Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Bird Management
2.4. Sample Collection and Parameter Determination
2.5. RNA Extraction, Reverse Transcription and Real-Time Quantitative PCR
2.6. Illumina Sequencing Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Ash and P of Excreta, Serum P and ALP Concentrations
3.3. P and Ash of Bone, and Tibia Strength
3.4. NaP-IIb and PiT-1, 2 mRNA Expressions in the Duodenum, Jejunum and Ileum of Broilers
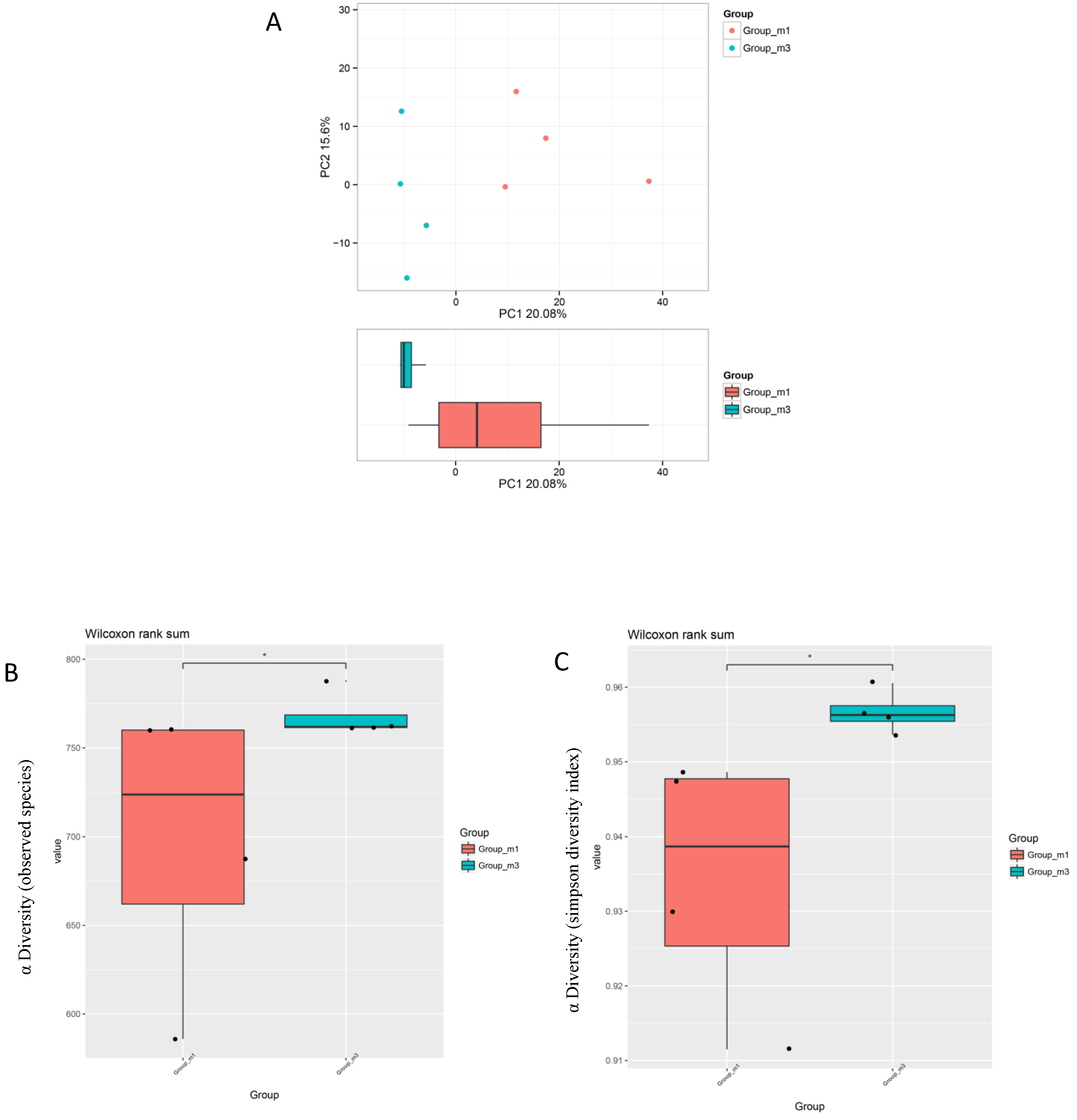
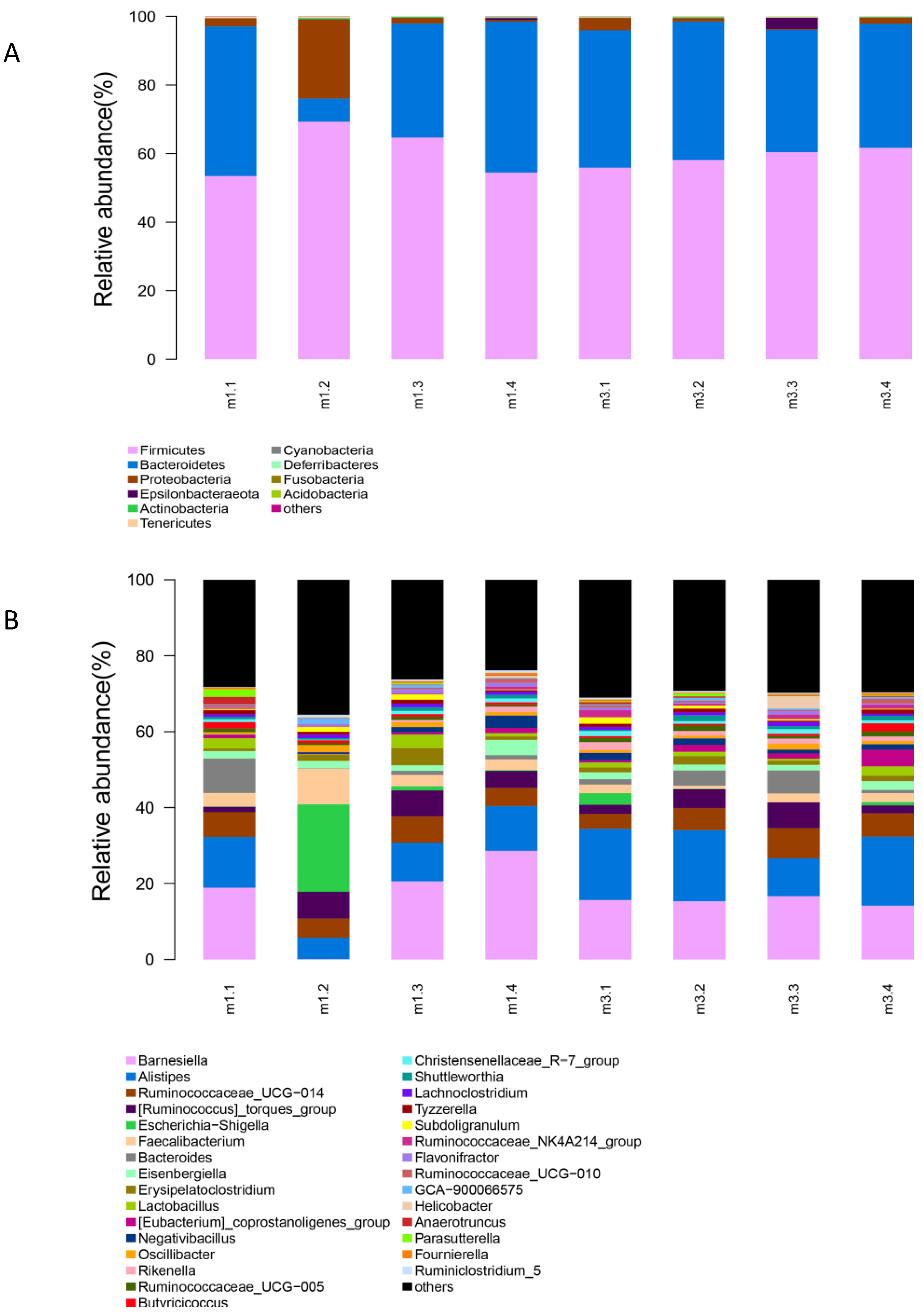
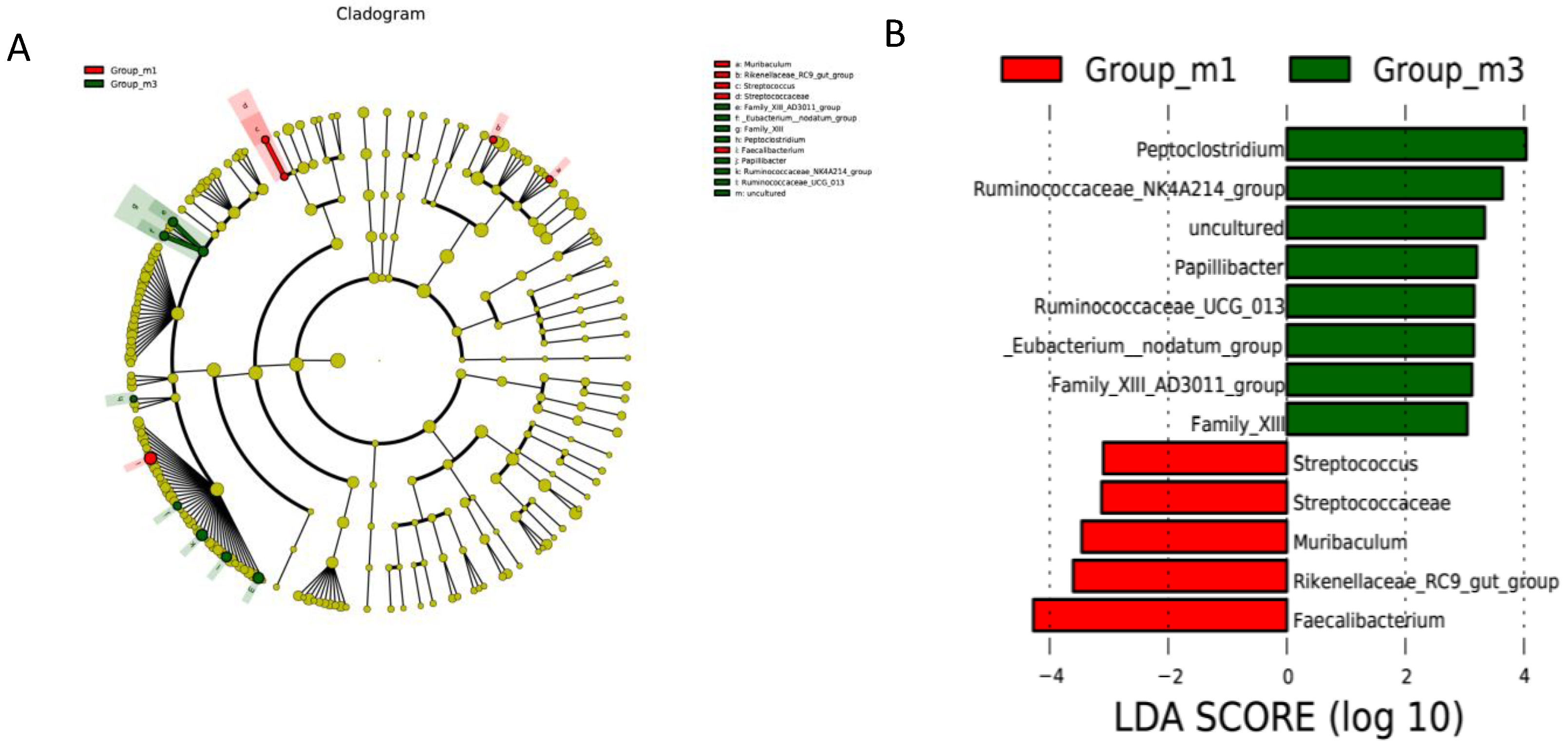
3.5. Gut Microbiota Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rath, N.C.; Huff, G.R.; Huff, W.E.; Balog, J.M. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000, 79, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Fleming; Robert, H. Nutritional factors affecting poultry bone health. P Nutr. Soc. 2008, 67, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Heaney, R.P. Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone 2003, 32, 532–540. [Google Scholar] [CrossRef]
- Gautier, A.E.; Walk, C.L.; Dilger, R.N. Influence of dietary calcium concentrations and the calcium-to-non-phytate phosphorus ratio on growth performance, bone characteristics, and digestibility in broilers. Poult. Sci. 2017, 96, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Bryden, W.L. Calcium and phosphorus metabolism and nutrition of poultry: Are current diets formulated in excess? Anim. Prod. Sci. 2017, 57, 2304–2310. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yang, T.Y.; Bryden, W.L. Phosphorus bioavailability: A key aspect for conserving this critical animal feed resource with reference to broiler nutrition. Agriculture 2016, 6, 25. [Google Scholar] [CrossRef]
- Bajagal, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in animal nutrition: Production, impact and regulation. In FAO Animal Production and Health Paper No. 179; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i5933e.pdf (accessed on 16 February 2020).
- Cao, G.T.; Zeng, X.F.; Chen, A.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef]
- Luo, J.J.; Zheng, A.J.; Meng, K.; Chang, W.H.; Bai, Y.G.; Li, K.; Cai, H.; Liu, G.; Yao, B. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteom. 2013, 91, 226–241. [Google Scholar] [CrossRef]
- Huang, L.Q.; Luo, L.Q.; Zhang, Y.R.; Wang, Z.; Xia, Z.F. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged broiler chickens. Probiotics Antimicrob. Proteins 2019, 11, 946–956. [Google Scholar] [CrossRef]
- Zheng, A.J.; Luo, J.J.; Meng, K.; Li, J.K.; Bryden, W.L.; Chang, W.H.; Zhang, S.; Wang, L.X.N.; Liu, G.; Yao, B. Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus). BMC Genom. 2016, 17, 89. [Google Scholar] [CrossRef]
- Zheng, A.J.; Luo, J.J.; Meng, K.; Li, J.K.; Zhang, S.; Li, K.; Liu, G.; Cai, H.; Bryden, W.L.; Yao, B. Proteome changes underpin improved meat quality and yield of chickens (Gallus gallus) fed the probiotic Enterococcus faecium. BMC Genom. 2014, 15, 1167. [Google Scholar] [CrossRef]
- Yan, F.F.; Murugesan, G.R.; Cheng, H.W. Effects of probiotic supplementation on performance traits, bone mineralization, cecal microbial composition, cytokines and corticosterone in laying hens. Animal 2018, 13, 33–41. [Google Scholar] [CrossRef]
- Yan, F.F.; Mohammed, A.; Murugesan, R.; Cheng, H.W. Effects of a dietary synbiotic inclusion on bone health in broilers subjected to cyclic heat stress episodes. Poult. Sci. 2018, 98, 1083–1089. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links between the microbiome and bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Mccabe, L.R.; Britton, R.A.; Parameswaran, N. Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Li, A.K.; Tao, H.H.; Yi, J.M. Study on the production technology and properties of microencapsulated Enterococcus faecium. JNWAFU 2009, 37, 51–62. [Google Scholar]
- Wang, T.T.; Li, A.K.; Yi, J.M.; Tao, H.H.; Wang, Y. Screening, identification and characterization of Enterococcus faecium for feed use. J. Chin. Cereal Oils Assoc. 2010, 25, 89–93, 97. (In Chinese) [Google Scholar]
- Aviagen. Available online: http://cn.aviagen.com/assets/Tech_Center/AA_Broiler/AA-Broiler-Pocket-Guide-2020-EN.pdf (accessed on 10 June 2019).
- Liu, S.B.; Liao, X.D.; Lu, L.; Li, S.F.; Wang, L.; Zhang, L.Y.; Jiang, Y.; Luo, X.G. Dietary non-phytate phosphorus requirement of broilers fed a conventional corn-soybean meal diet from 1 to 21 d of age. Poult. Sci. 2016, 96, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, H.Y.; Zhang, H.J.; Xu, L.; Wu, H.J.; Yan, H.J.; Gong, Y.S.; Qi, G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009, 88, 2033–2041. [Google Scholar] [CrossRef]
- Hu, Y.X.; Liao, X.D.; Wen, Q.; Lu, L.; Zhang, L.Y.; Luo, X.G. Phosphorus absorption and gene expression levels of related transporters in the small intestine of broilers. Br. J. Nutr. 2018, 119, 1346–1354. [Google Scholar] [CrossRef]
- Frost, T.J.; Roland, D.A. Research note: Current methods used in determination and evaluation of tibia strength: A correlation study involving birds fed various levels of cholecalciferol. Poult. Sci. 1991, 70, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.F.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative Real-Time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Shewita, R.; Elkatcha, M.; Soltan, M.; Sedeik, M. Impact of using Enterococcus faecium as a probiotic in broiler diets. AJVS 2016, 51, 102–113. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Jahromi, M.F.; Liang, J.B.; Ramasamy, K.; Sieo, C.C.; Ho, Y.W. Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE 2017, 12, e0175959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.L.; Zhan, X.A.; Zeng, X.F.; Zhou, L.; Cao, G.T.; Chen, A.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechno. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Oh, S.T.; Lee, Y.S.; Davis, E.; Zimmerman, N.; Rehberger, T.; Lillehoj, H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins 2017, 9, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafeez, H.M.; Saleh, E.S.E.; Tawfeek, S.S.; Youssef, I.M.I.; Abdeldaim, A.S.A. Effects of probiotic, prebiotic, and synbiotic with and without feed restriction on performance, hematological indices and carcass characteristics of broiler chickens. Asian-Australas. J. Anim. Sci. 2016, 30, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Yudiarti, T.; Isroli, I.; Widiastuti, E.; Kusumanti, E. Dietary supplementation of probiotics in poultry exposed to heat stress—A review. Ann. Anim. Sci. 2016, 17, 591–604. [Google Scholar] [CrossRef]
- Alloui, M.N.; Szczurek, W.; Sylwester, Ś. The usefulness of prebiotics and probiotics in modern poultry nutrition: A review. Ann. Anim. Sci. 2013, 13, 17–32. [Google Scholar] [CrossRef]
- Shini, S.; Zhang, D.; Aland, R.C.; Li, X.; Dart, P.J.; Callaghan, M.J.; Speight, R.E.; Bryden, W.L. Probiotic Bacillus amyloliquefaciens H57 ameliorates subclinical necrotic enteritis in broiler chicks by maintaining intestinal mucosal integrity and improving feed efficiency. Poult. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Wasserman, R.H.; Taylor, A.N. Intestinal absorption of phosphate in the chick: Effect of vitamin D3 and other parameters. J. Nutr. 1973, 103, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Proszkowiec-Weglarz, M.; Angel, R. Calcium and phosphorus metabolism in broilers: Effect of homeostatic; mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 2013, 22, 609–627. [Google Scholar] [CrossRef]
- Pérez-Conesa, D.; López, G.; Ros, G. Effect of probiotic prebiotic and synbiotic follow-up infant formulas on large intestine morphology and bone mineralisation in rats. J. Sci. Food Agr. 2007, 87, 1059–1068. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Castro, A.S.B.; Rodrigues, V.C.; Fernandes, S.A.; Fontes, E.A.F.; Oliveira, T.T.D.; Martino, H.S.D.; de Luces Fortes Ferreira, C.L. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food 2012, 15, 664–670. [Google Scholar] [CrossRef]
- Narva, M.; Nevala, R.; Poussa, T.; Korpela, R. The effect of Lactobacillus helveticus fermented milk on acute changes in calcium metabolism in postmenopausal women. Eur. J. Nutr. 2004, 43, 61–68. [Google Scholar] [CrossRef]
- Mutu, R.; Kocabagli, N.; Alp, M.; Acar, N.; Eren, M.; Gezen, S.S. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult. Sci. 2006, 85, 1621. [Google Scholar] [CrossRef]
- Gong, Y.; Slee, R.B.; Fukai, N.; Rawadi, G.; Roman-Roman, S.; Reginato, A.M.; Wang, H.; Cundy, T.; Glorieux, F.H.; Lev, D.; et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 107, 513–523. [Google Scholar] [CrossRef]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Romanroman, S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Hu, Y.X.; Liao, X.D.; Lu, L.; Li, S.F.; Zhang, L.Y.; Tan, H.Z.; Yang, L.; Suo, H.Q.; Luo, X.G. Kinetics of phosphorus absorption in ligated small intestinal segments of broilers. J. Anim. Sci. 2016, 94, 3312. [Google Scholar] [CrossRef]
- Andrabi, S.T.; Bhat, B.; Gupta, M.; Bajaj, B.K. Phytase-producing potential and other functional attributes of lactic acid bacteria isolates for prospective probiotic applications. Probiotics Antimicrob. Proteins 2016, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gheisar, M.M.; Hosseindoust, A.; Kim, I.H. Effects of dietary Enterococcus faecium on growth performance, carcass characteristics, faecal microbiota, and blood profile in broilers. Vet. Med. Czech. 2016, 61, 28–34. [Google Scholar] [CrossRef]
- Xiang, M.; Li, Y.Y.; Guo, Q.P.; Ren, Y.X.; Liang, S.Z.; Huang, Y. Effects of Enterococcus faecium on dominant phyla and genera of colonic microbial communities in suckling piglets. J. South. Agric. 2019, 50, 477–484. [Google Scholar]
- Bednorz, C.; Guenther, S.; Oelgeschlager, K.; Kinnemann, B.; Pieper, R.; Hartmann, S.; Tedin, K.; Semmler, T.; Neuman, K.; Schierack, P.; et al. Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli pathotypes that adhere to the gut mucosa. Appl. Environ. Microb. 2013, 79, 7896–7904. [Google Scholar] [CrossRef]
- Song, Y.L.; Kononen, E.; Rautio, M.; Liu, C.X.; Bryk, A.; Eerola, E.; Finegold, S.M. Alistipes onderdonkii sp. nov. and Alistipes shahii sp. nov., of human origin. Int. J. Syst. Evol. Microbiol. 2006, 56, 1985–1990. [Google Scholar] [CrossRef]
- Rautio, M.; Eerola, E.; Nen-Tunkelrott, M.V.I.; Molitoris, D.; Lawson, P.A.; Collins, M.D.; Lawson, P.; Collins, M.D.; Jousimies-Somer, H. Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources. Syst. Appl. Microbiol. 2003, 26, 182–188. [Google Scholar] [CrossRef]
- Mcintosh, C.M.; Chen, L.Q.; Shaiber, A.; Eren, A.M.; Alegre, M. Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 2018, 6, 96. [Google Scholar] [CrossRef]
- Collins, M.D.; Shah, H.N.; Mitsuoka, T. Reclassification of Bacteroides microfusus (Kaneuchi and Mitsuoka) in a new genus Rikenella, as Rikenella microfusus comb. nov. Syst. Appl. Microbiol. 1985, 6, 79–81. [Google Scholar] [CrossRef]
- Chen, D.F.; Jin, D.C.; Huang, S.M.; Wu, J.Y.; Xu, M.Q.; Liu, T.Y.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Kolsoom, P.; Rosita, J.; Golgis, K.; Reza, E. Effect of probiotics supplementation on bone mineral content and bone mass density. Sci. World J. 2014, 2014, 595962. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Adolphi, B.; Rochat, F.; Barclay, D.; de Vrese, M.; Açil, Y. Effects of probiotics, prebiotics, and synbiotics on mineral metabolism in ovariectomized rats—Impact of bacterial mass, intestinal absorptive area and reduction of bone turn-over. Nfs J. 2016, 3, 41–50. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Bottcher, M.; Ijazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Iwami, K.; Moriyama, T. Effects of short chain fatty acid, sodium butyrate, on osteoblastic cells and osteoclastic cells. Int. J. Biochem. 1993, 25, 1631–1635. [Google Scholar] [CrossRef]
| Ingredient | Starter (1–21 Days) (g/kg) | Grower (22–42 Days) (g/kg) |
|---|---|---|
| Corn | 593.1 | 604.2 |
| Soybean meal | 298.8 | 288.7 |
| Cotton seed meal | 50.0 | 30.0 |
| Soybean oil | 15.1 | 39.8 |
| L-Lysine | 1.5 | 0.9 |
| DL-Methionine | 1.4 | 1.6 |
| Limestone | 12.7 | 10.2 |
| CaHPO4 | 19.4 | 16.6 |
| NaCl | 3.0 | 3.0 |
| Choline chloride | 2.0 | 2.0 |
| Premix 1) | 1.3 | 1.3 |
| Zeolite powder | 1.7 | 1.7 |
| Total | 1000 | 1000 |
| Calculated nutrient level | ||
| Metabolic energy (MJ/kg) | 12.35 | 13.02 |
| Crude protein | 211.8 | 198.4 |
| Calcium | 10.1 | 8.5 |
| Available phosphorus | 4.5 | 4.0 |
| Total phosphorus | 6.9 | 6.3 |
| Lysine | 11.4 | 10.5 |
| Methionine | 4.9 | 4.8 |
| Methionine+Cysteine | 8.3 | 8.1 |
| Threonine | 7.7 | 2.2 |
| Gene | Primer Sequence (5’-3’) | Accession Number |
|---|---|---|
| NaP-IIb | F: CTGGATGCACTCCCTAGAGC R: TTATCTTTGGCACCCTCCTG | NM_204474.1 |
| PiT-1 | F: GCTCGTGGCTTCGTTCTTG R: GACCATTTGACGCCTTTCT | XM_015297502.1 |
| PiT-2 | F: GCAGCAGATACATCAACTC R: ATTTCCACTCCACCCTC | NM_001305398·1 |
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA | NM_205518.1 |
| Treatment | BW(g) | ADG(g/d) | ADFI(g/d) | F/G |
|---|---|---|---|---|
| Starter (1–21 days) | ||||
| Control | 771.11 ± 20.45 | 38.56 ± 1.02 | 51.04 ± 0.52 a | 1.374 ± 0.06 |
| E. faecium | 792.75 ± 18.28 | 39.64 ± 0.91 | 44.75 ± 1.49 b | 1.251 ± 0.02 |
| p Value | 0.460 | 0.453 | 0.019 | 0.102 |
| Grower (22–42 days) | ||||
| Control | 2207 ± 38.89 | 73.73 ± 2.79 | 152.47 ± 3.69 | 2.116 ± 0.03 |
| E. faecium | 2262 ± 25.48 | 76.36 ± 1.36 | 151.95 ± 5.07 | 2.071 ± 0.10 |
| p Value | 0.267 | 0.422 | 0.936 | 0.670 |
| Treatment | Ash (g/kg Excreta) | P (g/kg Excreta) | P (mmol/L) | ALP (U/L) |
|---|---|---|---|---|
| Starter (1–21 days) | ||||
| Control | 14.86 ± 0.36 | 1.24 ± 0.02 | 1.50 ± 0.06 | 3291 ± 178 b |
| E. faecium | 15.11 ± 0.32 | 1.31 ± 0.05 | 1.64 ± 0.04 | 4099 ± 123 a |
| p Value | 0.611 | 0.151 | 0.073 | 0.010 |
| Grower (22–42 days) | ||||
| Control | 15.58 ± 0.17 | 1.38 ± 0.03 a | 1.47 ± 0.05 | 1843 ± 176 b |
| E. faecium | 15.68 ± 0.21 | 1.28 ± 0.01 b | 1.52 ± 0.06 | 2787 ± 166 a |
| p Value | 0.172 | 0.050 | 0.513 | 0.005 |
| Treatment | P of Bone, % | Ash of Bone, % | Tibia Strength, g |
|---|---|---|---|
| Starter (1–21 days) | |||
| Control | 8.54 ± 0.55 | 53.51 ± 0.59 | 12868 ± 798 |
| E. faecium | 8.80 ± 0.16 | 54.25 ± 0.83 | 13408 ± 2440 |
| p Value | 0.847 | 0.491 | 0.840 |
| Grower (22–42 days) | |||
| Control | 8.34 ± 0.34 b | 52.16 ± 0.27 b | 23632 ± 1253 |
| E. faecium | 10.99 ± 0.40 a | 56.32 ± 1.84 a | 22896 ± 1712 |
| p Value | 0.002 | 0.035 | 0.736 |
| Treatment | NaP-IIb | PiT-1 | PiT-2 |
|---|---|---|---|
| Duodenum | |||
| Control | 1.000 ± 0.20 b | 5.861 ± 4.87 | 3.969 ± 2.97 |
| E. faecium | 2.335 ± 0.24 a | 6.106 ± 6.58 | 4.547 ± 0.73 |
| p Value | 0.013 | 0.255 | 0.878 |
| Jejunum | |||
| Control | 3.930 ± 1.13 b | 3.703 ± 3.08 | 1.791 ± 1.34 |
| E. faecium | 11.291 ± 1.16 a | 10.176 ± 4.16 | 3.705 ± 1.67 |
| p Value | 0.007 | 0.279 | 0.406 |
| Ileum | |||
| Control | 1.117 ± 0.13 b | 0.893 ± 0.08 b | 1.111 ± 0.05 a |
| E. faecium | 4.265 ± 0.59 a | 8.87 ± 2.08 a | 3.523 ± 0.54 b |
| p Value | 0.029 | 0.019 | 0.046 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Cai, H.; Zhang, A.; Chen, Z.; Chang, W.; Liu, G.; Deng, X.; Bryden, W.L.; Zheng, A. Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization. Animals 2020, 10, 1232. https://doi.org/10.3390/ani10071232
Wang W, Cai H, Zhang A, Chen Z, Chang W, Liu G, Deng X, Bryden WL, Zheng A. Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization. Animals. 2020; 10(7):1232. https://doi.org/10.3390/ani10071232
Chicago/Turabian StyleWang, Weiwei, Huiyi Cai, Anrong Zhang, Zhimin Chen, Wenhuan Chang, Guohua Liu, Xuejuan Deng, Wayne L. Bryden, and Aijuan Zheng. 2020. "Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization" Animals 10, no. 7: 1232. https://doi.org/10.3390/ani10071232
APA StyleWang, W., Cai, H., Zhang, A., Chen, Z., Chang, W., Liu, G., Deng, X., Bryden, W. L., & Zheng, A. (2020). Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization. Animals, 10(7), 1232. https://doi.org/10.3390/ani10071232








