The Relationship between Lung Inflammation and Aerobic Threshold in Standardbred Racehorses with Mild-Moderate Equine Asthma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Sample
- Collection of history;
- Complete clinical examination with lameness evaluation;
- Laboratory evaluation (blood count, biochemistry and blood gas analysis);
- Electrocardiogram;
- Thoracic ultrasound (and cardiac evaluation in the presence of any murmur);
- Incremental treadmill test with plasma lactate analysis and Holter registration;
- Dynamic endoscopy of the upper airways on treadmill;
- Endoscopy of the airways with collection of BALF;
- Gastroscopy.
- Neutrophils > 5% and/or;
- Mast-cells > 2% and/or;
- Eosinophils > 1%.
2.2. Incremental Treadmill Test, Blood Sample Collection and Processing and Calculation of VLA4
2.3. BALF Collection and Cytological Examination
2.4. Statistical Analysis
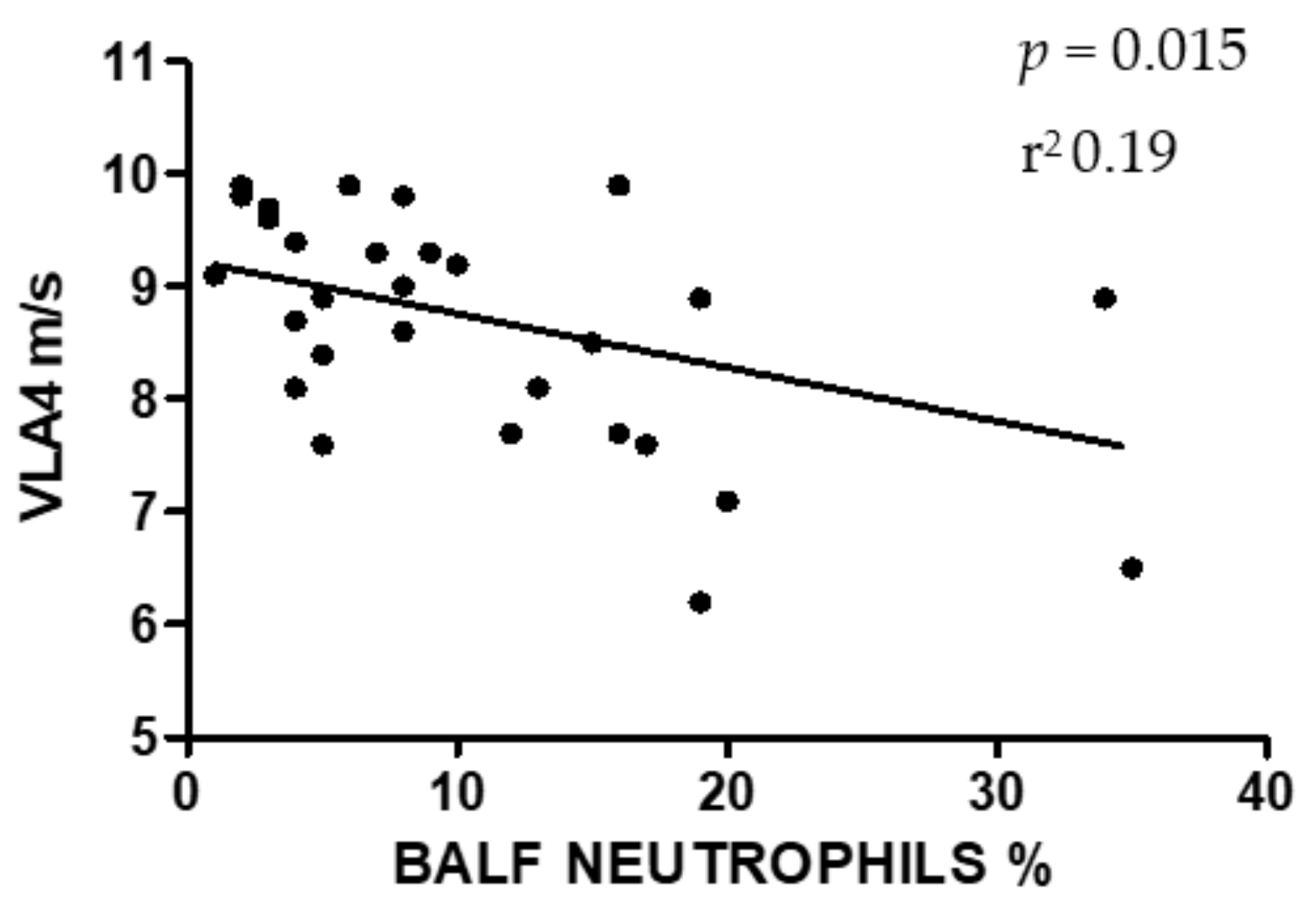
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lange-Consiglio, A.; Stucchi, L.; Zucca, E.; Lavoie, J.P.; Cremonesi, F.; Ferrucci, F. Insights into animal models for cell-based therapies in translational studies of lung diseases: Is the horse with naturally occurring asthma the right choice? Cytotherapy 2019, 21, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Couetil, L.L.; Cardwell, J.; Gerber, V.; Lavoie, J.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses—Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Christley, R.; Hodgson, D.R.; Rose, R.J.; Wood, J.L.N.; Reid, S.W.J.; Whitear, K.G.; Hodgson, J.L. A case-control study of respiratory disease in Thoroughbred racehorses in Sydney, Australia. Equine Vet. J. 2010, 33, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.M.; Wood, J.L.N.; Smith, K.C.; Newton, J.R. Descriptive results from a longitudinal study of airway inflammation in British National Hunt racehorses. Equine Vet. J. 2011, 43, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Gy, C.; le Clere, M.; Vargas, A.; Grimes, C.; Lavoie, J. Investigation of blood biomarkers for the diagnosis of mild to moderate asthma in horses. J. Vet. Intern. Med. 2019, 33, 1789–1795. [Google Scholar] [CrossRef]
- Fogarty, U.; Buckley, T. Bronchoalveolar lavage findings in horses with exercise intolerance. Equine Vet. J. 1991, 23, 434–437. [Google Scholar] [CrossRef]
- Richard, E.A.; Fortier, G.D.; Lekeux, P.M.; van Erck, E. Laboratory findings in respiratory fluids of the poorly-performing horse. Vet. J. 2010, 185, 115–122. [Google Scholar] [CrossRef]
- Lavoie, J.; Cesarini, C.; Lavoie-Lamoureux, A.; Moran, K.; Lutz, S.; Picandet, V.; Jean, D.; Marcoux, M. Bronchoalveolar Lavage Fluid Cytology and Cytokine Messenger Ribonucleic Acid Expression of Racehorses with Exercise Intolerance and Lower Airway Inflammation. J. Vet. Intern. Med. 2011, 25, 322–329. [Google Scholar] [CrossRef]
- Nolen-Walston, R.; Harris, M.; Agnew, M.E.; Martin, B.B.; Reef, V.B.; Boston, R.C.; Davidson, E.J. Clinical and diagnostic features of inflammatory airway disease subtypes in horses examined because of poor performance: 98 cases (2004–2010). J. Am. Vet. Med. Assoc. 2013, 242, 1138–1145. [Google Scholar] [CrossRef]
- Ivester, K.M.; Couetil, L.L.; Moore, G.E. An observational study of environmental exposures, airway cytology, and performance in racing thoroughbreds. J. Vet. Intern. Med. 2018, 32, 1754–1762. [Google Scholar] [CrossRef]
- Bond, S.L.; Greco-Otto, P.; MacLeod, J.; Galezowski, A.; Bayly, W.; Leguillette, R. Efficacy of dexamethasone, salbutamol, and reduced respirable particulate concentration on aerobic capacity in horses with smoke-induced mild asthma. J. Vet. Intern. Med. 2020, 34, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Widmer, A.; Doherr, M.G.; Tessier, C.; Koch, C.; Ramseyer, A.; Straub, R.; Gerber, V. Association of increased tracheal mucus accumulation with poor willingness to perform in show-jumpers and dressage horses. Vet. J. 2009, 182, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Gerber, V.; Ii, H.C.S.; Robinson, N.E. Owner assessment in judging the efficacy of airway disease treatment. Equine Vet. J. 2011, 43, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.G.B. Evaluation of exercise tolerance and fitness in the performance horse. In Equine Exercise Physiology; Snow, D.H., Persson, S.G.B., Rose, R.J., Eds.; Granta: Cambridge, UK, 1983; Volume 1, pp. 441–457. [Google Scholar]
- Couroucé, A.; Chatard, J.-C.; Auvinet, B. Estimation of performance potential of standardbred trotters from blood lactate concentrations measured in field conditions. Equine Vet. J. 1997, 29, 365–369. [Google Scholar] [CrossRef]
- le leu, C.; Cotrel, C.; Courouce-Malblanc, A. Relationships between physiological variables and race performance in French standardbred trotters. Vet. Rec. 2005, 156, 339–342. [Google Scholar] [CrossRef]
- Lindner, A. Relationships between racing times of Standardbreds and v4 and v2001. J. Anim. Sci. 2010, 88, 950–954. [Google Scholar] [CrossRef]
- Couetil, L.L.; Hoffman, A.M.; Hodgson, J.L.; Viel, L.; Wood, J.L.; Lavoie, J.-P.; Buechner-Maxwell, V. Inflammatory Airway Disease of Horses. ACVIM Consensus Statement. J. Vet. Intern. Med. 2007, 21, 356–361. [Google Scholar] [CrossRef]
- Stucchi, L.; Valli, C.; Stancari, G.; Zucca, E.; Ferrucci, F. Creatine-kinase reference intervals at rest and after maximal exercise in Standardbred racehorses. Comp. Exerc. Physiol. 2019, 15, 319–325. [Google Scholar] [CrossRef]
- Newell, J.; Higgins, D.; Madden, N.; Cruickshank, J.; Einbeck, J.; McMillan, K.; McDonald, R. Software for calculating blood lactate endurance markers. J. Sports Sci. 2007, 25, 1403–1409. [Google Scholar] [CrossRef]
- Ferro, E.; Ferrucci, F.; Zucca, E.; di Fabio, V.; Castoldi, S. Arterial blood gas analysis in 53 racehorses with a diagnosis of Small Airway Inflammatory Disease (SAID). J. Equine Vet. Sci. 2002, 22, 165–168. [Google Scholar] [CrossRef]
- Ferrucci, F.; Stucchi, L.; Salvadori, M.; Stancari, G.; Conturba, B.; Bronzo, V.; Ferro, E.; Zucca, E. Effetti dell’amikacina per via inalatoria in cavalli sportivi affetti da sindrome da calo di rendimento e confronto con la somministrazione endovenosa. Ippologia 2013, 24, 3–9. [Google Scholar]
- Fraipont, A.; van Erck, E.; Ramery, E.; Richard, E.A.; Denoix, J.-M.; Lekeux, P.; Art, T. Subclinical diseases underlying poor performance in endurance horses: Diagnostic methods and predictive tests. Vet. Rec. 2011, 169, 154. [Google Scholar] [CrossRef] [PubMed]
- Trilk, J.L.; Lindner, A.J.; Greene, H.M.; Alberghina, D.; Wickler, S.J. A lactate-guided conditioning programme to improve endurance performance. Equine Vet. J. 2010, 34, 122–125. [Google Scholar] [CrossRef]
- Couroucé, A.; Chrétien, M.; Valette, J.P. Physiological variables measured under field conditions according to age and state of training in French Trotters. Equine Vet. J. 2002, 34, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.G.B. Heart Rate and Blood Lactate Responses to Submaximal Treadmill Exercise in the Normally Performing Standardbred Trotter—Age and Sex Variations and Predictability from the Total Red Blood Cell Volume. Zent. Vet. A 1997, 44, 125–132. [Google Scholar] [CrossRef]
- Couëtil, L.L.; de Nicola, D.B. Blood gas, plasma lactate and bronchoalveolar lavage cytology analyses in racehorses with respiratory disease. Equine Vet. J. 1999, 31, 77–82. [Google Scholar] [CrossRef]
- Sanchez, A.; Couetil, L.L.; Ward, M.; Clark, S. Effect of Airway Disease on Blood Gas Exchange in Racehorses. J. Vet. Intern. Med. 2005, 19, 87–92. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Robinson, N.E.; Derksen, F.J.; Bertold, B.; Genovese, R.; Miller, R.; Rupp, H.D.F.; Carr, E.A.; Eberhart, S.W.; Boruta, D.; et al. Effect of tracheal mucus and tracheal cytology on racing performance in Thoroughbred racehorses. Equine Vet. J. 2006, 38, 300–304. [Google Scholar] [CrossRef]
- Koblinger, K.; Nicol, J.; McDonald, K.; Wasko, A.; Logie, N.; Weiss, M.; Léguillette, R. Endoscopic Assessment of Airway Inflammation in Horses. J. Vet. Intern. Med. 2011, 25, 1118–1126. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stucchi, L.; Alberti, E.; Stancari, G.; Conturba, B.; Zucca, E.; Ferrucci, F. The Relationship between Lung Inflammation and Aerobic Threshold in Standardbred Racehorses with Mild-Moderate Equine Asthma. Animals 2020, 10, 1278. https://doi.org/10.3390/ani10081278
Stucchi L, Alberti E, Stancari G, Conturba B, Zucca E, Ferrucci F. The Relationship between Lung Inflammation and Aerobic Threshold in Standardbred Racehorses with Mild-Moderate Equine Asthma. Animals. 2020; 10(8):1278. https://doi.org/10.3390/ani10081278
Chicago/Turabian StyleStucchi, Luca, Elena Alberti, Giovanni Stancari, Bianca Conturba, Enrica Zucca, and Francesco Ferrucci. 2020. "The Relationship between Lung Inflammation and Aerobic Threshold in Standardbred Racehorses with Mild-Moderate Equine Asthma" Animals 10, no. 8: 1278. https://doi.org/10.3390/ani10081278
APA StyleStucchi, L., Alberti, E., Stancari, G., Conturba, B., Zucca, E., & Ferrucci, F. (2020). The Relationship between Lung Inflammation and Aerobic Threshold in Standardbred Racehorses with Mild-Moderate Equine Asthma. Animals, 10(8), 1278. https://doi.org/10.3390/ani10081278




