Milk Replacer Supplementation with Docosahexaenoic Acid from Microalgae Does Not Affect Growth and Immune Status in Goat Kids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Treatments
2.2. Body Weight and Feed Intake Recording and Blood Collection
2.3. Immune Variables Measured in Plasma and Serum
2.4. Statistical Analysis
3. Results
3.1. Supplementation of a Microalgae-Derived Product Rich in DHA Did Not Affect Growth or Feed Intake in Goat Kids.
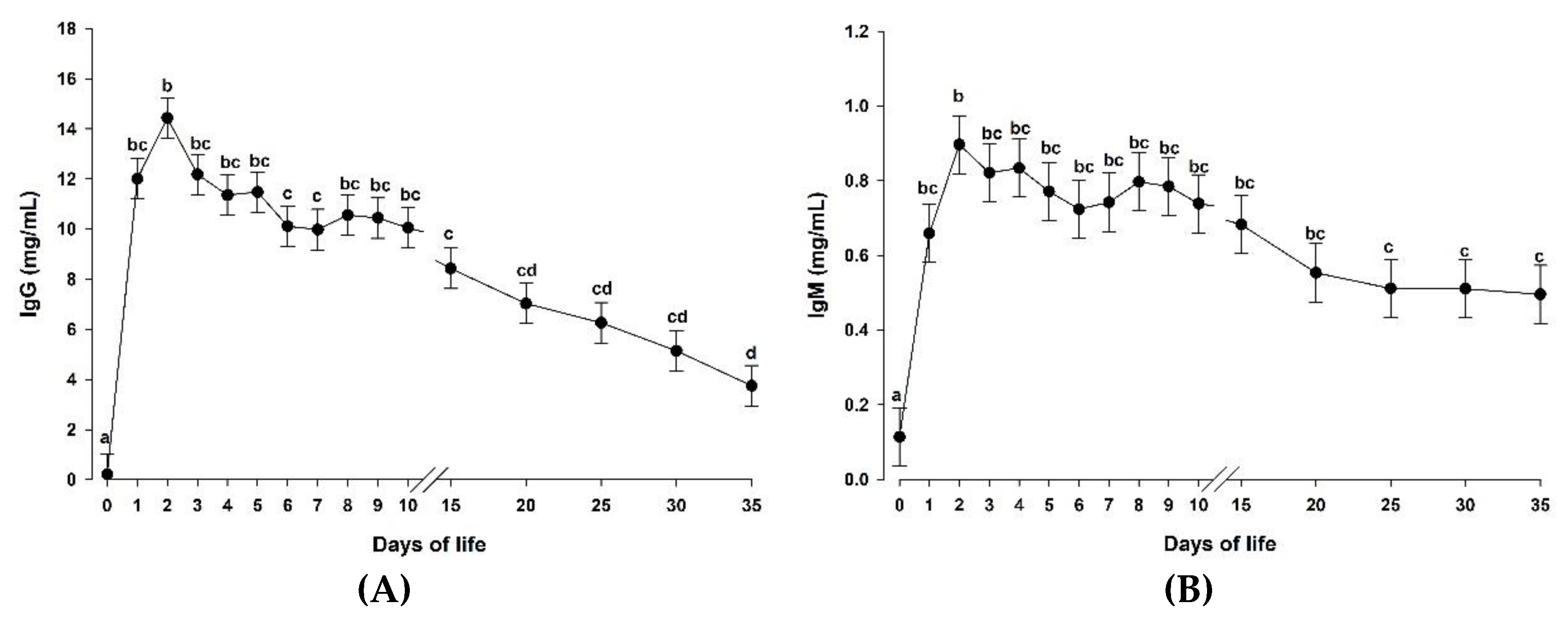
3.2. Supplementation of a Microalgae-Derived Product Rich in DHA Had No Effect on Blood Immunoglobulin Concentrations in Goat Kids
3.3. Neither the Complement System nor the Chitotriosidase Activities Were Affected in Goat Kids Supplemented with a Microalgae-Derived Product Rich in DHA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ian Givens, D.; Gibbs, R.A. Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them. Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain omega-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 429–434.
- Argüello, A. Trends in goat research, a review. J. Appl. Anim. Res. 2011, 39, 429–434. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Nally, J.E.; Lindahl, J.; Wanapat, M.; Alhidary, I.A.; Fangueiro, D.; Grace, D.; Ratto, M.; Bambou, J.C.; de Almeida, A.M. Dairy science and health in the tropics: Challenges and opportunities for the next decades. Trop. Anim. Health Prod. 2019, 51, 1009–1017. [Google Scholar] [CrossRef]
- Banskalieva, V.; Sahlu, T.; Goetsch, A.L. Fatty acid composition of goat muscles and fat depots: A review. Small Rumin. Res. 2000, 37, 255–268. [Google Scholar] [CrossRef]
- Rodrigues, S.; Teixeira, A. Consumers’ preferences for meat of Cabrito Transmontano. Effects of sex and carcass weight. Span. J. Agric. Res. 2010, 8, 936–945. [Google Scholar] [CrossRef]
- Garcia Navarro, M.C.; Ramos Morales, E.; De La Torre Adarve, G.; Fernandez Navarro, J.R.; Rodriguez Osorio, M.; Gill Extremera, F.; Sanz Sampelayo, M.R. Growth of Pre-ruminant Kid Goats and the Composition of Carcass Fat Deposits: Effects of Providing a PUFA-rich Fat in the Milk Replacer and Influence of the Kidding Season. Food Sci. Tech. Int. 2008, 14, 85–94. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Morales-delaNuez, A.; Hernandez-Castellano, L.E.; Sanchez-Macias, D.; Capote, J.; Castro, N.; Argüello, A. Docosahexaenoic acid in the goat kid diet: Effects on immune system and meat quality. J. Anim. Sci. 2012, 90, 3729–3738. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Castro, N.; Arguello, A. The colostrum proteome, ruminant nutrition and immunity: A review. Curr. Prot. Peptide Sci. 2014, 15, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.; Gomez-Gonzalez, L.A.; Earley, B.; Arguello, A. Use of clinic refractometer at farm as a tool to estimate the IgG content in goat colostrum. J. Appl. Anim. Res. 2018, 46, 1505–1508. [Google Scholar] [CrossRef]
- Morales-delaNuez, A.; Moreno-Indias, I.; Sanchez-Macias, D.; Capote, J.; Juste, M.C.; Castro, N.; Hernandez-Castellano, L.E.; Arguello, A. Sodium dodecyl sulfate reduces bacterial contamination in goat colostrum without negative effects on immune passive transfer in goat kids. J. Dairy Sci. 2011, 94, 410–415. [Google Scholar] [CrossRef]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Savoini, G.; Zorini, F.O.; Farina, G.; Agazzi, A.; Cattaneo, D.; Invernizzi, G. Effects of Fat Supplementation in Dairy Goats on Lipid Metabolism and Health Status. Animals 2019, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Moghadasian, M.H. Advances in dietary enrichment with n-3 fatty acids. Crit. Rev. Food. Sci. Nutr. 2008, 48, 402–410. [Google Scholar] [CrossRef]
- Leskanich, C.O.; Noble, R.C. The comparative roles of polyunsaturated fatty acids in pig neonatal development. Brit. J. Nutr. 1999, 81, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Betiku, O.C.; Barrows, F.T.; Ross, C.; Sealey, W.M. The effect of total replacement of fish oil with DHA-Gold((R)) and plant oils on growth and fillet quality of rainbow trout (Oncorhynchus mykiss) fed a plant-based diet. Aquacul. Nutr. 2016, 22, 158–169. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lordelo, M.M.; Costa, P.; Alves, S.P.; Benevides, W.S.; Bessa, R.J.B.; Lemos, J.P.C.; Pinto, R.M.A.; Ferreira, L.M.A.; Fontes, C.M.G.A.; et al. Effect of reduced dietary protein and supplementation with a docosahexaenoic acid product on broiler performance and meat quality. Br. Poult. Sci. 2014, 55, 752–765. [Google Scholar] [CrossRef]
- Lee, S.A.; Whenham, N.; Bedford, M.R. Review on docosahexaenoic acid in poultry and swine nutrition: Consequence of enriched animal products on performance and health characteristics. Anim. Nutr. 2019, 5, 11–21. [Google Scholar] [CrossRef]
- Moran, C.A.; Currie, D.; Keegan, J.D.; Knox, A. Tolerance of Broilers to Dietary Supplementation with High Levels of the DHA-Rich Microalga, Aurantiochytrium Limacinum: Effects on Health and Productivity. Animals 2018, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Vanbergue, E.; Peyraud, J.L.; Hurtaud, C. Effects of new n-3 fatty acid sources on milk fatty acid profile and milk fat properties in dairy cows. J. Dairy Res. 2018, 85, 265–272. [Google Scholar] [CrossRef]
- Argüello, A.; Castro, N.; Zamorano, M.J.; Castroalonso, A.; Capote, J. Passive transfer of immunity in kid goats fed refrigerated and frozen goat colostrum and commercial sheep colostrum. Small Rumin. Res. 2004, 54, 237–241. [Google Scholar] [CrossRef]
- Rodriguez, C.; Castro, N.; Capote, J.; Morales-Delanuez, A.; Moreno-Indias, I.; Sanchez-Macias, D.; Arguello, A. Effect of colostrum immunoglobulin concentration on immunity in Majorera goat kids. J. Dairy Sci. 2009, 92, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.D.; Potchoiba, M.J. Milk feeding and weaning of goat kids — A review. Small Rumin. Res. 1988, 1, 105–112. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Dodds, A.W.; Arguello, A.; Castro, N.; Sim, R.B. The complement system of the goat: Haemolytic assays and isolation of major proteins. BMC Vet. Res. 2012, 8, 91. [Google Scholar] [CrossRef]
- Arguello, A.; Castro, N.; Batista, M.; Moreno-Indias, I.; Morales-delaNuez, A.; Sanchez-Macias, D.; Quesada, E.; Capote, J. Chitotriosidase activity in goat blood and colostrum. J. Dairy Sci. 2008, 91, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castellano, L.E.; Ozcelik, R.; Hernandez, L.L.; Bruckmaier, R.M. Short communication: Supplementation of colostrum and milk with 5-hydroxy-l-tryptophan affects immune factors but not growth performance in newborn calves. J. Dairy Sci. 2017, 101, 794–800. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Moreno-Indias, I.; Morales-delaNuez, A.; Sánchez-Macías, D.; Torres, A.; Capote, J.; Argüello, A.; Castro, N. The effect of milk source on body weight and immune status of lambs. Livest. Sci. 2015, 175, 70–76. [Google Scholar] [CrossRef]
- Demiroren, E.; Shrestha, J.N.B.; Boylan, W.J. Breed and Environmental-Effects on Components of Ewe Productivity in Terms of Multiple Births, Artificial Rearing and 8-Month Breeding Cycles. Small Rumin. Res. 1995, 16, 239–249. [Google Scholar] [CrossRef]
- Napolitano, F.; Pacelli, C.; Girolami, A.; Braghieri, A. Effect of information about animal welfare on consumer willingness to pay for yogurt. J. Dairy Sci. 2008, 91, 910–917. [Google Scholar] [CrossRef]
- Emsen, E.; Yaprak, M.; Bilgin, O.C.; Emsen, B.; Ockerman, H.W. Growth performance of Awassi lambs fed calf milk replacer. Small Rumin. Res. 2004, 53, 99–102. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Martell-Jaizme, D.; Cugno, G.; Argüello, A.; Castro, N. The effect of colostrum period management on BW and immune system in lambs: From birth to weaning. Animal 2015, 9, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Csh. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Sanz Sampelayo, M.R.; Allegretti, L.; Gil Extremera, F.; Boza, J. Growth, body composition and energy utilisation in pre-ruminant goat kids: Effect of dry matter concentration in the milk replacer and animal age. Small Rumin. Res. 2003, 49, 61–67. [Google Scholar] [CrossRef]
- Calder, P.C. Long chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. 2013, 16, 425–433. [Google Scholar] [CrossRef]
- Lewis, G.S.; Wulster-Radcliffe, M.C.; Herbein, J.H. Fatty acid profiles, growth, and immune responses of neonatal lambs fed milk replacer and supplemented with fish oil or safflower oil. Small Rumin. Res. 2008, 79, 167–173. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 2010, 88, 2988–2997. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Ventosa, M.; Coelho, A.V.; Castro, N.; Arguello, A. The effect of colostrum intake on blood plasma proteome profile in newborn lambs: Low abundance proteins. BMC Vet. Res. 2014, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castellano, L.E.; Arguello, A.; Almeida, A.M.; Castro, N.; Bendixen, E. Colostrum protein uptake in neonatal lambs examined by descriptive and quantitative liquid chromatography-tandem mass spectrometry. J. Dairy Sci. 2015, 98, 135–147. [Google Scholar] [CrossRef]
- Mellado, M.; Pittroff, W.; Garcia, J.E.; Mellado, J. Serum IgG, blood profiles, growth and survival in goat kids supplemented with artificial colostrum on the first day of life. Trop. Anim. Health Prod. 2008, 40, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Batmaz, H.; Kacar, Y.; Topal, O.; Mecitoglu, Z.; Gumussoy, K.S.; Kaya, F. Evaluation of passive transfer in goat kids with Brix refractometer and comparison with other semiquantitative tests. Turk. J. Vet. Anim. Sci. 2019, 43, 596–602. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology, 10th ed.; Elsevier: St. Louis, MI, USA, 2017; pp. 247–260. [Google Scholar]
- Matthews, J.G. Diseases of the Goat, 4th ed.; Wiley-Blackwell: West Sussex, UK, 2016; pp. 61–71. [Google Scholar]
- Sijben, J.W.; Calder, P.C. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. P. Nutr. Soc. 2007, 66, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castellano, L.E.; Morales-delaNuez, A.; Sánchez-Macías, D.; Moreno-Indias, I.; Torres, A.; Capote, J.; Argüello, A.; Castro, N. The effect of colostrum source (goat vs. sheep) and timing of the first colostrum feeding (2h vs. 14h after birth) on body weight and immune status of artificially reared newborn lambs. J. Dairy Sci. 2015, 98, 204–210. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Moreno-Indias, I.; Sánchez-Macías, D.; Morales-delaNuez, A.; Torres, A.; Argüello, A.; Castro, C. Sheep and goats raised in mixed flocks have diverse immune status around parturition. J. Dairy Sci. 2019, 102, 8478–8485. [Google Scholar] [CrossRef]
- Castro, N.; Acosta, F.; Nino, T.; Vivas, J.; Quesada, E.; Capote, J.; Arguello, A. The effects of diet and age on serum complement system activity in goat kids. Livest. Sci. 2008, 119, 102–106. [Google Scholar] [CrossRef]
- Renkema, G.H.; Boot, R.G.; Au, F.L.; Donker-Koopman, W.E.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998, 251, 504–509. [Google Scholar] [CrossRef]
- van Eijk, M.; van Roomen, C.P.; Renkema, G.H.; Bussink, A.P.; Andrews, L.; Blommaart, E.F.; Sugar, A.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int. Immunol. 2005, 17, 1505–1512. [Google Scholar] [CrossRef]
- Morales-delaNuez, A.; Hernandez-Castellano, L.E.; Moreno-Indias, I.; Sanchez-Macias, D.; Arguello, A.; Castro, N. Use of glycerol and propylene glycol as additives in heat-treated goat colostrum. J. Dairy Sci. 2020, 103, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.; Malaguarnera, L.; Simpore, J.; Barone, R.; Whalen, M.; Musumeci, S. Chitotriosidase activity in colostrum from African and Caucasian women. Clin. Chem. Lab. Med. 2005, 43, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Macias, D.; Castro, N.; Morales-delaNuez, A.; Hernandez-Castellano, L.E.; Capote, J.; Arguello, A. Chemical composition and immune status of dairy goat colostrum fractions during the first 10 h after partum. Small Rumin. Res. 2012, 103, 220–224. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Torres, A.; Alavoine, A.; Ruiz-Diaz, M.D.; Arguello, A.; Capote, J.; Castro, N. Effect of milking frequency on milk immunoglobulin concentration (IgG, IgM and IgA) and chitotriosidase activity in Majorera goats. Small Rumin. Res. 2011, 98, 70–72. [Google Scholar] [CrossRef]
- Granot, E.; Jakobovich, E.; Rabinowitz, R.; Levy, P.; Schlesinger, M. DHA supplementation during pregnancy and lactation affects infants’ cellular but not humoral immune response. Mediat. Inflamm. 2011, 493925. [Google Scholar] [CrossRef]
- Gabler, N.K.; Spurlock, M.E. Integrating the immune system with the regulation of growth and efficiency. J. Anim. Sci. 2008, 86, 64–74. [Google Scholar] [CrossRef]
- Malaguarnera, L.; Musumeci, M.; Di Rosa, M.; Scuto, A.; Musumeci, S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J. Clin. Lab. Anal. 2005, 19, 128–132. [Google Scholar] [CrossRef]


| MR-NS | MR-DHA-9 | MR-DHA-18 | |
|---|---|---|---|
| Crude protein | 3.95 | 3.84 | 3.72 |
| Fat | 3.80 | 4.04 | 4.28 |
| Total fiber | 0.02 | 0.39 | 0.77 |
| Ash | 1.37 | 1.36 | 1.35 |
| Product specifications 1 | |
| DHA (C22:6n3) content | min. 18% |
| Total fat | min. 35% |
| Unsaponificable matter | max. 8% |
| Insoluble impurities | max. 5% |
| Free fatty acids | max. 5% |
| Moisture | max. 6% |
| Fatty acid profile | g/100g of DHA-Gold® |
| C14:0 | 5.90 |
| C16:0 | 13.1 |
| C22:5n6 | 6.84 |
| C22:6n3 | 19.4 |
| Variable | MR-NS | MR-DHA-9 | MR-DHA-18 | SEM | Fixed Effects (p-Value) | ||
|---|---|---|---|---|---|---|---|
| TRT | D | TRT × D | |||||
| BW (kg) | 4.27 | 4.17 | 4.22 | 0.38 | 0.926 | <0.001 | 0.255 |
| IgG (mg/mL) | 9.58 | 8.73 | 8.21 | 0.51 | 0.176 | <0.001 | 0.165 |
| IgM (mg/mL) | 0.73 | 0.66 | 0.57 | 0.05 | 0.069 | <0.001 | 0.937 |
| TC (hemolysis%) | 18.7 | 15.3 | 14.6 | 2.30 | 0.130 | <0.001 | 0.984 |
| AC (hemolysis%) | 9.71 | 8.74 | 7.94 | 2.51 | 0.502 | <0.001 | 0.420 |
| ChT (nmol/mL/h) | 1619 | 1605 | 1647 | 108 | 0.961 | 0.13 | 0.976 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Indias, I.; Hernández-Castellano, L.E.; Sánchez-Macías, D.; Morales-delaNuez, A.; Torres, A.; Argüello, A.; Castro, N. Milk Replacer Supplementation with Docosahexaenoic Acid from Microalgae Does Not Affect Growth and Immune Status in Goat Kids. Animals 2020, 10, 1233. https://doi.org/10.3390/ani10071233
Moreno-Indias I, Hernández-Castellano LE, Sánchez-Macías D, Morales-delaNuez A, Torres A, Argüello A, Castro N. Milk Replacer Supplementation with Docosahexaenoic Acid from Microalgae Does Not Affect Growth and Immune Status in Goat Kids. Animals. 2020; 10(7):1233. https://doi.org/10.3390/ani10071233
Chicago/Turabian StyleMoreno-Indias, Isabel, Lorenzo E. Hernández-Castellano, Davinia Sánchez-Macías, Antonio Morales-delaNuez, Alexandr Torres, Anastasio Argüello, and Noemí Castro. 2020. "Milk Replacer Supplementation with Docosahexaenoic Acid from Microalgae Does Not Affect Growth and Immune Status in Goat Kids" Animals 10, no. 7: 1233. https://doi.org/10.3390/ani10071233
APA StyleMoreno-Indias, I., Hernández-Castellano, L. E., Sánchez-Macías, D., Morales-delaNuez, A., Torres, A., Argüello, A., & Castro, N. (2020). Milk Replacer Supplementation with Docosahexaenoic Acid from Microalgae Does Not Affect Growth and Immune Status in Goat Kids. Animals, 10(7), 1233. https://doi.org/10.3390/ani10071233







