Time Course-Dependent Study on Equine Herpes Virus 9-Induced Abortion in Syrian Hamsters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Animals and Treatments
2.3. Collection and Processing of Samples
2.4. Histopathology and Immunohistochemistry
2.5. Enzyme-Linked Immunosorbent Assay for Detection of Cytokines (IFN-γ and TNF-α)
2.6. DNA Extraction and Polymerase Chain Reaction
2.7. Statistical Analysis
3. Results
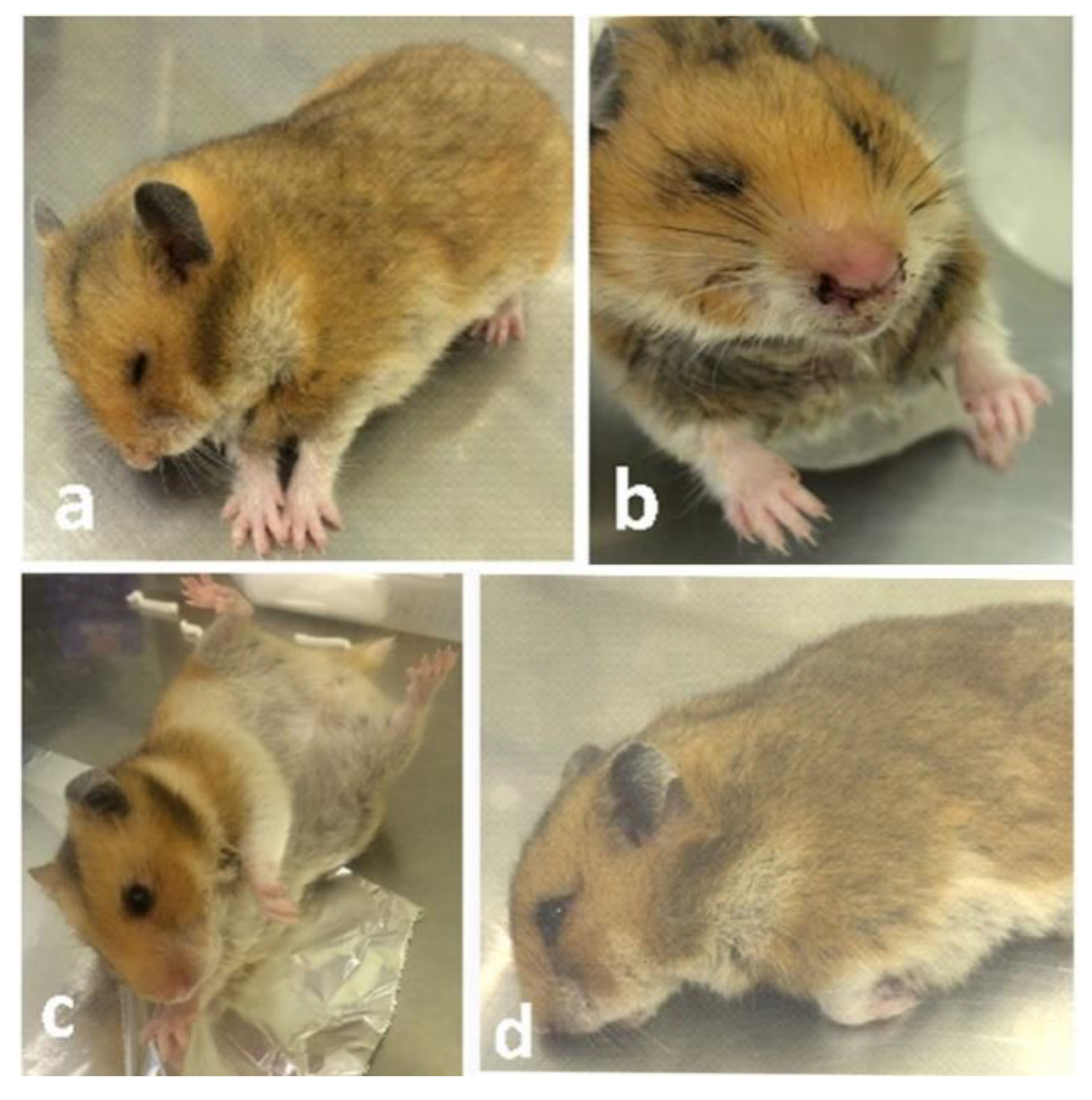
3.1. Clinical Signs and Gross Findings
3.1.1. Inoculation of EHV-9 in First Trimester Hamsters
3.1.2. Inoculation of EHV-9 in Late-Trimester Hamsters
3.2. Histopathology and Immunohistochemistry
3.2.1. Infection During the First Trimester of Gestation
3.2.2. Infection During the Third Trimester of Gestation
3.3. Detection of Viral DNA by PCR
3.4. Detection of Cytokines (IFN-γ and TNF-α) by ELISA Kits
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fukushi, H.; Tomita, T.; Taniguchi, A.; Ochiai, Y.; Kirisawa, R.; Matsumura, T.; Yanai, T.; Masegi, T.; Yamaguchi, T.; Hirai, K. Gazelle herpesvirus 1: A new neurotropic herpesvirus immunologically related to equine herpesvirus 1. Virology 1997, 227, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Sakai, T.; Fukushi, H.; Hirai, K.; Narita, M.; Sakai, H.; Masegi, T. Neuropathological study of gazelle herpesvirus 1 (equine herpesvirus 9) infection in Thomson’s gazelles (Gazella thomsoni). J. Comp. Pathol. 1998, 119, 159–168. [Google Scholar] [CrossRef]
- Fukushi, H.; Taniguchi, A.; Yasuda, K.; Yanai, T.; Masegi, T.; Yamaguchi, T.; Hirai, K. A hamster model of equine herpesvirus 9 induced encephalitis. J. Neurovirology 2000, 6, 314–319. [Google Scholar] [CrossRef] [PubMed]
- El-Habashi, N.; El-Nahass, E.; Fukushi, H.; Nayel, M.; Hibi, D.; Sakai, H.; Yanai, T. Effects of equine herpesvirus-9 infection in pregnant mice and hamsters. J. Comp. Pathol. 2011, 144, 103–112. [Google Scholar] [CrossRef] [PubMed]
- El-Habashi, N.; El-Nahass, E.; Haridy, M.; Nayel, M.; Abdelaziz, A.; Fukushi, H.; Kuroda, K.; Sakai, H.; Yanai, T. Pathological Findings in Equine Herpesvirus 9-Induced Abortion in Rats. J. Comp. Pathol. 2014, 151, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Kodama, A.; Yanai, T.; Yomemaru, K.; Sakai, H.; Masegi, T.; Yamada, S.; Fukushi, H.; Kuraishi, T.; Hattori, S.; Kai, C. Acute neuropathogenicity with experimental infection of equine herpesvirus 9 in common marmosets (Callithrix jacchus). J. Med. Primatol. 2007, 36, 335–342. [Google Scholar] [CrossRef]
- Yanai, T.; Fujishima, N.; Fukushi, H.; Hirata, A.; Sakai, H.; Masegi, T. Experimental infection of equine herpesvirus 9 in dogs. Vet. Pathol. Online 2003, 40, 263–267. [Google Scholar] [CrossRef]
- Yanai, T.; Tujioka, S.; Sakai, H.; Fukushi, H.; Hirai, K.; Masegi, T. Experimental infection with equine herpesvirus 9 (EHV-9) in cats. J. Comp. Pathol. 2003, 128, 113–118. [Google Scholar] [CrossRef]
- Taniguchi, A.; Fukushi, H.; Yanai, T.; Masegi, T.; Yamaguchi, T.; Hirai, K. Equine herpesvirus 9 induced lethal encephalomyelitis in experimentally infected goats. Arch. virol. 2000, 145, 2619–2627. [Google Scholar] [CrossRef]
- Narita, M.; Uchimura, A.; Kimura, K.; Tanimura, N.; Yanai, T.; Masegi, T.; Fukushi, H.; Hirai, K. Brain lesions and transmission of experimental equine herpesvirus type 9 in pigs. Vet. Pathol. Online 2000, 37, 476–479. [Google Scholar] [CrossRef] [Green Version]
- Narita, M.; Uchimura, A.; Kawanabe, M.; Fukushi, H.; Hirai, K. Invasion and spread of equine herpesvirus 9 in the olfactory pathway of pigs after intranasal inoculation. J. Comp. Pathol. 2001, 124, 265–272. [Google Scholar] [CrossRef]
- El-Habashi, N.; El-Nahass, E.-S.; Namihira, Y.; Hagiwara, H.; Fukushi, H.; Narita, M.; Hirata, A.; Sakai, H.; Yanai, T. Neuropathogenicity of equine herpesvirus 9 in cattle. J. Equine Vet. Sci. 2011, 31, 72–77. [Google Scholar] [CrossRef]
- El-Habashi, N.; El-Nahass, E.-S.; Fukushi, H.; Hibi, D.; Sakai, H.; Sasseville, V.; Yanai, T. Experimental intranasal infection of equine herpesvirus 9 (EHV-9) in suckling hamsters: Kinetics of viral transmission and inflammation in the nasal cavity and brain. J. Neurovirology 2010, 16, 242–248. [Google Scholar] [CrossRef]
- Hoenerhoff, M.J.; Janovitz, E.B.; Richman, L.K.; Murphy, D.A.; Butler, T.C.; Kiupel, M. Fatal herpesvirus encephalitis in a reticulated giraffe (Giraffa camelopardalis reticulata). Vet. Pathol. 2006, 43, 769–772. [Google Scholar] [CrossRef]
- Kasem, S.; Yamada, S.; Kiupel, M.; Woodruff, M.; Ohya, K.; Fukushi, H. Equine herpesvirus type 9 in giraffe with encephalitis. Emerg. Infect. Dis. 2008, 14, 1948–1949. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.A.; Schrenzel, M.D.; Tucker, T.; Pessier, A.P.; Bicknese, B.; Busch, M.D.; Wise, A.G.; Maes, R.; Kiupel, M.; McKnight, C.; et al. Meningoencephalitis in a polar bear caused by equine herpesvirus 9 (EHV-9). Vet. Pathol. 2009, 46, 1138–1143. [Google Scholar] [CrossRef]
- Schrenzel, M.D.; Tucker, T.A.; Donovan, T.A.; Busch, M.D.; Wise, A.G.; Maes, R.K.; Kiupel, M. New hosts for equine herpesvirus 9. Emerg. Infect. Dis. 2008, 14, 1616–1619. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, H.; Yanai, T. Virology and Pathology of Encephalitis in Alien Hosts by Neurotropic Equine Herpesvirus 9; INTECH Open Access Publisher: London, UK, 2011. [Google Scholar]
- Abdelgawad, A.; Hermes, R.; Damiani, A.; Lamglait, B.; Czirjak, G.A.; East, M.; Aschenborn, O.; Wenker, C.; Kasem, S.; Osterrieder, N.; et al. Comprehensive Serology Based on a Peptide ELISA to Assess the Prevalence of Closely Related Equine Herpesviruses in Zoo and Wild Animals. PLoS ONE 2015, 10, e0138370. [Google Scholar] [CrossRef] [PubMed]
- Chiswick, E.L.; Duffy, E.; Japp, B.; Remick, D. Detection and quantification of cytokines and other biomarkers. Methods Mol. Biol. 2012, 844, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.C.; Blunden, A.S.; Whitwell, K.E.; Dunn, K.A.; Wales, A.D. A survey of equine abortion, stillbirth and neonatal death in the UK from 1988 to 1997. Equine Vet. J. 2003, 35, 496–501. [Google Scholar] [CrossRef]
- Dunowskaa, M. A review of equid herpesvirus 1 for the veterinary practitioner. Part A: Clinical presentation, diagnosis and treatmen. N.Z. Vet. J. 2014, 4, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Bryansa, J.T.B. Molecular epidemiology, pathogenesis and prophylaxis of equine herpesvirus-1 infections. In Progress in Veterinary Microbiology and Immunology; Pandey, R., Ed.; Karger: Basel, Switzerland, 1986; pp. 78–144. [Google Scholar]
- Mumford, J.A.; Rossdale, P.D.; Jessett, D.M.; Gann, S.J.; Ousey, J.; Cook, R.F. Serological and virological investigations of an equid herpesvirus 1 (EHV-1) abortion storm on a stud farm in 1985. J. Reprod. Fertil Suppl. 1987, 35, 509–518. [Google Scholar] [PubMed]
- Kapranos, N.C.; Kotronias, D.C. Detection of herpes simplex virus in first trimester pregnancy loss using molecular techniques. In Vivo 2009, 23, 839–842. [Google Scholar]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: Implications for transmission and pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haimovici, F.; Hill, J.A.; Anderson, D.J. The effects of soluble products of activated lymphocytes and macrophages on blastocyst implantation events in vitro. Biol. Reprod 1991, 44, 69–75. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Beer, A.E.; Kim, S.H.; Mantouvalos, H.P. Immunopathology of the implantation site utilizing monoclonal antibodies to natural killer cells in women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 1999, 41, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.G.; Munoz, R.; Tejerizo, G.; Montes, M.J.; Gómez-Molina, F.; Abadía-Molina, A.C. Decidual lymphocytes of human spontaneous abortions induce apoptosis but not necrosis in JEG-3 extravillous trophoblast cells. Biol. Reprod. 2002, 67, 1211–1217. [Google Scholar] [CrossRef] [Green Version]
- Awan, A.; Baxi, M.; Field, H. EHV 1-induced abortion in mice and its relationship to stage of gestation. Res. Vet. Sci. 1995, 59, 139–145. [Google Scholar] [CrossRef]
- Walker, C.; Perotti, V.; Love, D.; Whalley, J. Infection with equine herpesvirus 1 (EHV-1) strain HVS25A in pregnant mice. J. Comp. Pathol. 1999, 120, 15–27. [Google Scholar] [CrossRef]
- Smith, K.C.; Whitwell, K.E.; Mumford, J.A.; Gower, S.M.; Hannant, D.; Tearle, J.P. An immunohistological study of the uterus of mares following experimental infection by Equid herpesvirus 1. Equine Vet. J. 1993, 25, 36–40. [Google Scholar] [CrossRef]
- Smith, K.C.; Whitwell, K.E.; Binns, M.M.; Dolby, C.A.; Hannant, D.; Mumford, J.A. Abortion of virologically negative foetuses following experimental challenge of pregnant pony mares with Equid herpesvirus 1. Equine Vet. J. 1992, 24, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Edington, N.; Smyth, B.; Griffiths, L. The role of endothelial cell infection in the endometrium, placenta and foetus of equid herpesvirus 1 (EHV-1) Abortions. J. Comp. Pathol. 1991, 104, 379–387. [Google Scholar] [CrossRef]
- Burek, J.; Roos, R.; Narayan, O. Virus-induced abortion. Studies of equine herpesvirus 1 (abortion virus) in hamsters. Lab. Investig. 1975, 33, 400–406. [Google Scholar] [PubMed]
- Awan, A.; Gibson, J.; Field, H. A murine model for studying EHV-1-induced abortion. Res. Vet. Sci. 1991, 51, 94–99. [Google Scholar] [CrossRef]
- Bryans, J.; Swerczek, T.; Darlington, R.; Crowe, M. Neonatal foal disease associated with perinatal infection by equine herpesvirus I. J. Equine Med. Surg. 1977, 1, 20–26. [Google Scholar]
- Hartley, W.; Dixon, R. An outbreak of Foal Perinatal Mortality due to Equid Herpesvirus Type I: Pathological Observations. Equine Vet. J. 1979, 11, 215–218. [Google Scholar] [CrossRef]
- Kukreja, A.; Walker, C.; Fitzmaurice, T.; Awan, A.; Love, D.; Whalley, J.; Field, H. Protective effects of equine herpesvirus-1 (EHV-1) glycoprotein B in a murine model of EHV-1-induced abortion. Vet. Microbiol. 1998, 62, 303–311. [Google Scholar] [CrossRef]
- Dixon, R.; Hartley, W.; Hutchins, D.; Lepherd, E.; Feilen, C.; Jones, R.; Love, D.N.; Sabine, M.; Wells, A.L. Perinatal foal mortality associated with a herpesvirus. Aust. Vet. J. 1978, 54, 103–105. [Google Scholar] [CrossRef]
- Gardiner, D.W.; Lunn, D.P.; Goehring, L.S.; Chiang, Y.-W.; Cook, C.; Osterrieder, N.; McCue, P.; Del Piero, F.; Hussey, S.B.; Hussey, G.S. Strain impact on equine herpesvirus type 1 (EHV-1) abortion models: Viral loads in fetal and placental tissues and foals. Vaccine 2012, 30, 6564–6572. [Google Scholar] [CrossRef]
- Gerber, J.; Marron, A.E.; Bass, E.; Beckenhauer, W. Effect of age and pregnancy on the antibody and cell-mediated immune responses of horses to equine herpesvirus 1. Can. J. Comp. Med. 1977, 41, 471. [Google Scholar]
- Khan, M.M. Role of cytokines. In Immunopharmacology; Springer Science: Berlin, Germany, 2008. [Google Scholar]
- Dane, K.; Patton, T.; Andrea, K.; Soboll, G. Cytokine responses to EHV-1 infection in immune and non-immune ponies. Vet. Immunol. Immunopathol. 2006, 111, 109–116. [Google Scholar]
- Lo´pez-Gatius, F.; Almería, S.; Donofrio, G.; Nogareda, C.; García-Ispierto, I.; Bech-Sàbat, G.; Santolaria, P.; Yániz, J.L.; Pabón, M.; De Sousa, N.M.; et al. Protection against abortion linked to gamma interferon production in pregnant dairy cows naturally infected with Neospora caninum. Theriogenology 2007, 68, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.S.; Watanabe, K.; Furuoka, H.; Suzuki, H.; Watarai, M. Interferon-γ promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Group | Date of Gestation at Time of Inoculation | (Number of Animals)/Date of Sampling | Dam’s Number Referred to in Text |
|---|---|---|---|
| Group 1 (control group) | (3) at 8th day of gestation (3) at 11th day of gestation (3) at 13th day of gestation (3) at 15th day of gestation | ||
| Group 2 (1st trimester group) | 5th day of gestation | (3) at 8th day of gestation/(3 day post inoculation dpi) (3) at 11th day of gestation/(6 dpi) (3) at 16th day of gestation/(11 dpi) | 1, 2 and 3 4, 5 and 6 7, 8 and 9 |
| Group 3 (3rd trimester group) | (3) at 13th day of gestation/(3 dpi) (3) at 16 day of gestation/(5 dpi) | 10, 11 and 12 13, 14 and 15 |
| Dam No. | Clinical Signs | Gross Lesions | Number of Dead Fetuses/Total Number of Fetuses | Number of Fetuses in Corresponding Control |
|---|---|---|---|---|
| 1 | Depression | Petechial hemorrhages on lungs Pregnancy in one horn | 0/4 | 0/10 |
| 2 | Nasal discharge, mild nervous manifestation (tilting, tremors of head, etc.) | 6 pregnancy cysts in the right horn; 2 appeared as transparent empty vesicles. Left horn had one pregnancy cyst | 0/7 | 0/11 |
| 3 | Nasal discharge and depression | Congested uterus | 0/11 | 0/10 |
| 4 | Nasal discharge, convulsions | Petechial hemorrhage on lungs, 3 pale, smaller pregnancy cysts | 3/11 | 0/11 |
| 5 | Nasal discharge, nervous manifestation | Hepatomegaly, pinpoint necrotic foci on the surface of the liver, 2 pregnancy cysts were pale and smaller in size than others. | 2/11 | 0/13 |
| 6 | Nasal discharge, paddling of the hind limb | Ascites and congested uterus | 1/14 | 0/10 |
| 7 | Ruffled hair, nasal discharge, nervous manifestation | Congested uterus with dark red areas. 3 undersized dark congested dead fetuses. Petechial hemorrhage on lungs. | 3/10 | 0/8 |
| 8 | Nervous manifestation | One dead undersized dark fetus | 1/11 | 0/10 |
| 9 | Nervous manifestation | Pinpoint pale areas on the lungs. No gross changes on uterus or fetuses | 0/11 | 0/10 |
| 10 | Nasal discharges, convulsions, and tremors | The liver was congested with sharp demarcation of hepatic lobules, pregnancy in one horn | 3/6 | 0/6 |
| 11 | Ruffled fur, nasal discharge, and convulsions | Congested blackish uterine wall | 3/10 | 0/10 |
| 12 | Nervous manifestation with hind limb paralysis | Hepatomegaly with hepatic congestion | 4/10 | 0/11 |
| 13 | Convulsions | Congested liver and brain. Dark red uterus contained 4 undersized dead dark fetuses | 4/11 | 0/8 |
| 14 | Nervous manifestation and recumbency | Ascites and congested enlarged liver | 5/10 | 0/10 |
| 15 | Respiratory distress and nervous manifestations | Dark red uterus contained 3 undersized fetuses | 3/9 | 0/10 |
| Dam No. | IHC | PCR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Lungs | Liver | Fetus a | Placent a | Blood | Brain | Lungs | Liver | Uterus b | Placenta | Fetus | |
| 1 | + | − | − | − | − | − | + | − | − | − | NA | NA |
| 2 | + | − | − | − | − | − | + | − | − | − | NA | NA |
| 3 | + | −/+ | − | − | + | + | + | − | − | − | NA | NA |
| 4 | + | + | − | − | −/+ | + | + | + | − | − | NA | NA |
| 5 | + | + | + | − | + | + | + | + | + | + | NA | NA |
| 6 | + | − | − | − | −/+ | + | + | − | − | − | NA | NA |
| 7 | + | + | + | − | ++ | − | + | + | + | + | + | − |
| 8 | + | − | + | − | ++ | − | + | − | + | + | + | − |
| 9 | + | + | − | − | + | − | + | + | − | + | + | − |
| 10 | + | − | +/− | − | + | + | + | − | − | + | + | − |
| 11 | + | − | − | − | − | − | + | − | − | − | − | − |
| 12 | ++ | +/− | + | H/+ | + | + | + | − | + | + | − | − |
| 13 | + | + | + | H/+ P/+ | ++ | + | + | + | + | + | + | P/+ H/+ |
| 14 | + | + | + | H/+ P/+ | ++ | + | + | + | + | + | + | P/+ H/+ |
| 15 | +/− | − | + | Ep/+ H/+ P/+ In/+ | + | + | + | − | + | + | + | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abas, O.; Abdo, W.; Kasem, S.; Alwazzan, A.; Saleh, A.G.; Saleh, I.G.; Fukushi, H.; Yanai, T.; Haridy, M. Time Course-Dependent Study on Equine Herpes Virus 9-Induced Abortion in Syrian Hamsters. Animals 2020, 10, 1369. https://doi.org/10.3390/ani10081369
Abas O, Abdo W, Kasem S, Alwazzan A, Saleh AG, Saleh IG, Fukushi H, Yanai T, Haridy M. Time Course-Dependent Study on Equine Herpes Virus 9-Induced Abortion in Syrian Hamsters. Animals. 2020; 10(8):1369. https://doi.org/10.3390/ani10081369
Chicago/Turabian StyleAbas, Osama, Walied Abdo, Samy Kasem, Abdulatif Alwazzan, Asmaa G. Saleh, Ibrahim G. Saleh, Hideto Fukushi, Tokuma Yanai, and Mohie Haridy. 2020. "Time Course-Dependent Study on Equine Herpes Virus 9-Induced Abortion in Syrian Hamsters" Animals 10, no. 8: 1369. https://doi.org/10.3390/ani10081369
APA StyleAbas, O., Abdo, W., Kasem, S., Alwazzan, A., Saleh, A. G., Saleh, I. G., Fukushi, H., Yanai, T., & Haridy, M. (2020). Time Course-Dependent Study on Equine Herpes Virus 9-Induced Abortion in Syrian Hamsters. Animals, 10(8), 1369. https://doi.org/10.3390/ani10081369






