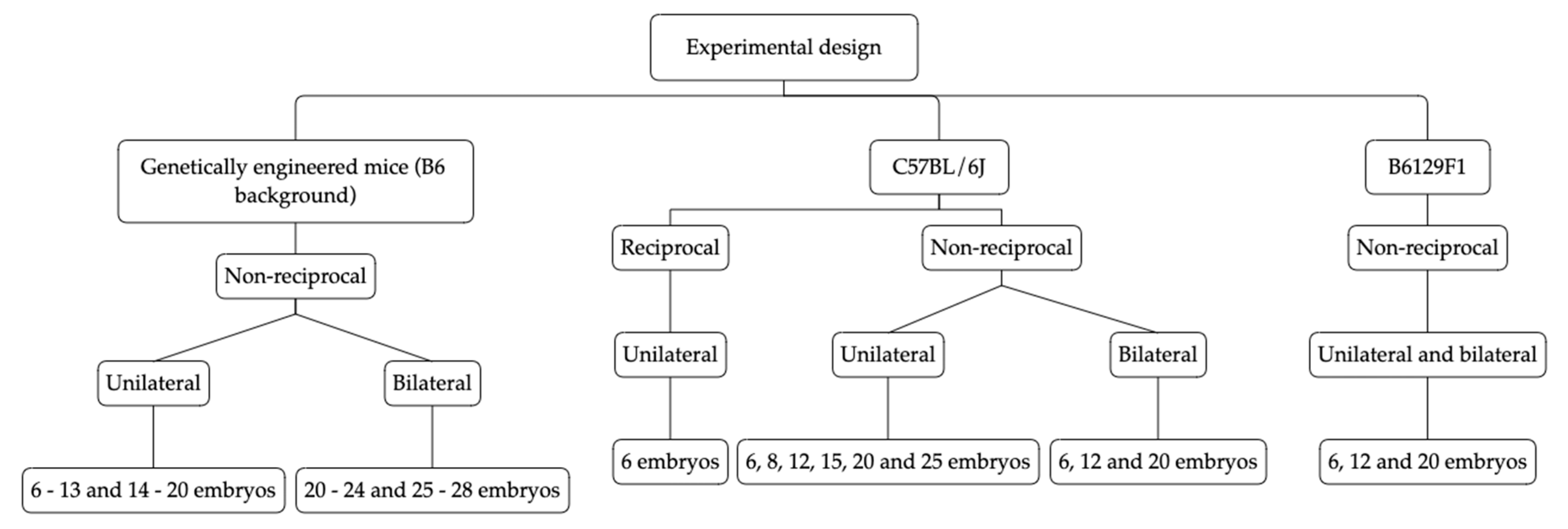
2.2. Experimental Groups
2.3. Superovulation
2.4. Embryo Collection
2.5. Embryo Transfer
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Jara, P.; Fontela, T.; López-Mimbela, E.; Cereceda, M.; Del Olmo, D.; Moreno, M. Optimization of the balance between effort and yield in unilateral surgical transfer of mouse embryos. Lab. Anim. 2017, 51, 622–628. [Google Scholar] [CrossRef]
- Dorsch, M.; Wittur, I.; Garrels, W. Success of embryo transfer in mice with freshly collected and cryopreserved two-cell embryos with different genetic backgrounds correlated with the number of transferred embryos: A 5-year retrospective analysis. Lab. Anim. 2019, 53, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, E.; Volland, R.; Landsberger, A.; Manz, S.; Na, E.; Urban, I.; Michel, G. Reproductive Performance after Unilateral or Bilateral Oviduct Transfer of 2-Cell Embryos in Mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 110–114. [Google Scholar] [PubMed]
- Johnson, L.W.; Moffatt, R.J.; Bartol, F.F.; Pinkert, C.A. Optimization of embryo transfer protocols for mice. Theriogenology 1996, 46, 1267–1276. [Google Scholar] [CrossRef]
- Heykants, M.; Mahabir, E. Estrous cycle staging before mating led to increased efficiency in the production of pseudopregnant recipients without negatively affecting embryo transfer in mice. Theriogenology 2016, 85, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Mochida, K.; Ogonuki, N.; Hirose, M.; Tomishima, T.; Inoue, K.; Ogura, A. Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J. Reprod. Dev. 2017, 63, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Mamrot, J.; Pangestu, M.; Walker, D.; Gardner, D.K.; Dickinson, H. Confirmed dioestrus in pseudopregnant mice using vaginal exfoliative cytology improves embryo transfer implantation rate. Reprod. Biomed. Online 2015, 31, 538–543. [Google Scholar] [CrossRef][Green Version]
- Wakuda, K.; Takakura, K.; Nakanishi, K.; Kita, N.; Shi, H.; Hirose, M.; Noda, Y. Embryo-dependent induction of embryo receptivity in the mouse endometrium. Reproduction 1999, 115, 315–324. [Google Scholar] [CrossRef]
- Li, S.-J.; Wang, T.-S.; Qin, F.-N.; Huang, Z.; Liang, X.-H.; Gao, F.; Song, Z.; Yang, Z.-M. Differential regulation of receptivity in two uterine horns of a recipient mouse following asynchronous embryo transfer. Sci. Rep. 2015, 5, 15897. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Tsai, C.-Y.; Huang, L.-W.; Seow, K.-M.; Hwang, J.-L.; Tzeng, C.-R. Reduced uterine receptivity for mouse embryos developed from in-vitro matured oocytes. J. Assist. Reprod. Genet. 2014, 31, 1713–1718. [Google Scholar] [CrossRef]
- Lee, M.; Ahn, J.I.; Lee, A.R.; Ko, D.W.; Yang, W.S.; Lee, G.; Ahn, J.Y.; Lim, J.M. Adverse Effect of Superovulation Treatment on Maturation, Function and Ultrastructural Integrity of Murine Oocytes. Mol. Cells 2017, 40, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Karagenc, L.; Yalcin, E.; Ulug, U.; Bahceci, M. Administration of increasing amounts of gonadotrophin compromises preimplantation development of parthenogenetic mouse embryos. Reprod. Biomed. Online 2004, 8, 628–634. [Google Scholar] [CrossRef]
- Van der Auwera, I.; D’Hooghe, T. Superovulation of female mice delays embryonic and fetal development. Hum. Reprod. 2001, 16, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, I.; Pijnenborg, R.; Koninckx, P.R. The influence of in-vitro culture versus stimulated and untreated oviductal environment on mouse embryo development and implantation. Hum. Reprod. 1999, 14, 2570–2574. [Google Scholar] [CrossRef][Green Version]
- Ertzeid, G.; Storeng, R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum. Reprod. 2001, 16, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Schwegler, H.; Hanke, J.; Yilmazer-Hanke, D.M. Pregnancy rates, prenatal and postnatal survival of offspring, and litter sizes after reciprocal embryo transfer in DBA/2JHd, C3H/HeNCrl and NMRI mice. Theriogenology 2012, 77, 1883–1893. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Gourabi, H.; Eimani, H.; Barekati, Z.; Akhlaghi, A. Superovulation, in vitro fertilization (IVF) and in vitro development (IVD) protocols for inbred BALB/cJ mice in comparison with outbred NMRI mice. Reprod. Med. Biol. 2012, 11, 185–192. [Google Scholar] [CrossRef]
- Van Keuren, M.L.; Saunders, T.L. Rederivation of transgenic and gene-targeted mice by embryo transfer. Transgenic Res. 2004, 13, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.L.; Fujiwara, Y. The use of buprenorphine as an analgesic after rodent embryo transfer. Lab Anim. 2008, 37, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.R.; Myers, P.H.; Goulding, E.H.; Blankenship, T.L.; Grant, M.F.; Forsythe, D.B. The effects of perioperative analgesia on litter size in Crl:CD1(ICR) mice undergoing embryo transfer. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 423–426. [Google Scholar]
- Schlapp, G.; Goyeneche, L.; Fernández, G.; Menchaca, A.; Crispo, M. Administration of the nonsteroidal anti-inflammatory drug tolfenamic acid at embryo transfer improves maintenance of pregnancy and embryo survival in recipient mice. J. Assist. Reprod. Genet. 2015, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, T.; Arras, M.; Sauer, M.; Saleh, L.; Rülicke, T.; Jirkof, P. Voluntary intake of paracetamol-enriched drinking water and its influence on the success of embryo transfer in mice. Res. Vet. Sci. 2017, 111, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.M.; Austin, J.; Wilkerson, J.; Carbone, L. Effects of multimodal analgesia on the success of mouse embryo transfer surgery. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 466–470. [Google Scholar] [PubMed]
- Hogan, B.; Costantini, F.; Lacy, E. Manipulating the Mouse Embryo: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1986. [Google Scholar]
- Norton, W.B.; Scavizzi, F.; Smith, C.N.; Dong, W.; Raspa, M.; Parker-Thornburg, J.V. Refinements for embryo implantation surgery in the mouse: Comparison of injectable and inhalant anesthesias—Tribromoethanol, ketamine and isoflurane—On pregnancy and pup survival. Lab. Anim. 2016, 50, 335–343. [Google Scholar] [CrossRef]
- Lamas, S.; Franquinho, F.; Morgado, M.; Gartner, F.; Amorim, I. Harvesting of Mouse Embryos at 0.5 Dpc as a Tool to Reduce Animal Use: Data from C57BL/6J, B6* 129 and FVB/NJ Strains. Open J. Anim. Sci. 2020, 10, 254–265. [Google Scholar] [CrossRef][Green Version]
- Rülicke, T.; Haenggli, A.; Rappold, K.; Moehrlen, U.; Stallmach, T. No transuterine migration of fertilised ova after unilateral embryo transfer in mice. Reprod. Fertil. Dev. 2006, 18, 885–891. [Google Scholar] [CrossRef]
- Strobel, M.C.; Reinholdt, L.G.; Malcolm, R.D.; Pritchett-Corning, K. Chapter 31—Genetic Monitoring of Laboratory Mice and Rats. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1403–1416. [Google Scholar] [CrossRef]
- Barkley, M.; FitzGerald, R. Influence of embryonic and maternal genotype on gestational events in the mouse. Reproduction 1990, 89, 285–291. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Matsuo, M.; Gebril, M.; Akaeda, S.; Hiraoka, T.; Osuga, Y. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 2019, 18, 234–240. [Google Scholar] [CrossRef]
- Matsumoto, H. Molecular and cellular events during blastocyst implantation in the receptive uterus: Clues from mouse models. J. Reprod. Dev. 2017, 63, 445–454. [Google Scholar] [CrossRef]
- Schwarzer, C.; Esteves, T.C.; Araúzo-Bravo, M.J.; Le Gac, S.; Nordhoff, V.; Schlatt, S.; Boiani, M. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum. Reprod. 2012, 27, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Paolo, R.; Richard, M.S. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction 2004, 128, 301–311. [Google Scholar] [CrossRef]
- Ozturk, S.; Yaba-Ucar, A.; Sozen, B.; Mutlu, D.; Demir, N. Superovulation alters embryonic poly(A)-binding protein (Epab) and poly(A)-binding protein, cytoplasmic 1 (Pabpc1) gene expression in mouse oocytes and early embryos. Reprod. Fertil. Dev. 2016, 28, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Huang, H.; Guan, Y.; Li, M.; Jiang, X.; Yu, M.; Yang, X. Effects of superovulation, in vitro fertilization, and oocyte in vitro maturation on imprinted gene Grb10 in mouse blastocysts. Arch. Gynecol. Obstet. 2018, 298, 1219–1227. [Google Scholar] [CrossRef]
- Uysal, F.; Ozturk, S.; Akkoyunlu, G. Superovulation alters DNA methyltransferase protein expression in mouse oocytes and early embryos. J. Assist. Reprod. Genet. 2018, 35, 503–513. [Google Scholar] [CrossRef]
- Auerbach, A.; Norinsky, R.; Ho, W.; Losos, K.; Guo, Q.; Chatterjee, S.; Joyner, A. Strain-Dependent Differences in the Efficiency of Transgenic Mouse Production. Transgenic Res. 2003, 12, 59–69. [Google Scholar] [CrossRef]
- Moler, T.L.; Donahue, S.E.; Anderson, G.B.; Bradford, G.E. Effects of Maternal and Embryonic Genotype on Prenatal Survival in Two Selected Mouse Lines. J. Anim. Sci. 1980, 51, 300–303. [Google Scholar] [CrossRef]
| Reciprocity | Surgical Technique | Number of Transfers | Total Number of Embryos Transferred | Pregnancy Rate (%) | Mean Number of Pups Born per Litter a |
|---|---|---|---|---|---|
| Non-reciprocal | Unilateral | 17 | 6 to 13 | 100 | 5.5 ± 0.4 |
| 22 | 14 to 22 | 77.3 | 7.1 ± 0.4 | ||
| Bilateral | 23 | 20 to 24 | 73.9 | 6.4 ± 0.4 | |
| 12 | 25 to 28 | 83.3 | 5.5 ± 0.6 |
| Reciprocity | Surgical Technique | Total n of Embryos Transferred | Number of Transfers | Number of Pregnant Females | Pregnancy Rate (%) | Number of Pups Born per Litter a | Success Rate (%) a |
|---|---|---|---|---|---|---|---|
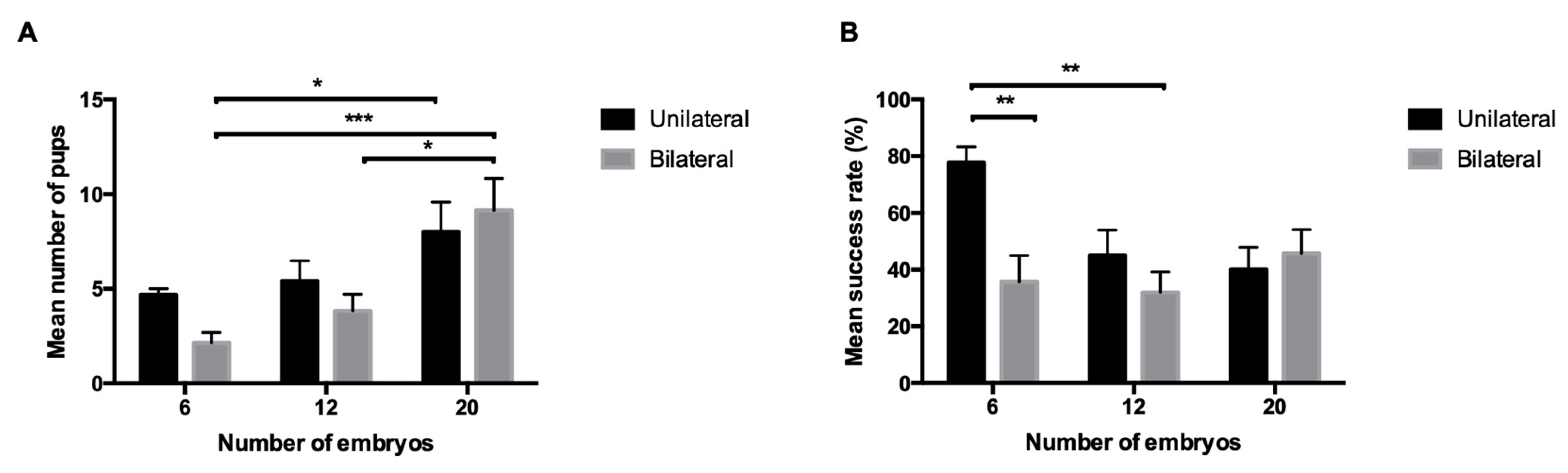
| Non-reciprocal | Unilateral | 6 | 7 | 6 | 85.7 | 4.6 ± 0.3 | 77.8 ± 5.6 |
| 8 | 7 | 5 | 71.4 | 4.4 ± 0.6 | 55 ± 7.5 | ||
| 12 | 7 | 5 | 71.4 | 5.4 ± 1.08 | 45 ± 9 | ||
| 15 | 7 | 5 | 71.4 | 6.4 ± 0.4 | 42.7 ± 2.7 | ||
| 20 | 6 | 5 | 83.3 | 8 ± 1.6 | 40 ± 7.9 | ||
| 25 | 6 | 5 | 83.3 | 6 ± 0.9 | 24 ± 3.6 | ||
| Bilateral | 6 | 7 | 7 | 100 | 2.1 ± 0.6 | 35.7 ± 9.2 | |
| 12 | 6 | 6 | 100 | 3.8 ± 0.9 | 31.9 ± 7.3 | ||
| 20 | 7 | 7 | 100 | 9.1 ± 1.7 | 45.7 ± 8.4 | ||
| Reciprocal | Unilateral | 6 | 7 | 6 | 85.7 | 3.8 ± 0.5 | 63.9 ± 8 |
| Reciprocity | Surgical Technique | Total Number of Embryo’s Transferred | C57BL/6J a | B6129F1 a |
|---|---|---|---|---|
| Non-reciprocal | Unilateral | 6 | 1.3 ± 0.1 | 3.1 ± 0.9 |
| 8 | 1.8 ± 0.5 | – | ||
| 12 | 2.8 ± 0.8 | 1.6 ± 0.2 | ||
| 15 | 2.4 ± 0.17 | – | ||
| 20 | 3 ± 0.7 | 3.7 ± 1.3 | ||
| 25 | 4.5 ± 0.6 | – | ||
| Bilateral | 6 | 3.8 ± 0.8 | 1.6 ± 0.4 | |
| 12 | 4.7 ± 1.6 | 2.1 ± 0.3 | ||
| 20 | 3.3 ± 1.2 | 4.6 ± 1.5 | ||
| Reciprocal | Unilateral | 6 | 1.7 ± 0.3 | – |
| Reciprocity | Surgical Technique | Total n of Embryos Transferred | Number of Transfers | Number of Pregnant Females | Pregnancy Rates (%) | Number of Pups Born per Litter a | Success Rate (%) a |
|---|---|---|---|---|---|---|---|
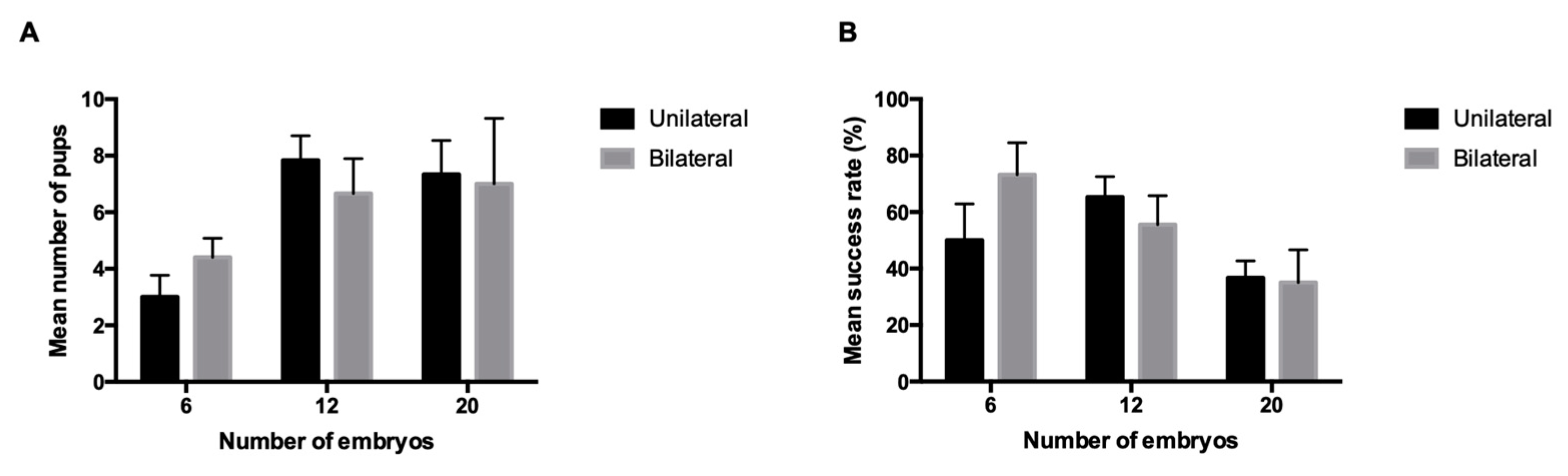
| Non-reciprocal | Unilateral | 6 | 7 | 6 | 85.7 | 3 ± 0.8 | 50 ± 12.9 |
| 12 | 7 | 6 | 85.7 | 7.8 ± 0.9 | 65.3 ± 7.2 | ||
| 20 | 7 | 6 | 85.7 | 7 ± 2.2 | 36.7 ± 6 | ||
| Bilateral | 6 | 7 | 5 | 71.4 | 4.4 ± 0.7 | 73.3 ± 11.3 | |
| 12 | 7 | 6 | 85.7 | 6.7 ± 1.2 | 55.6 ± 10.2 | ||
| 20 | 6 | 5 | 83.3 | 7.3 ± 2.2 | 35 ± 11.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamas, S.; Franquinho, F.; Morgado, M.; Mesquita, J.R.; Gärtner, F.; Amorim, I. C57BL/6J and B6129F1 Embryo Transfer: Unilateral and Bilateral Transfer, Embryo Number and Recipient Female Background Control for the Optimization of Embryo Survival and Litter Size. Animals 2020, 10, 1424. https://doi.org/10.3390/ani10081424
Lamas S, Franquinho F, Morgado M, Mesquita JR, Gärtner F, Amorim I. C57BL/6J and B6129F1 Embryo Transfer: Unilateral and Bilateral Transfer, Embryo Number and Recipient Female Background Control for the Optimization of Embryo Survival and Litter Size. Animals. 2020; 10(8):1424. https://doi.org/10.3390/ani10081424
Chicago/Turabian StyleLamas, Sofia, Filipa Franquinho, Marlene Morgado, João R. Mesquita, Fátima Gärtner, and Irina Amorim. 2020. "C57BL/6J and B6129F1 Embryo Transfer: Unilateral and Bilateral Transfer, Embryo Number and Recipient Female Background Control for the Optimization of Embryo Survival and Litter Size" Animals 10, no. 8: 1424. https://doi.org/10.3390/ani10081424
APA StyleLamas, S., Franquinho, F., Morgado, M., Mesquita, J. R., Gärtner, F., & Amorim, I. (2020). C57BL/6J and B6129F1 Embryo Transfer: Unilateral and Bilateral Transfer, Embryo Number and Recipient Female Background Control for the Optimization of Embryo Survival and Litter Size. Animals, 10(8), 1424. https://doi.org/10.3390/ani10081424





