Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Assessment of Fertility Parameters
2.3. Metabolite Extraction
2.4. LC–MS Analysis
LC–MS Raw Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Metabolomic Analysis of Seminal Plasma before and after Cryopreservation
3.2. Metabolomic Analysis of Spermatozoa before and after Cryopreservation
3.3. Metabolomic Analysis of Seminal Plasma from HF and LF Bulls
3.4. Metabolomic Analysis of Spermatozoa from HF and LF Bulls
4. Discussion
4.1. Metabolomic Analysis of Seminal Plasma before and after Cryopreservation
4.2. Metabolomic Analysis of Spermatozoa before and after Cryopreservation
4.3. Metabolomic Analysis of Seminal Plasma from HF and LF Bulls
4.4. Metabolomic Analysis of Spermatozoa from HF and LF Bulls
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- García-Vázquez, F.A.; Gadea, J.; Matás, C.; Holt, W.V. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J. Androl. 2016, 18, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Nongbua, T.; Valeanu, S.; Verde, I.L.; Lundstedt-Enkel, K.; Edman, A.; Johannisson, A. Sperm quality variables as indicators of bull fertility may be breed dependent. Anim. Reprod. Sci. 2017, 185, 42–52. [Google Scholar] [CrossRef]
- Ugur, M.R.; Abdelrahman, A.S.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in Cryopreservation of Bull Sperm. Front. Veter-Sci. 2019, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- Cormier, N.; Sirard, M.; Bailey, J.L. Premature capacitation of bovine spermatozoa is initiated by cryopreservation. J. Androl. 1997, 18, 461–468. [Google Scholar] [PubMed]
- Longobardi, V.; Zullo, G.; Salzano, A.; De Canditiis, C.; Cammarano, A.; De Luise, L.; Puzio, M.V.; Neglia, G.; Gasparrini, B. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology 2017, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Sinha, R.; Rahim, A.; Ganaie, B.A.; Singh, A.; Shah, N. Spermatozoa molecules in relation to bulls fertility. Iran. J. Appl. Anim. Sci. 2017, 7, 371–375. [Google Scholar]
- Cancel, A.M.; Chapman, D.A.; Killian, G.J. Osteopontin is the 55-kilodalton fertility-associated protein in Holstein bull seminal plasma. Biol. Reprod. 1997, 57, 1293–1301. [Google Scholar] [CrossRef]
- Brown, K.I.; Crabo, B.G.; Graham, E.F.; Pace, M.M. Some factors affecting loss of intracellular enzymes from spermatozoa. Cryobiology 1971, 8, 220–224. [Google Scholar] [CrossRef]
- Roychoudhury, P.N.; Gowda, H.C.; Pareek, P.K. Effect of Different Glycerol Levels on the Release of Glutamic Oxaloacetic Transaminase (GOT) from Deep Frozen Ram Spermatozoa. Andrologia 1975, 7, 211–215. [Google Scholar] [CrossRef]
- Ollero, M.; Bescós, O.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Loss of plasma membrane proteins of bull spermatozoa through the freezing-thawing process. Theriogenology 1998, 49, 547–555. [Google Scholar] [CrossRef]
- Al Naib, A.; Hanrahan, J.; Lonergan, P.; Fair, S. In vitro assessment of sperm from bulls of high and low field fertility. Theriogenology 2011, 76, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Mason, M.C.; Govindaraju, A.; Belser, L.; Kaya, A.; Stokes, J.; Rowe, D.; Memili, E. Interrelationships Between Apoptosis and Fertility in Bull Sperm. J. Reprod. Dev. 2013, 59, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mocé, E.; Graham, J. In vitro evaluation of sperm quality. Anim. Reprod. Sci. 2008, 105, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Pastuszak, A.W.; Lamb, D.J. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil. Steril. 2014, 99, 998–1007. [Google Scholar] [CrossRef]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-associated metabolites in bull seminal plasma and blood serum: 1H nuclear magnetic resonance analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef]
- Muñoz, M.; Uyar, A.; Correia, E.; Diez, C.; Fernández-González, A.; Caamaño, J.N.; Trigal, B.; Carrocera, S.; Seli, E.; Gómez, E.; et al. Non-invasive assessment of embryonic sex in cattle by metabolic fingerprinting of in vitro culture medium. Metabolomics 2013, 10, 443–451. [Google Scholar] [CrossRef]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics–the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Dunn, W.; Bailey, N.J.C.; Johnson, H.E. Measuring the metabolome: Current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef]
- Menezes, E.B.; Velho, A.L.C.; Santos, F.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Uncovering sperm metabolome to discover biomarkers for bull fertility. BMC Genom. 2019, 20, 714. [Google Scholar] [CrossRef]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic markers of fertility in bull seminal plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gago, R.; Domínguez, J.C.; Martinez-Pastor, F. Seminal plasma applied post-thawing affects boar sperm physiology: A flow cytometry study. Theriogenology 2013, 80, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Jørgensen, N.; Andersson, A.-M. Parabens in urine, serum and seminal plasma from healthy Danish men determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Expo. Sci. Environ. Epidemiol. 2011, 21, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef]
- Rubessa, M.; Boccia, L.; Campanile, G.; Longobardi, V.; Albarella, S.; Tateo, A.; Zicarelli, L.; Gasparrini, B. Effect of energy source during culture on in vitro embryo development, resistance to cryopreservation and sex ratio. Theriogenology 2011, 76, 1347–1355. [Google Scholar] [CrossRef]
- Chen, X.; Hu, C.; Dai, J.; Chen, L. Metabolomics Analysis of Seminal Plasma in Infertile Males with Kidney-Yang Deficiency: A Preliminary Study. Evid. Based Complement Altern. Med. 2015, 2015, 892930. [Google Scholar] [CrossRef]
- Wheeler, M.B.; Seidel, G.E. Capacitation of bovine spermatozoa by lysophospholipids and trypsin. Gamete Res. 1989, 22, 193–204. [Google Scholar] [CrossRef]
- Kyono, K.; Hoshi, K.; Saito, A.; Tsuiki, A.; Hoshiai, H.; Suzuki, M. Effects of phospholipase A2, lysophosphatidyl choline, and fatty acid on the acrosome reaction of human spermatozoa. Tohoku J. Exp. Med. 1984, 144, 257–263. [Google Scholar] [CrossRef]
- Nimptsch, A.; Pyttel, S.; Paasch, U.; Mohr, C.; Heinrich, J.-M.; Schiller, J. A MALDI MS Investigation of the Lysophosphatidylcholine/Phosphatidylcholine Ratio in Human Spermatozoa and Erythrocytes as a Useful Fertility Marker. Lipids 2014, 49, 287–293. [Google Scholar] [CrossRef]
- Castro, L.S.; Hamilton, T.R.D.S.; Mendes, C.M.; Nichi, M.; Barnabe, V.; Visintin, J.A.; Assumpção, M.E. Sperm cryodamage occurs after rapid freezing phase: Flow cytometry approach and antioxidant enzymes activity at different stages of cryopreservation. J. Anim. Sci. Biotechnol. 2016, 7, 17. [Google Scholar] [CrossRef]
- Kidd, M.; Ferket, P.; Garlich, J. Nutritional and osmoregulatory functions of betaine. World Poult. Sci. J. 1997, 53, 125–139. [Google Scholar] [CrossRef]
- Petronini, P.G.; De Angelis, E.M.; Borghetti, P.; Borghetti, A.F.; Wheeler, K.P. Modulation by betaine of cellular responses to osmotic stress. Biochem. J. 1992, 282, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Lindeberg, H.; Kurten, A.; Koskinen, E.; Katila, T. Freezing of stallion semen with addition of glycine betaine. J. Vet. Med. 1999, 46, 87–90. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Balestrieri, A.; Neglia, G.; Monaco, A.; Tatullo, M.; Casale, R.; Limone, A.; Balestrieri, M.L.; Campanile, G. Antioxidant and Anti-Inflammatory Activities of Buffalo Milk δ-Valerobetaine. J. Agric. Food Chem. 2019, 67, 1702–1710. [Google Scholar] [CrossRef]
- Attia, Y.A.; El-Naggar, A.S.; Abou-Shehema, B.M.; Abdella, A.A. Effect of Supplementation with Trimethylglycine (Betaine) and/or Vitamins on Semen Quality, Fertility, Antioxidant Status, DNA Repair and Welfare of Roosters Exposed to Chronic Heat Stress. Animals 2019, 9, 547. [Google Scholar] [CrossRef]
- Sánchez-Partida, L.G.; Setchell, B.P.; Maxwell, W.M.C. Effect of compatible solutes and diluent composition on the post-thaw motility of ram sperm. Reprod. Fertil. Dev. 1998, 10, 347–357. [Google Scholar] [CrossRef]
- Sanchez-Partida, L.; Maxwell, W.; Paleg, L.; Setchell, B. Proline and glycine betaine in cryoprotective diluents for ram spermatozoa. Reprod. Fertil. Dev. 1992, 4, 113–118. [Google Scholar] [CrossRef]
- Koskinen, E.; Junnila, M.; Katila, T.; Soini, H. A Preliminary Study on the Use of Betaine as a Cryoprotective Agent in Deep Freezing of Stallion Semen. J. Vet. Med. 1989, 36, 110–114. [Google Scholar] [CrossRef]
- Trimeche, A.; Yvon, J.M.; Vidament, M.; Palmer, E.; Magistrini, M. Effects of glutamine, proline, histidine and betaine on post-thaw motility of stallion spermatozoa. Theriogenology 1999, 52, 181–191. [Google Scholar] [CrossRef]
- Lugar, D.W.; Krom, W.A.; Mings, P.D.; Stewart, K.R. Effects of supplemental betaine to semen extenders on semen quality in boars. Transl. Anim. Sci. 2018, 2, 195–204. [Google Scholar] [CrossRef]
- Zhang, B.R.; Buhr, M.; Kroetsch, T.; Leibo, S.P. Glycine betaine improves survival of fresh bovine spermatozoa. Reprod. Fertil. Dev. 2001, 13, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Szramko, E.; Jeroch, H.; Matusevicius, P. Effects of Betaine on Energy Utilization in Growing Pigs—A Review. Ann. Anim. Sci. 2012, 12, 291–300. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Alvarez, E.; Enríquez, J.A.; López-Pérez, M.J. Association between seminal plasma carnitine and sperm mitochondrial enzymatic activities. Int. J. Androl. 2001, 24, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E. Carnitine, acetylcarnitine and the activity of carnitine acyltransferases in seminal plasma and spermatozoa of men, rams and rats. J. Reprod. Fertil. 1979, 56, 667–673. [Google Scholar] [CrossRef]
- Casillas, E.R. The distribution of carnitine in male reproductive tissues and its effect on palmitate oxidation by spermatozoal particles. Biochim. Biophys. Acta 1972, 280, 545–551. [Google Scholar] [CrossRef]
- Casillas, E.R.; Villalobos, P.; Gonzales, R. Distribution of carnitine and acylcarnitine in the hamster epididymis and in epididymal spermatozoa during maturation. J. Reprod. Fertil. 1984, 72, 197–201. [Google Scholar] [CrossRef]
- Casillas, E.R. Accumulation of carnitine by bovine spermatozoa during maturation in the epididymis. J. Biol. Chem. 1973, 248, 8227–8232. [Google Scholar]
- Jeulin, C.; Lewin, L. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum. Reprod. Updat. 1996, 2, 87–102. [Google Scholar] [CrossRef]
- Golan, R.; Weissenberg, R.; Lewin, L.M. Carnitine and acetylcarnitine in motile and immotile human spermatozoa. Int. J. Androl. 1984, 7, 484–494. [Google Scholar] [CrossRef]
- Johansen, L.; Bohmer, T. Motility related to the presence of carnitine and carnitine/acetylcarnitine in human spermatozoa. Int. J. Androl. 1979, 2, 202–210. [Google Scholar] [CrossRef]
- Stradaioli, G.; Lakamy, S.; Zelli, R.; Chiodi, P.; Monaci, M. Effect of L-carnitine administration on the seminal characteristics of oligoasthenospermic stallions. Theriogenology 2004, 62, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Başpınar, N.; Taşpınar, M.; Çoyan, K.; Bilgili, A.; Akalın, P.P.; Büyükleblebici, S.; Aydos, S.; et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-J.; Yang, J.; Chang, S.; Xu, W.-M.; Yin, T.-L.; Long, W. Acetyl-L-carnitine: An effective antioxidant against cryo-damage on human spermatozoa with asthenospermia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 915–921. [Google Scholar] [CrossRef]
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Montagnon, D.; Valtat, B.; Vignon, F.; Koll-Back, M.H. Secretory proteins of human seminal vesicles and their relationship to lipids and sugars. Andrologia 1990, 22, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Vignon, F.; Koll-Back, M.H.; Clavert, A.; Cranz, C. Lipid Composition of Human Seminal Plasma. Arch. Androl. 1989, 22, 49–53. [Google Scholar] [CrossRef]
- Beer-Ljubic, B.; Aladrovic, J.; Marenjak, T.S.; Laskaj, R.; Majić-Balić, I.; Milinković-Tur, S. Cholesterol concentration in seminal plasma as a predictive tool for quality semen evaluation. Theriogenology 2009, 72, 1132–1140. [Google Scholar] [CrossRef]
- Argov, N.; Sklan, D.; Zeron, Y.; Roth, Z. Association between seasonal changes in fatty-acid composition, expression of VLDL receptor and bovine sperm quality. Theriogenology 2007, 67, 878–885. [Google Scholar] [CrossRef]
- Brinsko, S.P.; Love, C.; Bauer, J.; MacPherson, M.; Varner, D. Cholesterol-to-phospholipid ratio in whole sperm and seminal plasma from fertile stallions and stallions with unexplained subfertility. Anim. Reprod. Sci. 2007, 99, 65–71. [Google Scholar] [CrossRef]
- García, M.F.; Favre, R.N.; Stornelli, M.C.; Rearte, R.; Mitacek, M.C.G.; De La Sota, R.L.; Stornelli, M.A. Relationship between semen quality and seminal plasma cholesterol, triacylglycerols and proteins in the domestic cat. J. Feline Med. Surg. 2019, 1–8. [Google Scholar] [CrossRef]
- Sebastian, S.M.; Selvaraj, S.; Aruldhas, M.M.; Govindarajulu, P. Pattern of neutral and phospholipids in the semen of normospermic, oligospermic and azoospermic men. J. Reprod. Fertil. 1987, 79, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Deepinder, F.; Chowdary, H.T.; Agarwal, A. Role of metabolomic analysis of biomarkers in the management of male infertility. Expert Rev. Mol. Diagn. 2007, 7, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR spectroscopic studies on human seminal plasma: A probative discriminant function analysis classification model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Egea, R.; Garrido, N.; Sota, N.; Meseguer, M.; Remohí, J.; Dominguez, F. Sperm lipidic profiles differ significantly between ejaculates resulting in pregnancy or not following intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2018, 35, 1973–1985. [Google Scholar] [CrossRef]
- Mitra, J.; Chowdhury, M. Association of glycerylphosphorylcholine with human sperm and effect of capacitation on their metabolism. Reprod. Fertil. Dev. 1994, 6, 679–685. [Google Scholar] [CrossRef]
- Chowdhury, M.; Mitra, J. Glycerylphosphorylcholine (GPC) diesterase related alterations in the oxygen consumption profile of rat spermatozoa in differing functional states. Int. J. Androl. 1992, 15, 345–354. [Google Scholar] [CrossRef]
- Mann, T.; Lutwak-Mann, C. Male Reproductive Function and the Composition of Semen: General Considerations. In Male Reproductive Function and Semen; Springer: Berlin/Heidelberg, Germany, 1981; pp. 1–37. [Google Scholar]
- Riffo, M.; Párraga, M. Role of phospholipase A2 in mammalian sperm-egg fusion: Development of hamster oolemma fusibility by lysophosphatidylcholine. J. Exp. Zool. 1997, 279, 81–88. [Google Scholar] [CrossRef]
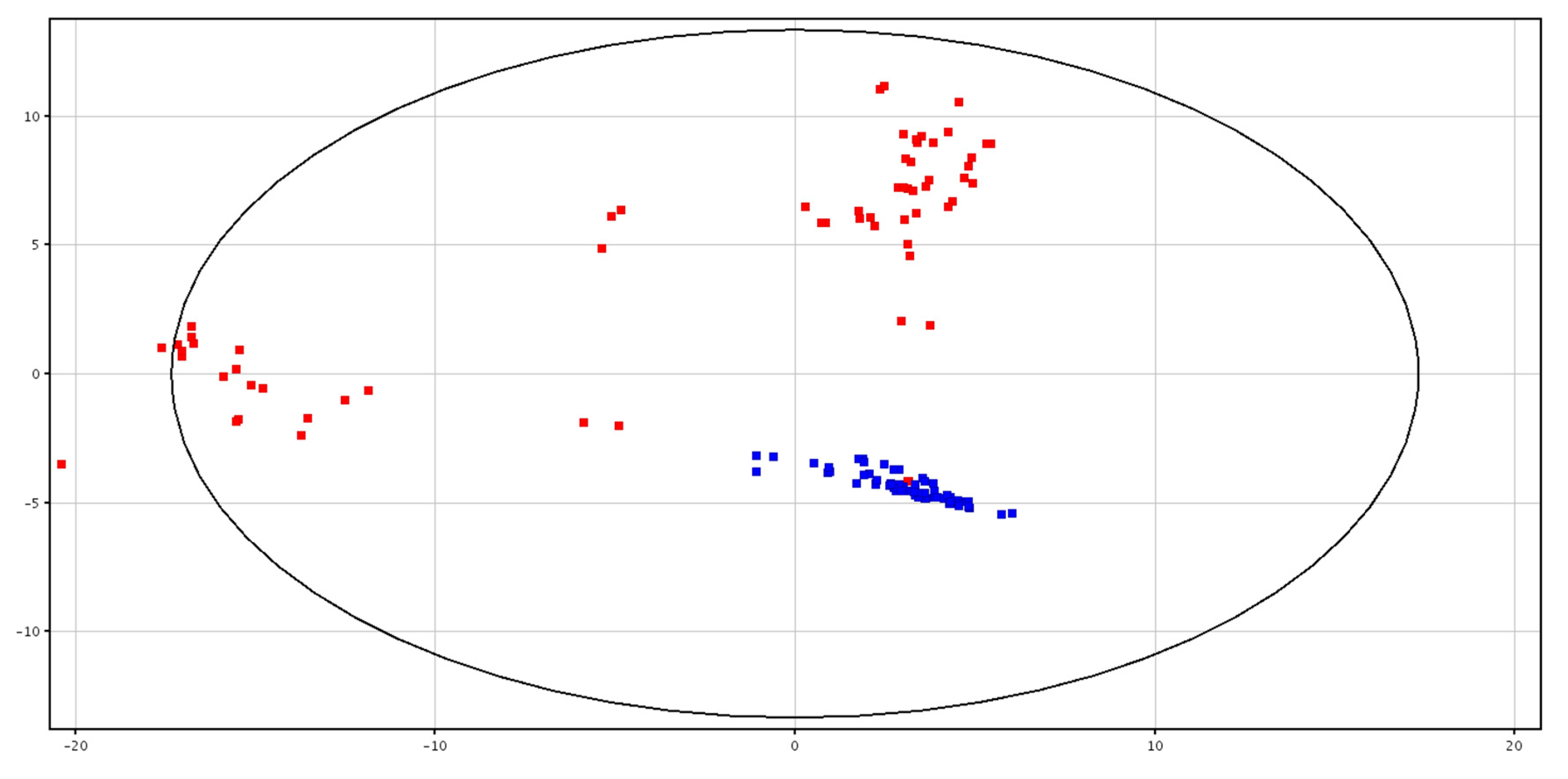
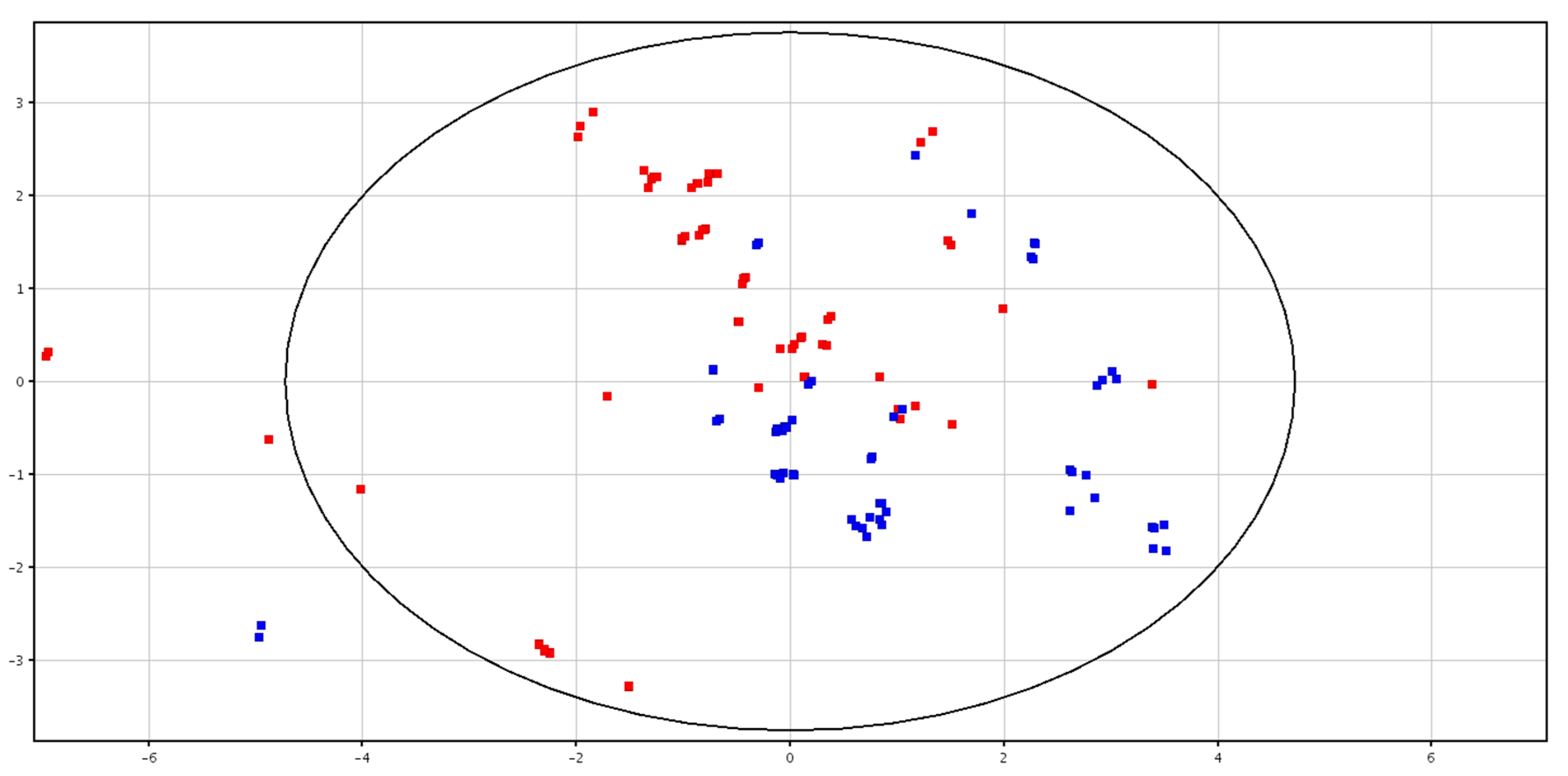
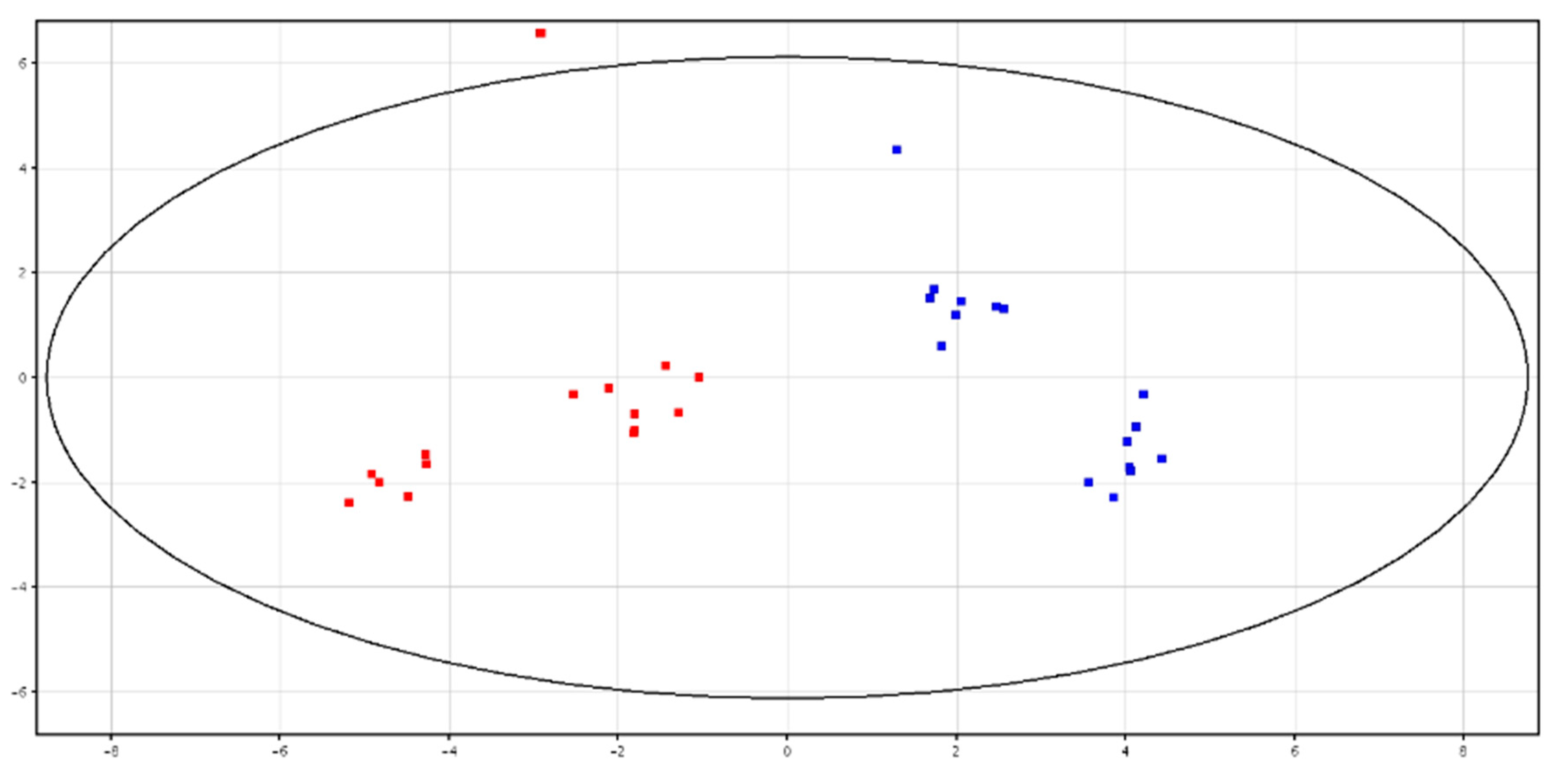
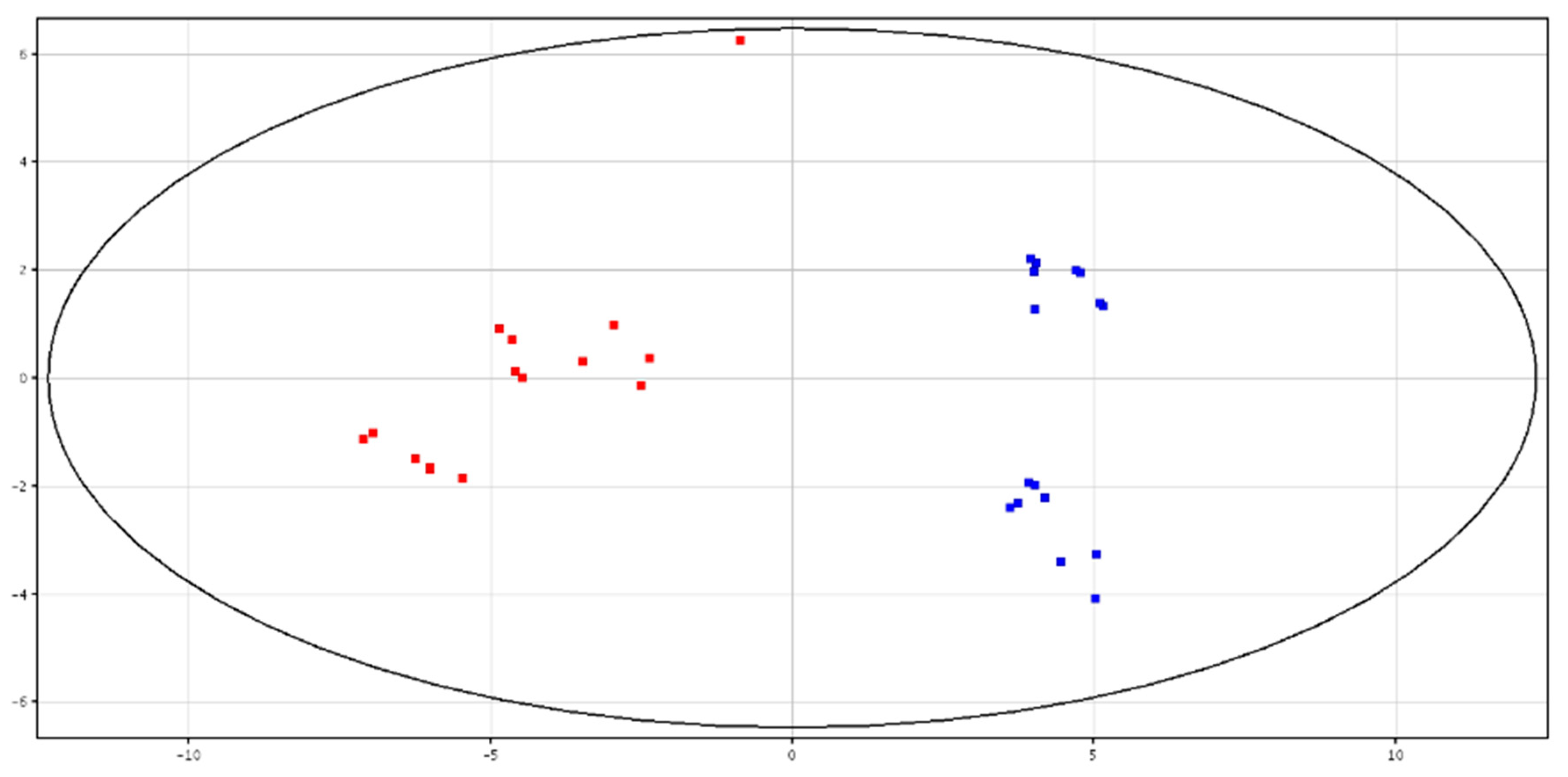
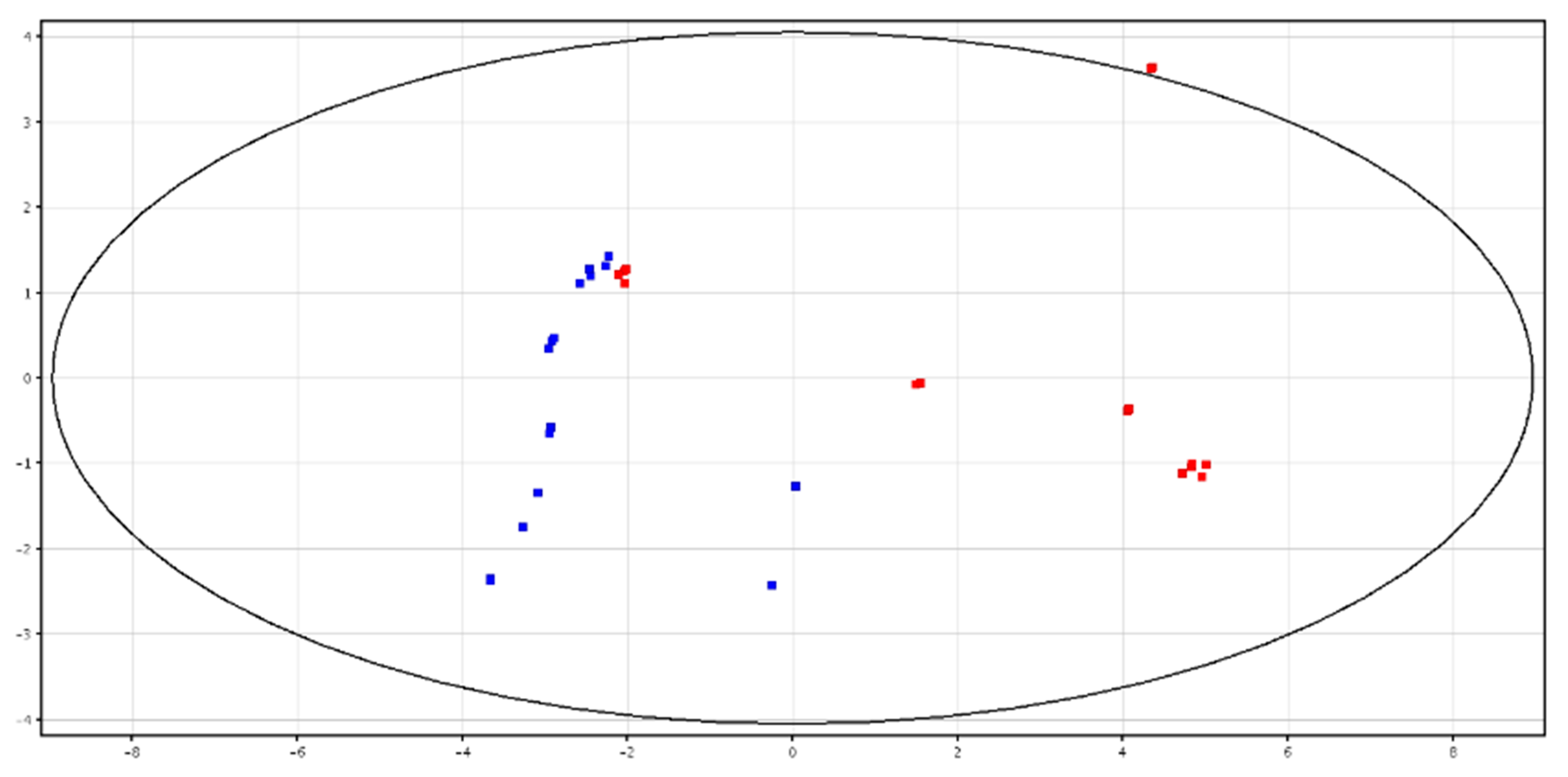
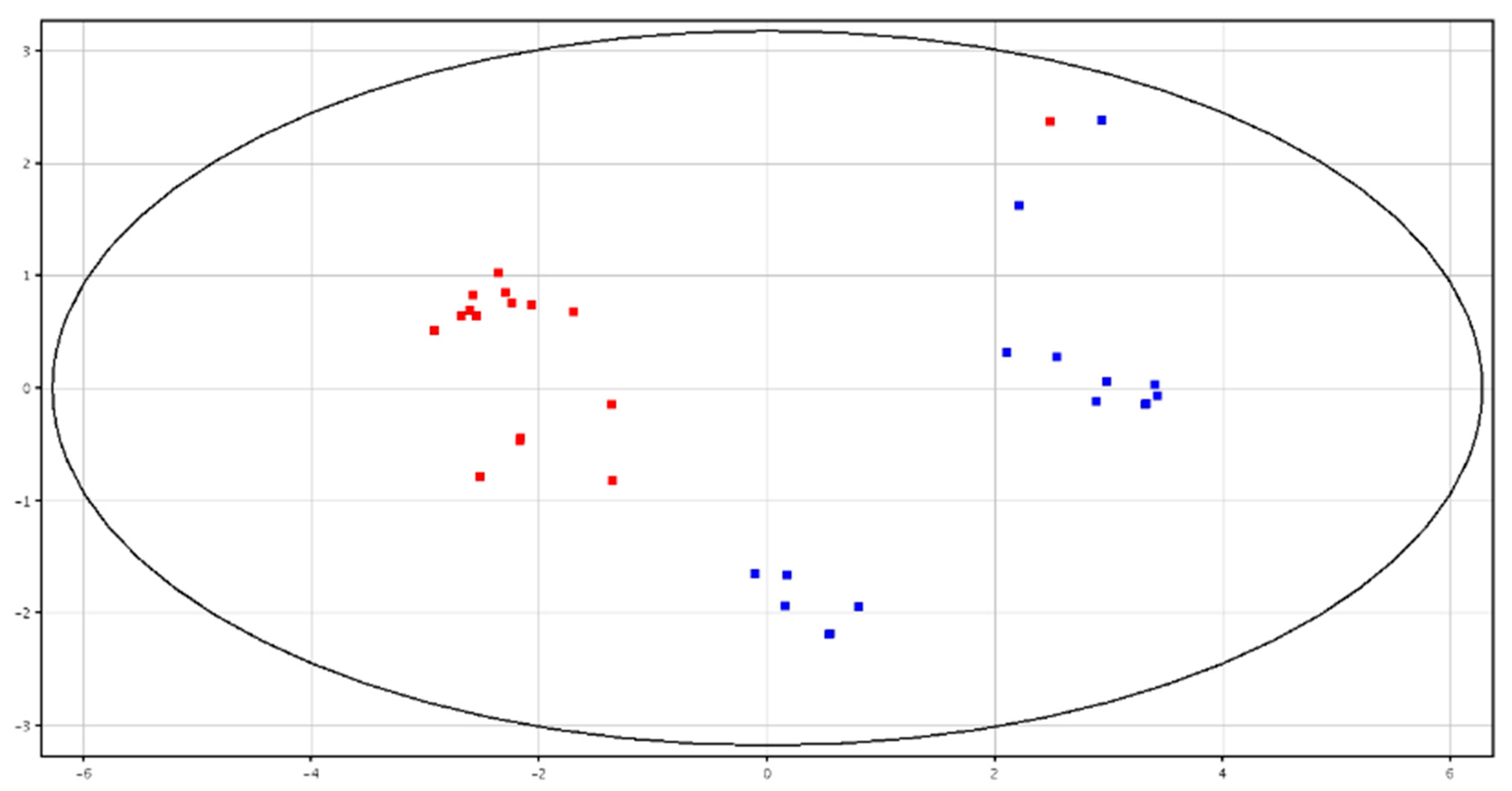
| F-P vs. CRY-P | F-S vs. CRY-S | ||||
|---|---|---|---|---|---|
| Compound | Mass (Da) | Log2FC | pCorr | Log2FC | pCorr |
| Glycine Betaine a | 117.0789 | 1.68 | 3.43 × 10−2 | ||
| LysoPC (0:0/18:2(9Z,12Z)) *,a | 519.334 | 1.54 | 1.34 × 10−31 | ||
| Pyro-l-glutaminyl-l-glutamine a | 257.1015 | 1.26 | 3.49 × 10−2 | ||
| Parameters | HF (Mean ± SE) | LF (Mean ± SE) |
|---|---|---|
| Viability (%) * | 80.0 ± 2.0 A | 46.7 ± 6.7 B |
| Total Anomalies (%) * | 7.8 ± 1.3 | 9.7 ± 2.5 |
| HOS+ (%) * | 67.8 ± 1.8 A | 55.0 ± 2.4 B |
| Tunel + (%) * | 10.5 ± 2.2 | 14.6 ± 1.9 |
| Cleavage (%) ** | 75.3 ± 1.8 A | 54.9 ± 3.5 B |
| Blastocysts (%) ** | 31.1 ± 1.8 A | 13.4 ± 2.8 B |
| Compound | Mass (Da) | F-P | CRY-P | F-S | CRY-S | ||||
|---|---|---|---|---|---|---|---|---|---|
| Log2FC | pCorr | Log2FC | pCorr | Log2FC | pCorr | Log2FC | pCorr | ||
| 2,3-Diacetoxypropyl stearate B | 44.3283 | 1.43 | 2.13 × 10−3 | ||||||
| Glycine Betaine A | 117.0789 | 1.77 | 7.97 × 10−32 | 1.32 | 1.39 × 10−2 | ||||
| Butyrylcarnitine A | 231.1473 | 1.14 | 7.76 × 10−3 | 1.32 | 3.84 × 10−3 | ||||
| Glycerol tripropanoate B | 260.1239 | 1.63 | 8.55 × 10−3 | ||||||
| GPC *,B | 257.1032 | 1.07 | 1.31 × 10−1 | 1.54 | 8.73 × 10−3 | 2.23 | 3.95 × 10−3 | ||
| l-Acetylcarnitine B | 203.1159 | 1.00 | 6.04 × 10−1 | ||||||
| l-Carnitine A | 161.1049 | 1.38 | 4.60 × 10−31 | ||||||
| LysoPC (16:0/0:0) *,B | 495.3346 | 2.38 | 1.20 × 10−3 | ||||||
| LysoPC (P-16:0/0:0) *,A | 479.3382 | 2.04 | 1.61 × 10−2 | ||||||
| Piperidine A | 85.892 | 1.32 | 1.098 × 10−5 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longobardi, V.; Kosior, M.A.; Pagano, N.; Fatone, G.; Staropoli, A.; Vassetti, A.; Vinale, F.; Campanile, G.; Gasparrini, B. Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility. Animals 2020, 10, 1065. https://doi.org/10.3390/ani10061065
Longobardi V, Kosior MA, Pagano N, Fatone G, Staropoli A, Vassetti A, Vinale F, Campanile G, Gasparrini B. Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility. Animals. 2020; 10(6):1065. https://doi.org/10.3390/ani10061065
Chicago/Turabian StyleLongobardi, Valentina, Michal A. Kosior, Nunzia Pagano, Gerardo Fatone, Alessia Staropoli, Anastasia Vassetti, Francesco Vinale, Giuseppe Campanile, and Bianca Gasparrini. 2020. "Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility" Animals 10, no. 6: 1065. https://doi.org/10.3390/ani10061065
APA StyleLongobardi, V., Kosior, M. A., Pagano, N., Fatone, G., Staropoli, A., Vassetti, A., Vinale, F., Campanile, G., & Gasparrini, B. (2020). Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility. Animals, 10(6), 1065. https://doi.org/10.3390/ani10061065









