Long-Term Effects Following Fresh/Vitrified Embryo Transfer Are Transmitted by Paternal Germline in a Large Size Rabbit Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Statements
2.2. Experimental Design
2.3. Statistical Analyses
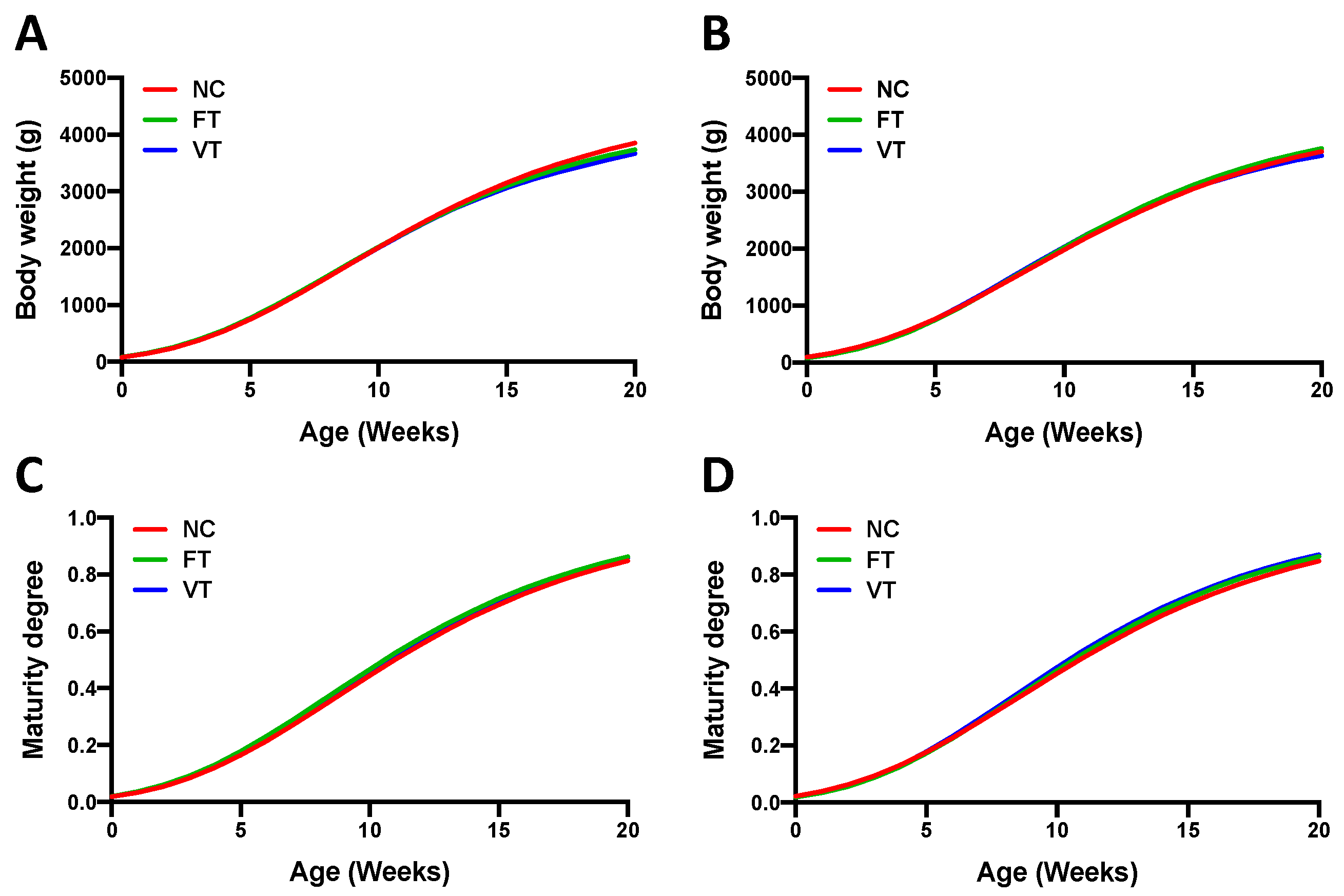
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ibeas, P.; Heras, S.; Gómez-Redondo, I.; Planells, B.; Fernández-González, R.; Pericuesta, E.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Gutiérrez-Adán, A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019, 86, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Canovas, S.; Ross, P.J.; Kelsey, G.; Coy, P. DNA Methylation in Embryo Development: Epigenetic Impact of ART (Assisted Reproductive Technologies). BioEssays 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018, 33, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Canovas, S.; Ivanova, E.; Romar, R.; García-Martínez, S.; Soriano-Úbeda, C.; García-Vázquez, F.A.; Saadeh, H.; Andrews, S.; Kelsey, G.; Coy, P. DNA methylation and gene expression changes derived from assisted reproductive technologies can be decreased by reproductive fluids. Elife 2017, 6. [Google Scholar] [CrossRef]
- García-Martínez, S.; Hurtado, M.A.S.; Gutiérrez, H.; Margallo, F.M.S.; Romar, R.; Latorre, R.; Coy, P.; Albors, O.L. Mimicking physiological O 2 tension in the female reproductive tract improves assisted reproduction outcomes in pig. Mol. Hum. Reprod. 2018, 24, 260–270. [Google Scholar] [CrossRef]
- Campo, H.; García-Domínguez, X.; López-Martínez, S.; Faus, A.; Vicente Antón, J.S.; Marco-Jiménez, F.; Cervelló, I. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 2019, 89, 126–138. [Google Scholar] [CrossRef]
- Sparks, A.E.T. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin. Reprod. Med. 2015, 33, 128–144. [Google Scholar] [CrossRef]
- Dulioust, E.; Toyama, K.; Busnel, M.C.; Moutier, R.; Carlier, M.; Marchaland, C.; Ducot, B.; Roubertoux, P.; Auroux, M. Long-term effects of embryo freezing in mice. Proc. Natl. Acad. Sci. USA 1995, 92, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, X.; Vicente, J.S.; Marco-Jiménez, F. Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals 2020, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Rinaudo, P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017, 8, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.; Rinaudo, P. From Embryos to Adults: A DOHaD Perspective on In Vitro Fertilization and Other Assisted Reproductive Technologies. Healthcare 2016, 4, E51. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, X.; Marco-Jiménez, F.; Peñaranda, D.S.; Vicente, J.S. Long-Term Phenotypic and Proteomic Changes Following Vitrified Embryo Transfer in the Rabbit Model. Animals 2020, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Ozanne, S.E. Transgenerational developmental programming. Hum. Reprod. Update 2014, 20, 63–75. [Google Scholar] [CrossRef]
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019, 10, 3922. [Google Scholar] [CrossRef]
- Mahsoudi, B.; Li, A.; O’Neill, C. Assessment of the Long-Term and Transgenerational Consequences of Perturbing Preimplantation Embryo Development in Mice1. Biol. Reprod. 2007, 77, 889–896. [Google Scholar] [CrossRef][Green Version]
- Lavara, R.; Baselga, M.; Marco-Jiménez, F.; Vicente, J.S. Long-term and transgenerational effects of cryopreservation on rabbit embryos. Theriogenology 2014, 81, 988–992. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Marco-Jiménez, F.; Peñaranda, D.S.; Diretto, G.; García-carpintero, V.; Cañizares, J.; Vicente, J.S.; Marco-Jiménez, F. Long-term and transgenerational phenotypic, transcriptional and metabolic effects in rabbit males born following vitrified embryo transfer. Sci. Rep. 2020, 10, 11313. [Google Scholar] [CrossRef]
- Laubach, Z.M.; Perng, W.; Dolinoy, D.C.; Faulk, C.D.; Holekamp, K.E.; Getty, T. Epigenetics and the maintenance of developmental plasticity: Extending the signalling theory framework. Biol. Rev. 2018, 93, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Kohda, T.; Ishino, F. Embryo manipulation via assisted reproductive technology and epigenetic asymmetry in mammalian early development. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zacchini, F.; Sampino, S.; Stankiewicz, A.M.; Haaf, T.; Ptak, G.E. Assessing the epigenetic risks of assisted reproductive technologies: A way forward. Int. J. Dev. Biol. 2019, 63, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Estany, J.; Baselga, M.; Blasco, A.; Camacho, J. Mixed model methodology for the estimation of genetic response to selection in litter size of rabbits. Livest. Prod. Sci. 1989, 21, 67–75. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Marco-Jimenez, F.; Viudes-de-Castro, M.P.; Vicente, J.S. Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Vis. Exp. 2019, 147, e58055. [Google Scholar] [CrossRef]
- Vicente, J.S.; García-Ximénez, F. Osmotic and cryoprotective effects of a mixture of DMSO and ethylene glycol on rabbit morulae. Theriogenology 1994, 42, 1205–1215. [Google Scholar] [CrossRef]
- Ventura-Juncá, P.; Irarrázaval, I.; Rolle, A.J.; Gutiérrez, J.I.; Moreno, R.D.; Santos, M.J. In vitro fertilization (IVF) in mammals: Epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol. Res. 2015, 48, 68. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef]
- Calle, A.; Miranda, A.; Fernandez-Gonzalez, R.; Pericuesta, E.; Laguna, R.; Gutierrez-Adan, A. Male Mice Produced by In Vitro Culture Have Reduced Fertility and Transmit Organomegaly and Glucose Intolerance to Their Male Offspring1. Biol. Reprod. 2012, 87, 1–9. [Google Scholar] [CrossRef]
- Mitchell, E.; Klein, S.L.; Argyropoulos, K.V.; Sharma, A.; Chan, R.B.; Toth, J.G.; Barboza, L.; Bavley, C.; Bortolozzi, A.; Chen, Q.; et al. Behavioural traits propagate across generations via segregated iterative-somatic and gametic epigenetic mechanisms. Nat. Commun. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Gidenne, T.; Combes, S.; Feugier, A.; Jehl, N.; Arveux, P.; Boisot, P.; Briens, C.; Corrent, E.; Fortune, H.; Montessuy, S.; et al. Feed restriction strategy in the growing rabbit. 2. Impact on digestive health, growth and carcass characteristics. Animal 2009, 3, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kohda, T. Effects of embryonic manipulation and epigenetics. J. Hum. Genet. 2013, 58, 416–420. [Google Scholar] [CrossRef] [PubMed]
| Body Weight (g) | Naturally-Conceived | Fresh-Transferred | Vitrified-Transferred |
|---|---|---|---|
| Paternal crossbred Animals (n) | (718) | (701) | (748) |
| Birth | 52.7 ± 0.51 | 53.3 ± 0.62 | 52.7 ± 0.50 |
| Weaning | 559.5 ± 5.59 | 569.8 ± 7.54 | 562.7 ± 4.53 |
| Prepuberty | 1476.8 ± 18.44 | 1494.6 ± 16.57 | 1468.3 ± 14.49 |
| Puberty | 3108.1 ± 31.01 a | 3011.3 ± 27.87 b | 3009.5 ± 30.49 b |
| Adulthood | 3924.2 ± 41.49 a | 3758.9 ± 35.75 b | 3769.1 ± 38.97 b |
| Maternal crossbred Animals (n) | (136) | (98) | (145) |
| Birth | 55.3 ± 0.93 | 54.6 ± 1.12 | 52.9 ± 0.90 |
| Weaning | 570.5 ± 10.233 a | 514.6 ± 11.05 b | 525.6 ± 10.62 b |
| Prepuberty | 1668.7 ± 20.58 | 1704.4 ± 22.18 | 1751.8 ± 20.85 |
| Puberty | 3124.0 ± 55.76 | 3162.6 ± 57.21 | 3133.5 ± 55.76 |
| Adulthood | 3655.2 ± 82.99 | 3707.3 ± 82.99 | 3628.0 ± 80.88 |
| Gompertz Parameters * | Naturally-Conceived | Fresh-Transferred | Vitrified-Transferred |
|---|---|---|---|
| Paternal crossbred animals | |||
| a | 4536.6 ± 42.98 a | 4329.4 ± 44.83 b | 4349.6 ± 42.50 b |
| b | 4.1 ± 0.04 | 3.9 ± 0.05 | 3.9 ± 0.04 |
| k | 0.16 ± 0.002 | 0.16 ± 0.003 | 0.16 ± 0.003 |
| Maternal crossbred animals | |||
| a | 4372.7 ± 75.95 | 4354.1 ± 109.71 | 4157.6 ± 94.133 |
| b | 3.8 ± 0.07 | 4.0 ± 0.14 | 3.9 ± 0.13 |
| k | 0.16 ± 0.004 | 0.16 ± 0.007 | 0.17 ± 0.007 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Dominguez, X.; Vicente, J.S.; Viudes-de-Castro, M.P.; Marco-Jiménez, F. Long-Term Effects Following Fresh/Vitrified Embryo Transfer Are Transmitted by Paternal Germline in a Large Size Rabbit Cohort. Animals 2020, 10, 1272. https://doi.org/10.3390/ani10081272
Garcia-Dominguez X, Vicente JS, Viudes-de-Castro MP, Marco-Jiménez F. Long-Term Effects Following Fresh/Vitrified Embryo Transfer Are Transmitted by Paternal Germline in a Large Size Rabbit Cohort. Animals. 2020; 10(8):1272. https://doi.org/10.3390/ani10081272
Chicago/Turabian StyleGarcia-Dominguez, Ximo, José Salvador Vicente, María P. Viudes-de-Castro, and Francisco Marco-Jiménez. 2020. "Long-Term Effects Following Fresh/Vitrified Embryo Transfer Are Transmitted by Paternal Germline in a Large Size Rabbit Cohort" Animals 10, no. 8: 1272. https://doi.org/10.3390/ani10081272
APA StyleGarcia-Dominguez, X., Vicente, J. S., Viudes-de-Castro, M. P., & Marco-Jiménez, F. (2020). Long-Term Effects Following Fresh/Vitrified Embryo Transfer Are Transmitted by Paternal Germline in a Large Size Rabbit Cohort. Animals, 10(8), 1272. https://doi.org/10.3390/ani10081272





