Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity
Simple Summary
Abstract
1. Introduction
2. A Synopsis of Trapping Standards
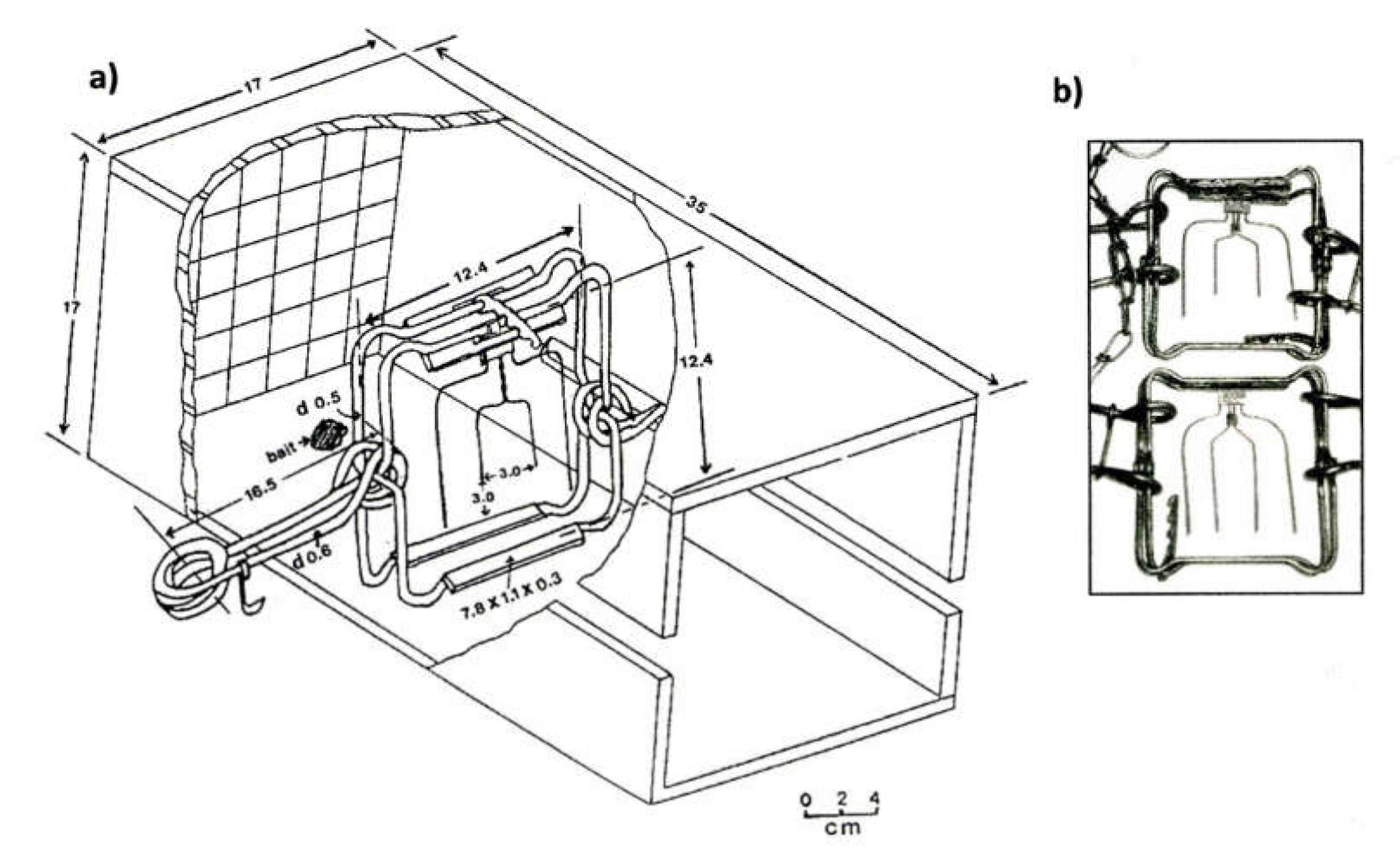
2.1. Test Procedures
2.2. Comparisons
3. Hypothesis 1: The List of Mammal Species Included in the AIHTS Is Incomplete
3.1. Furbearers
3.2. Rodents
3.3. Other Non-Furbearing Species
3.4. Animals Used in Research and Conservation Programs
4. Hypothesis 2: The AIHTS Have Relatively Low Animal Welfare Performance Thresholds of Acceptance of Traps and Do Not Reflect State-of-the-Art Trapping Technology
4.1. Thresholds of Acceptance
4.2. Times of Irreversible Loss of Consciousness (TIUs)
4.3. Trap Exemptions
5. Hypothesis 3: The AIHTS Animal Welfare Indicators and Injuries Are Insufficient
6. Hypothesis 4: The AIHTS Testing Procedures Are Neither Thorough Nor Transparent
7. Hypothesis 5: The AIHTS Protocols for the Use of Certified Traps Are Inadequate
8. Hypothesis 6: The AIHTS Procedures for the Handling and Dispatching of Animals Are Nonexistent
8.1. Animal Handling and Dispatching
8.2. Trap Visit Intervals
9. Hypothesis 7: The AIHTS Criteria to Assess Trap Efficiency and Species Selectivity Are Inappropriate
9.1. Capture Efficiency
9.2. Species Selectivity
10. Discussion
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barrett, M.W.; Proulx, G.; Jotham, N. Wild fur industry under challenge–the Canadian response. Trans. North Am. Wildl. Nat. Resour. Conf. 1988, 53, 180–190. [Google Scholar]
- Proulx, G. Intolerable Cruelty—The Truth behind Killing Neck Snares and Strychnine; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 2018. [Google Scholar]
- ECGCGRF (European Community, Government of Canada, and Government of the Russian Federation). Agreement on international humane trapping standards. Off. J. Eur. Communities 1997, 42, 43–57. [Google Scholar]
- Anonymous. International agreement in the form of an agreed minute between the European Community and the United States of America on humane trapping standards. Off. J. Eur. Communities 1998, L219, 26–37. [Google Scholar]
- Harrop, S.R. The agreements on international humane trapping standards—Background, critique and the texts. J. Int. Wildl. Law Policy 1998, 1, 387–394. [Google Scholar] [CrossRef]
- ISO 10990-4. Part 4: Methods for testing killing trap systems used on land and underwater. In Animal (Mammal) Traps; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- ISO 10990-5. Part 5: Methods for testing restraining traps. In Animal (Mammal) Traps; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Talling, J.C.; Inglis, I.R. Improvements to Trapping Standards. DG ENV. 2009. Available online: http://ec.europa.eu/environment/biodiversity/animal_welfare/hts/pdf/final_report.pdf (accessed on 26 March 2020).
- Powell, R.A.; Proulx, G. Trapping and marking terrestrial mammals for research: Integrating ethics, standards, techniques, and common sense. Inst. Lab. Anim. Res. J. 2003, 44, 259–276. [Google Scholar] [CrossRef]
- Proulx, G.; Cattet, M.R.L.; Powell, R.A. Humane and efficient capture and handling methods for carnivores. In Carnivore Ecology and Conservation: A Handbook of Techniques; Boitani, L., Powell, R.A., Eds.; Oxford University Press: London, UK, 2012; pp. 70–129. [Google Scholar]
- Proulx, G.; Rodtka, D. Steel-jawed leghold traps and killing neck snares: Similar injuries command a change to agreement on international humane trapping standards. J. Appl. Anim. Welf. Sci. 2017, 20, 198–203. [Google Scholar] [CrossRef]
- Proulx, G.; Rodtka, D.; Barrett, M.W.; Cattet, M.; Dekker, D.; Moffatt, E.; Powell, R.A. Humaneness and selectivity of killing neck snares used to capture canids in Canada: A review. Can. Wildl. Biol. Manag. 2015, 4, 55–65. [Google Scholar]
- Virgós, E.; Lozano, J.; Cabezas-Díaz, S.; Macdonald, D.W.; Zalewski, A.; Atienza, J.C.; Proulx, G.; Ripple, W.J.; Rosalino, L.M.; Santos-Reis, M.; et al. A poor international standard for trap selectivity threatens global carnivore and biodiversity conservation. Biodivers. Conserv. 2016. [Google Scholar] [CrossRef]
- Iossa, G.; Soulsbury, C.D.; Harris, S. Mammal trapping: A review of animal welfare standards of killing and restraining traps. Anim. Welf. 2007, 16, 1–18. [Google Scholar]
- Proulx, G.; Rodtka, D. Killing traps and snares in North America: The need for stricter checking time periods. Animals 2019, 9, 570. [Google Scholar] [CrossRef]
- Game and Wildlife Conservation Trust. Agreement on International Humane Trapping Standards (AIHTS). 2020. Available online: https://www.gwct.org.uk/advisory/faqs/aihts/ (accessed on 26 March 2020).
- Hampton, J.O.; Hyndman, T.H.; Laurence, M.; Perry, A.L.; Adams, P.; Collins, T. Animal welfare and the use of procedural documents: LImitations and refinement. Wildl. Res. 2016, 43, 599–603. [Google Scholar] [CrossRef]
- Paquet, P.C.; Darimont, C.T. Wildlife conservation and animal welfare: Two sides of the same coin? Anim. Welf. 2010, 19, 177–190. [Google Scholar]
- Field, K.A.; Paquet, P.C.; Artelle, K.; Proulx, G.; Brook, R.K.; Darimont, C.T. Publication reform to safeguard wildlife from researcher harm. PLoS Biol. 2019, 17, e3000193. [Google Scholar] [CrossRef]
- Baker, S.E.; Ellwood, S.A.; Tagarielli, V.L.; Macdonald, D.W. Mechanical performance of rat, mouse and mole spring traps, and possible implications for welfare performance. PLoS ONE 2012, 7, e39334. [Google Scholar] [CrossRef]
- Jacobs, M.H. Wildlife value orientations in the Netherlands. Hum. Dimens. Wildl. 2007, 12, 359–365. [Google Scholar] [CrossRef]
- Manfredo, M.J.; Teel, T.L.; Dietsch, A.M. Implications of human value shift and persistence for biodiversity conservation. Conserv. Biol. 2016, 30, 287–296. [Google Scholar] [CrossRef]
- Proulx, G.; Barrett, M.W. Animal welfare concerns and wildlife trapping: Ethics, standards and commitments. Trans. West. Sect. Wildl. Soc. 1989, 25, 1–6. [Google Scholar]
- Hampton, J.O.; Fisher, P.M.; Warburton, B. Reconsidering humaneness. Conserv. Biol. 2020. [Google Scholar] [CrossRef]
- Proulx, G. Improved trapping standards for marten and fisher. In Martes: Taxonomy, Ecology, Techniques and Management; Proulx, G., Bryant, H.N., Woodard, P.M., Eds.; Provincial Museum of Alberta: Edmonton, AB, Canada, 1997; pp. 362–371. [Google Scholar]
- Cook, S.R.; Proulx, G. Mechanical evaluation and performance improvement of the rotating jaw Conibear 120 trap. ASTM J. Test. Eval. 1989, 17, 190–195. [Google Scholar]
- Zelin, S.; Jofriet, J.C.; Percival, K.; Abdinoor, D.J. Evaluation of humane traps: Momentum thresholds for four furbearers. J. Wildl. Manag. 1983, 47, 863–868. [Google Scholar] [CrossRef]
- Warburton, B.; Hall, J.V. Impact momentum and clamping force thresholds for developing standards for possum kill traps. N. Z. J. Zool. 1995, 22, 39–44. [Google Scholar] [CrossRef]
- Proulx, G.; Onderka, D.K.; Kolenosky, A.J.; Cole, P.J.; Drescher, R.K.; Badry, M.J. Injuries and behavior of raccoons (Procyon lotor) captured in the Soft CatchTM and the EGGTM traps in simulated natural environments. J. Wildl. Dis. 1993, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Proulx, G.; Cook, S.R.; Barrett, M.W. Assessment and preliminary development of the rotating-jaw Conibear 120 trap to effectively kill marten (Martes americana). Can. J. Zool. 1989, 67, 1074–1079. [Google Scholar] [CrossRef]
- Gilbert, F.F. Assessment of Furbearer Response to Trapping Devices, Proceedings of the Worldwide Furbearer Conference, Frostburg, MD, USA, 3–11 August 1980; Chapman, J.A., Pursley, D., Eds.; Worldwide Furbearer Conference Inc.: Frostburg, MD, USA, 1981; pp. 1599–1611. [Google Scholar]
- Proulx, G.; Drescher, R.K. Assessment of rotating-jaw traps to humanely kill raccoons. J. Wildl. Dis. 1994, 30, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hiltz, M.; Roy, L.D. Use of anaesthetized animals to test humaneness of killing traps. Wildl. Soc. Bull. 2001, 29, 606–611. [Google Scholar]
- Proulx, G.; Barrett, M.W.; Cook, S.R. The C120 Magnum: An effective quick-kill trap for marten. Wildl. Soc. Bull. 1989, 17, 294–298. [Google Scholar]
- Pawlina, I.; Proulx, G. Factors affecting trap efficiency: A review. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 95–115. [Google Scholar]
- Barrett, M.W.; Proulx, G.; Hobson, D.; Nelson, D.; Nolan, J.W. Field evaluation of the C120 Magnum trap for marten. Wildl. Soc. Bull. 1989, 17, 299–306. [Google Scholar]
- Proulx, G. (Alpha Wildlife Research & Management Ltd., Sherwood Park, Alberta, T8H 1W3, Canada). Unpublished notes gathered during his participation as a technical expert in ISO meetings in Brussels, Belgium, 1992.
- Obbard, M.L.; Jones, J.G.; Newman, R.; Booth, A.; Satterthwaite, A.J.; Linscombe, G. Furbearer harvests in North America. In Wild Furbearer Management and Conservation in North America; Novak, M., Baker, J.A., Obbard, M.E., Malloch, B., Eds.; Ontarion Trappers Association: North Bay, ON, Canada, 1987; pp. 1007–1034. [Google Scholar]
- Proulx, G. The impact of human activities on North American mustelids. In Mustelids in a Modern World: Management and Conservation Aspects of Small Carnivore: Human Interactions; Griffiths, H.I., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2000; pp. 53–75. [Google Scholar]
- Mørk, T.; Bohlin, J.; Fuglei, E.; Asbakk, K.; Tryland, M. Rabies in the Arctic fox population, Svalbard, Norway. J. Wildl. Dis. 2011, 47, 945–957. [Google Scholar] [CrossRef]
- Proulx, G.; Barrett, M.W. A review of the 1985–88 humane trapping research program at the Alberta Environmental Centre, Vegreville, Alberta. In Proceedings of the International Symposium on Trapping Wild Furbearers, Edmonton, AB, Canada, 14–16 November 1988. Chap. III. [Google Scholar]
- Proulx, G.; Barrett, M.W.; Cook, S.R. The C120 Magnum with pan trigger: A humane trap for mink (Mustela vison). J. Wildl. Dis. 1990, 26, 511–517. [Google Scholar] [CrossRef]
- Drickamer, L.C.; Mikesic, D.G. Differences in trapping and killing efficiency of Sherman, Victor and Museum Special traps for house mice. Am. Midl. Nat. 1993, 130, 397–401. [Google Scholar] [CrossRef]
- Proulx, G.; Barrett, M.W. Evaluation of the Bionic® trap to quickly kill fisher (Martes pennanti) in simulated natural environments. J. Wildl. Dis. 1993, 29, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Proulx, G.; Barrett, M.W. Field testing the C120 Magnum trap for mink. Wildl. Soc. Bull. 1993, 21, 421–426. [Google Scholar]
- Proulx, G.; Pawlina, I.M.; Onderka, D.K.; Badry, M.J.; Seidel, K. Field evaluation of the No. 1-1/2 steel-jawed leghold and the Sauvageau 2001-8 traps to humanely capture Arctic fox. Wildl. Soc. Bull. 1994, 22, 179–183. [Google Scholar]
- Proulx, G.; Kolenosky, A.J.; Cole, P.J. Assessment of the Kania® trap to humanely kill red squirrels (Tamiasciurus hudsonicus) in enclosures. J. Wildl. Dis. 1993, 29, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Proulx, G.; Kolenosky, A.J.; Badry, M.J.; Cole, P.J.; Drescher, R.K. Assessment of the Sauvageau 2001-8 trap to effectively kill Arctic fox. Wildl. Soc. Bull. 1993, 21, 132–135. [Google Scholar]
- Proulx, G. Testing the ARC 90-16 Kania Trap for Red Squirrels on Traplines; Report submitted to The Fur Institute of Canada, Ottawa; Alpha Wildlife Research & Management Ltd.: Sherwood Park, AB, Canada, 1996. [Google Scholar]
- Mason, G.; Littin, K. The humaneness of rodent pest control. Anim. Welf. 2003, 12, 1–37. [Google Scholar]
- Proulx, G. A northern pocket gopher (Thomomys talpoides) border control strategy: Promising approach. Crop Prot. 1997, 16, 279–284. [Google Scholar] [CrossRef]
- Marsh, R.E. Current (1994) ground squirrel control practices in California, Proceedings of the 16th Vertebrate Pest Conference, Santa Clara, CA, USA, 28 February, 1–3 March, 1994; Halverson, W.S., Crabb, A.C., Eds.; University of California: Davis, CA, USA, 1994; pp. 61–65. [Google Scholar]
- Proulx, G. Evaluation of the experimental PG trap to effectively kill northern pocket gophers. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 89–93. [Google Scholar]
- Mengak, M.T.; Guynn, D.C., Jr. Pitfalls and snap traps for sampling small mammals and herpetofauna. Am. Midl. Nat. 1987, 118, 284–288. [Google Scholar] [CrossRef]
- Delehanty, B.; Boonstra, R. Impact of live trapping on stress profiles of Richardson’s ground squirrel (Spermophilus richardsonii). Gen. Comp. Endocrinol. 2009, 160, 176–182. [Google Scholar] [CrossRef]
- Sikes, R.S.; Gannon, W.L.; The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Proulx, G. Animal welfare concerns in wildlife research and management. Can. Wildl. Biol. Manag. 2017, 6, 1–3. [Google Scholar]
- Beringer, J.; Hansen, L.P.; Sheriff, S.L. Evaluation of two capture techniques for white-tailed deer. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 165–174. [Google Scholar]
- Bergvall, U.A.; Jäderberg, L.; Kjellander, P. The use of box-traps for wild roe deer: Behaviour, injuries and recaptures. Eur. J. Wildl. Res. 2017, 63, 67. [Google Scholar] [CrossRef]
- Proulx, G. Review of current mammal trap technology in North America. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 1–46. [Google Scholar]
- Proulx, G.; Barrett, M.W. Ethical considerations in the selection of traps to harvest martens and fishers. In Martens, Sables, and Fishers: Biology and Conservation; Buskirk, S.W., Harestad, A.S., Raphael, M.G., Powell, R.A., Eds.; Cornell University Press: Ithaca, NY, USA, 1994; pp. 192–196. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Fleiss, J.L. Statistical Methods for Rates and Proportions; John and Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Dulberg, C. (Health Sciences, University of Ottawa, Ottawa, ON, Canada). Personal communication, 1992.
- Proulx, G.; Kolenosky, A.J.; Cole, P.J.; Drescher, R.K. A humane killing trap for lynx (Felis lynx): The Conibear 330 with clamping bars. J. Wildl. Dis. 1995, 31, 57–61. [Google Scholar] [CrossRef]
- The Spring Traps Approval (England) Order 2018. Available online: http://www.legislation.gov (accessed on 9 December 2019).
- Baker, S.E.; Sharp, T.M. Welfare in commensal rodent trapping: One step forward, two steps back. Anim. Welf. 2015, 24, 369–371. [Google Scholar]
- Blue Angel—The German Ecolabel. Non-Toxic Pest Control and Prevention DE-UZ 34 Basic Award Criteria, Edition January 2017, Version 2. 2017. Available online: https://produktinfo.blauer-engel.de/uploads/criteriafile/en/DE-UZ%20034-201701-en%20Criteria.pdf (accessed on 15 May 2020).
- Blue Angel—Rodent traps. Available online: https://www.blauer-engel.de/en/products/home-living/pest-control-biocide-free-indoor/rodent-traps (accessed on 15 May 2020).
- Baker, S.E. A voluntary trap approval scheme to end trap welfare inequality in the UK. Anim. Welf. 2017, 26, 131–133. [Google Scholar]
- Baker, S.E.; Macdonald, D.W.; Ellwood, S.A. Double Standards in Spring Trap Welfare: Ending Inequality for Rats (Rodentia: Muridae), Mice (Rodentia: Muridae) and Moles (Insectivora: Talpidae) in the United Kingdom, Proceedings of the Ninth International Conference on Urban Pests, Birmingham, UK, 9–12 July 2017; Davies, M.P., Pfeiffer, C., Robinson, W.H., Eds.; Pureprint Group: Uckfield, UK, 2017. [Google Scholar]
- Pontu, N.; Warburton, B. Evaluation of the Effectiveness of the Waddington Backcracker Trap for Killing Stoats; Doc Science Internal Series 132; Department of Conservation: Wellington, New Zealand, 2003. [Google Scholar]
- Association of Fish & Wildlife Agencies (AFWA]. Best Management Practices for trapping American marten in the United States. Mimeograph. 2014. Available online: https://www.fishwildlife.org/application/files/ (accessed on 26 March 2019).
- Association of Fish & Wildlife Agencies (AFWA). Undated. Best Management Practices for Trapping Mink in the United States. Mimeograph. Available online: https://www.fishwildlife.org/application/files/2015/2105/2663/MinkRV3.pdf (accessed on 26 March 2019).
- Association of Fish & Wildlife Agencies (AFWA). Best Management Practices for Trapping Raccoons in the United States. Mimeograph. 2014. Available online: https://www.dec.ny.gov/docs/wildlife_pdf/trapbmpsraccoon.pdf (accessed on 9 December 2019).
- ISO. Part 4, Non-mechanically powered killing snares. In Animal (Mammal Traps); ISO: Geneva, Switzerland, 1995; ISO/TC 191/N 99. [Google Scholar]
- Statistics Canada. Fur Statistics; Statistics Canada: Ottawa, ON, Canada, 2011. [Google Scholar]
- Fox, C.H.; Papouchis, C.M. Cull of the Wild—A Contemporary Analysis of Wildlife Trapping in the United States; Animal Protection Institute: Sacramento, CA, USA, 2004. [Google Scholar]
- Andrews, E.J.; Bennett, B.T.; Clark, J.D.; Houpt, K.A.; Pascoe, P.J.; Robinson, G.W.; Boyce, J.R. Report of the AVMA panel on euthanasia. J. Am. Vet. Med Assoc. 1993, 202, 229–249. [Google Scholar]
- Ludders, J.W.; Schmidt, R.H.; Dein, F.J.; Klein, P.N. Drowning is not euthanasia. Wildl. Soc. Bull. 1999, 27, 666–670. [Google Scholar]
- Mason, G.; Mendl, M. Why is there no simple way of measuring animal welfare? Anim. Welf. 1993, 2, 301–319. [Google Scholar]
- Mellor, D.J.; Reid, C.S.W. Concepts of animal well-being and predicting the impact of procedures on experimental animals. In Improving the Well-Being of Animals in the Research Environment; Baker, R.M., Jenkin, G., Mellor, D.J., Eds.; Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART): Glen Osmond, Australia, 1994; pp. 3–18. [Google Scholar]
- Sharp, T.; Saunders, G. A model for Assessing the Relative Humaneness of Pest Animal Control Methods, 2nd ed.; ACT, Australian Government Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2011. [Google Scholar]
- Seddon, P.J.; van Heezik, Y.; Maloney, R.E. Short- and medium-term evaluations of foothold trap injuries in two species of fox in Saudi Arabia. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 67–77. [Google Scholar]
- Cattet, M.; Boulanger, J.; Stenhouse, G.; Powell, R.A.; Reynolds-Hogland, M.J. An evaluation of long-term capture effects in ursids: Implications for wildlife welfare and research. J. Mammal. 2008, 89, 973–990. [Google Scholar] [CrossRef]
- Cattet, M.; Stenhouse, G.; Bollinger, T. Exertional myopathy in a grizzly bear (Ursus arctos) captured by leghold snare. J. Wildl. Dis. 2008, 44, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Gese, E.M.; Terletzky, P.A.; Erb, J.D.; Fuller, K.C.; Grabarkewitz, J.P.; Hart, J.P.; Humpal, C.; Sampson, B.A.; Young, J.K. Injury scores and spatial responses of wolves following capture: Cable restraints versus foothold traps. Wildl. Soc. Bull. 2019, 43, 42–52. [Google Scholar] [CrossRef]
- Warburton, B.; Gregory, N.; Bunce, M. Stress response of Australian brushtail possums captured in foothold and cage traps. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 53–56. [Google Scholar]
- Cattet, M.; Wismer, D.; Stenhouse, G. Wildlife Health: An Integral Component of Forest Management; Final report (EOI-FFI-17-04); Forest Resource Improvement Association of Alberta: Edmonton, AB, Canada, 2020. [Google Scholar]
- Onderka, D.K. Pathological examination as an aid for trap selection guidelines: Usefulness and limitations. In Mammal Trapping; Proulx, G., Ed.; Alpha Wildlife Publications: Sherwood Park, AB, Canada, 1999; pp. 47–51. [Google Scholar]
- Fur Institute of Canada (FIC). Certified Traps—AIHTS Implementation in Canada. 2019. Available online: https://fur.ca/certified-traps/ (accessed on 26 March 2020).
- Hiltz, M.; Roy, L.D. Rating Killing Traps Against Humane Trapping Standards Using Computer Simulations. Proceedings of the 19th Vertebrate Pest Conference, San Diego, California; Salmon, L.P., Crabb, A.C., Eds.; University of California: Davis, CA, USA, 2000; pp. 197–201. [Google Scholar]
- Proulx, G.; Barrett, M.W. Evaluation of mechanically improved Conibear 220TM traps to quickly kill fisher (Martes pennanti) in simulated natural environments. J. Wildl. Dis. 1993, 29, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Akl, E.A.; Bryant, D.; Xie, F.; Apalone, G.; Ter Riet, G. Discussing study limitations in reports and biomedical studies—The need for more transparency. Health Qual. Life Outcomes 2012, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.F.; Williams, M. Irreproducibility in preclinical biomedical research: Perceptions, uncertainties, and knowledge gaps. Trends Pharm. Res. 2016, 37, 290–302. [Google Scholar] [CrossRef]
- Serfass, T.L.; Wright, L.; Pearce, K.; Duplaix, N. Animal welfare issues pertaining to the trapping of otters for research, conservation, and fur. In Marine Mammal Welfare; Butterworth, A., Ed.; Springer Nature: Cham, Switzerland, 2017; pp. 543–571. [Google Scholar]
- Naylor, B.J.; Novak, M. Catch efficiency and selectivity of various traps and sets used for capturing American martens. Wildl. Soc. Bull. 1994, 22, 489–496. [Google Scholar]
- Fournier, G.; Canac-Marquis, P. Best Trapping Practices. Fédération des trappeurs gestionnaires du Québec: Québec, QC, Canada, 2018. Available online: https://mffp.gouv.qc.ca/wp-content/uploads/best-trapping-pratices-juillet-2018.pdf (accessed on 26 March 2020).
- Utah Today’s Trapper Course. American Marten Sets. Available online: https://www.hunter-ed.com/utah/studyGuide/American-Marten-Sets/221046_700121056/ (accessed on 26 March 2020).
- Giroux, A. The role of the trapper today. In Wild Furbearer Management and Conservation in North America; Novak, M., Baker, J.A., Obbard, M.E., Malloch, B., Eds.; The Ontario Trappers Association: North Bay, ON, Canada, 1987; pp. 55–58. [Google Scholar]
- Meyer, S. Being Kind to Animal Pests. A Non-Nonsense Guide to Humane Animal Control with Cage Traps; Meyer Pub Co: Garrison, IA, USA, 1991. [Google Scholar]
- Southworth, P. Moment a Hunter Has to Free the Hissing and Clawing Mountain Lion He Trapped by Mistake While Trying to Catch Coyotes in the Utah Wilderness. Daily Mail. 4 March 2018. Available online: https://www.dailymail.co.uk/news/article-5460953/Utah-trapper-frees-mountain-lions-paw-catching-mistake.html (accessed on 15 May 2020).
- Alaska Outdoors Supersite. Methods for dispatching trapped animals. Available online: http://forums.outdoorsdirectory.com/showthread.php/136907-Methods-for-Dispatching-Trapped-Animals (accessed on 26 March 2020).
- Wildlife removal, San Jose, CA. Available online: http://www.sanjosepestwildlife.com/squirrel-kill.html (accessed on 26 March 2020).
- Taxidwermy.net—Forum Dispatching Trapped Animals with Minimal Skull/Hide Damage. Available online: https://www.taxidermy.net/threads/356106/ (accessed on 23 July 2020).
- Warburton, B. Leghold Traps. A Guideline for Capturing Possums, Ferrets and Feral Cats Using Leghold Traps; National Pest Control Agencies: Wellington, New Zealand, 2015. [Google Scholar]
- American Association of Zoo Veterinarians (AAZV). Guidelines for Euthanasia of Nondomestic Animals; AAZV: Yulee, FL, USA, 2006. [Google Scholar]
- Leary, S.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals, 2020th ed; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Vantassel, S.; Hiller, T.L.; Powell, K.D.J.; Hyngstrom, S.E. Using advancements in cable-trapping to overcome barriers to furbearer management in the United States. J. Wildl. Manag. 2010, 74, 934–939. [Google Scholar] [CrossRef]
- Larkin, R.P.; VanDeelen, T.R.; Sabick, R.M.; Gosselink, T.E.; Warner, R.E. Electronic signaling for prompt removal of an animal from a trap. Wildl. Soc. Bull. 2003, 31, 392–398. [Google Scholar]
- Santos, N.; Rio-Maior, H.; Nakamura, M.; Roque, S.; Brandão, R.; Álvares, F. Characterization and minimization of the stress response to trapping in free-ranging wolves (Canis lupus): Insights from physiology and behavior. Stress 2017, 20, 513–522. [Google Scholar] [CrossRef]
- Krause, T. Editor’s notes. Am. Trapp. 1977, 17, 6–7. [Google Scholar] [CrossRef]
- Miskosky, R. Never let the truth get in the way of a good story. Alta. Outdoorsmen 2016, 17, 6–7. [Google Scholar]
- Baker, S.E.; Macdonald, D.W. Not so humane mole tube traps. Anim. Welf. 2012, 21, 613–615. [Google Scholar]
- The Animal Welfare Act 2006: What it means for wildlife. WML-GU02, England. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/798010/wml-gu02-animal-welfare-act-wildlife-managment.pdf (accessed on 23 July 2020).
- Warburton, B. Evaluation of seven trap models as humane and catch-efficient possum trap. N. Z. J. Zool. 1982, 9, 409–418. [Google Scholar] [CrossRef]
- Krause, T. Restraining trap research. Am. Trapp. 1989, 29, 28–29. [Google Scholar]
- Skinner, D.L.; Todd, A.W. Evaluating efficiency of footholding devices for coyote capture. Wildl. Soc. Bull. 1990, 18, 166–175. [Google Scholar]
- Messineo, J. The EGG (trap) and I. Am. Trapp. 1991, 31, 33–35. [Google Scholar]
- Hubert, G.F., Jr.; Hungerford, L.L.; Proulx, G.; Bluett, R.D.; Bowman, L. Evaluation of two restraining traps to capture raccoons in non-drowning water sets. Wildl. Soc. Bull. 1996, 24, 699–708. [Google Scholar]
- Canadian General Standards Board. Animal (Mammal) Traps—Mechanically Powered, Trigger-Activated Killing Traps for Use on Land; National Standards of Canada: Ottawa, ON, Canada, 1996. [Google Scholar]
- Proulx, G. A preliminary evaluation of four types of traps to capture northern pocket gophers, Thomomys talpoides. Can. Field-Nat. 1997, 111, 640–643. [Google Scholar]
- Munõz-Igualada, J.; Shivik, J.A.; Domínguez, F.G.; González, L.M.; Aranda-Moreno, A.; Olalla, M.F.; García, C.A. Traditional and new cable restraint systems to capture fox in central Spain. J. Wildl. Manag. 2010, 74, 181–187. [Google Scholar] [CrossRef]
- Rodríguez, A.; Delibes, M. Patterns and causes of non-natural mortality in the Iberian lynx during a 40-year period of range contraction. Biol. Conserv. 2004, 118, 151–161. [Google Scholar] [CrossRef]
- Cabezas-Díaz, S.; Lozano, J.; Virgós, E. The declines of the wild rabbit (Oryctolagus cuniculus) and the Iberian lynx (Lynx pardinus) in Spain: Redirecting conservation efforts. In Handbook of Nature Conservation: Global, Environmental and Economic Issues; Aronoff, J.B., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2009; pp. 283–310. [Google Scholar]
- Marshall, C. Runners Injured in Animal Snares. BBC News Environment Correspondent. 2015. Available online: https://www.bbc.com/news/science-environment-32503789 (accessed on 15 May 2020).
- Fur Institute of Canada (FIC). An Abridged Chronology of Events Concerning EU Regulation #3254/91; Mimeograph, Fur Institute of Canada: Ottawa, ON, Canada, 1996. [Google Scholar]
- NAWAC guidelines 09. Assessing the Welfare Performance of RESTRAINING and kill Traps. 2019. Available online: https://www.mpi.govt.nz/dmsdocument/8521-nawac-guideline-09-assessing-the-welfare-performance-of-restraining-and-kill-traps (accessed on 15 May 2020).
- Cook, S.R.; Proulx, G. Use of a digital waveform analyzer, accelerometers, and a load cell to measure momentum and clamping forces of killing traps for furbearers. Astm. J. Test. Eval. 1989, 17, 186–189. [Google Scholar]
- Cattet MRL. Falling through the cracks: Shortcomings in the collaboration between biologists and veterinarians and their consequences for wildlife. ILAR J. 2013, 54. [Google Scholar] [CrossRef]
- McDonald, J. Cornerstone of U.S. conservation: The Pitman-Robertson Act celebrates 75 years. Wildl. Prof. 2012, 6, 74–78. [Google Scholar]
- Dronova, N.; Shestakov, A.S. Conservation and Socioeconomic Aspects of the Fur Trade in the Russian Far East. TRAFFIC Europe–Russia. Rufford Maurice Laing Foundation and WWF Germany. 2015. Available online: ttps://www.researchgate.net/publication/305281177_Trapping_a_Living_Conservation_and_Socio-Economic_Aspects_of_the_Fur_Trade_in_the_Russian_Far_East (accessed on 15 May 2020).
| Subject | Standards | 1985-93 Canadian Research Program [10] | ||||
|---|---|---|---|---|---|---|
| ISO [6,7] | AIHTS [3] | |||||
| Restraining Traps | Killing Traps | Restraining Traps | Killing Traps | Restraining Traps | Killing Traps | |
| Legal significance | None | Binding agreement—each party should take the necessary steps to ensure that the respective competent authorities (a) establish appropriate processes for certifying traps in accordance with the standards; (b) ensure that the trapping methods conducted in their respective territories are in accordance with the standards; (c) prohibit the use of traps that are not certified in accordance with the standards; (d) require manufacturers to identify certified traps and provide instructions for their appropriate setting, safe operation, and maintenance. | None | |||
| Definition | Device used to capture and restrain a mammal. A restraining trap system encompasses equipment (trap and trigger) and set (site modifications, lures, and baits). | Device for use on land or underwater to kill a mammal. A killing trap system encompasses equipment (trap and trigger) and set (site modifications, lures, and baits). | Traps designed and set with the intention of not killing the trapped animal but restricting its movements to such an extent that a human can make direct contact with it. | Traps designed and set with the intention of killing a trapped animal of the target species. | As per ISO and AIHTS | |
| List of species | All mammal species | Coyote (Canis latrans) Wolf (Canis lupus) North American beaver (Castor canadensis) European beaver (Castor fiber) Bobcat (Felis rufus) North American otter (Lontra canadensis) European otter (Lutra lutra) Canada lynx (Lynx canadensis) European lynx (Lynx lynx) American marten (Martes americana) Fisher (Pekania pennanti) Sable (Martes zibellina) Pine marten (Martes martes) European badger (Meles meles) Ermine (Mustela erminea) Raccoon dog (Nyctereutes procyonoides) Muskrat (Ondatra zibethicus) Raccoon (Procyon lotor) North American badger (Taxidea taxus) | All mammal species Species for which acceptable traps have been developed by researchers according to performance levels presented below: Arctic fox (Vulpes lagopus) Red squirrel (Tamiasciurus hudsonicus) Northern pocket gopher (Thomomys talpoides) Canada lynx American marten Fisher American mink (Neovison vison) Raccoon | |||
| Testing procedure | Field testing Pathological evaluation | Mechanical evaluation Kill tests with anaesthetized animals. Kill tests in compounds. Field tests. Pathological evaluations. Inspection and testing for user safety and traps. | Compound tests to evaluate behavioural, physiological, and biochemical parameters. Field tests (vs. a control trap) to assess selectivity and user safety. Pathological evaluations. | Approach tests to ensure a proper positioning of the animals in the traps. Kill tests in compounds to assess loss of consciousness. Field tests (vs. a control trap) to assess selectivity and user safety. Pathological evaluations. | Mechanical evaluation to compare impact momentum and clamping forces of different trap models. Compound tests to assess behavioural and physiological parameters. Field tests (vs. a control trap) to assess selectivity and user safety. Pathological evaluations. | Mechanical evaluation to assess the potential of traps.Approach tests to ensure a proper positioning of the animals in the traps. Kill tests with anaesthetized animals to assess loss of consciousness in non-reactive animals. Kill tests in compounds to assess loss of consciousness in conscious animals. Field tests to verify compound test findings. Pathological evaluations in compound and field tests. Inspection and testing for user safety and traps. |
| Test report | Humaneness: report on the position of each animal in the trap and evaluation of the condition of the captured animals according to a trauma scale, with scores proportional to the severity of the injuries. Field tests: number of captures of target and non-target species. User safety: field notes. | Humaneness: report on strike location and time to loss of corneal and palpebral reflexes and heartbeat. Field tests: number of captures of target and non-target species. Compound and field tests: pathological observations. User safety: field notes. | Humaneness Behavioural indicators of poor welfare:
| Humaneness The time of occurrence and insensibility produced by the killing technique based on the loss of corneal and palpebral reflexes or any other specifically proven suitable substitute parameter: 45 s—Mustela erminea 120 s—Martes spp. 300 s—all other species listed above. | Humaneness: report on the position of each animal in the trap and evaluation of the condition of the captured animals according to a trauma scale with scores proportional to the severity of the injuries; total injuries must amount to <50 points on the scale. Field tests: Number of captures of target and non-target species. User safety: field notes. | Humaneness The number of animals tested and the proportion that lost insensibility based on the loss of corneal and palpebral reflexes. 3 min—all species except large carnivores. 5 min—Red fox (Vulpes Vulpes) |
| Number of tests | Unspecified. The number of replicates in the tests shall be sufficient to determine if the differences are statistically significant at the level to be determined by the authority implementing the test. Comparison of selectivity (number of captured target animals divided by the total number of captured animals) with a control trap and user safety as specified by the authority implementing the standard. | Unspecified. Capability of a killing trap, as part of the killing trap system, to kill an animal within a time period and to meet the requirements related to mechanical properties, comparison of selectivity (number of captured target animals divided by the total number of captured animals) with a control trap model, and user safety as specified by the authority implementing the standard. | The number of specimens of the same target species from which the data are derived is at least 20. | The number of specimens of the same target species from which the data are derived is at least 12. | ≥9 specimens for compound tests. >30 specimens for field tests where capture durations ≤24 h. | ≥6 specimens for approach tests ≥9 specimens for compound kill tests. >30 specimens for field tests. |
| Minimum successful compound tests required to meet performance thresholds | None | None | At least 16 (80%) of 20 animals show none of the indicators listed above. | At least 10 (80%) of 12 animals are unconscious and insensible within the time limit and remain in this state until death. | 9/9 (100%), or 13/14 (93%), or 21/24(88%), etc. (proportions based on the normal approximation to the binomial distribution). | |
| Predicted performance threshold at population level (95% confidence level) resulting from the number of successful tests—one-tailed binomial test | n/a | n/a | 57% | 49% | 71% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proulx, G.; Cattet, M.; Serfass, T.L.; Baker, S.E. Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity. Animals 2020, 10, 1262. https://doi.org/10.3390/ani10081262
Proulx G, Cattet M, Serfass TL, Baker SE. Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity. Animals. 2020; 10(8):1262. https://doi.org/10.3390/ani10081262
Chicago/Turabian StyleProulx, Gilbert, Marc Cattet, Thomas L. Serfass, and Sandra E. Baker. 2020. "Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity" Animals 10, no. 8: 1262. https://doi.org/10.3390/ani10081262
APA StyleProulx, G., Cattet, M., Serfass, T. L., & Baker, S. E. (2020). Updating the AIHTS Trapping Standards to Improve Animal Welfare and Capture Efficiency and Selectivity. Animals, 10(8), 1262. https://doi.org/10.3390/ani10081262




