Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Data Analysis
2.3. Descriptive Statistics
2.4. Initial Associations
2.5. Poisson Regression
3. Results
3.1. Descriptive Statistics
3.2. Initial Associations
3.3. Poisson Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Boden, L.; Parkin, T.D.; Yates, J.; Mellor, D.; Kao, R.R. Summary of current knowledge of the size and spatial distribution of the horse population within Great Britain. BMC Vet. Res. 2012, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Blue Cross. 2018. Available online: www.bluecross.org.uk/sites/default/files/NEHS (accessed on 31 March 2020).
- Greve, L.; Dyson, S.J. The interrelationship of lameness, saddle slip and back shape in the general sports horse population. Equine Vet. J. 2014, 46, 687–694. [Google Scholar] [CrossRef]
- Rhodin, M.; Roepstorff, L.; French, A.; Keegan, K.G.; Pfau, T.; Egenvall, A. Head and pelvic movement asymmetry during lungeing in horses showing symmetrical movement on the straight. Equine Vet. J. 2016, 48, 315–320. [Google Scholar] [CrossRef]
- Dyson, S.; Berger, J.; Ellis, A.D.; Mullard, J. Development of an ethogram for a pain scoring system in ridden horses and its application to determine the presence of musculoskeletal pain. J. Vet. Behav. 2018, 23, 47–57. [Google Scholar] [CrossRef]
- Dyson, S.; Berger, J.; Ellis, A.; Mullard, J. Behavioural observations and comparisons of non-lame horses and lame horses before and after resolution of lameness by diagnostic analgesia. J. Vet. Behav. Clin. Appl. Res. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Dyson, S.; Van Dijk, J. Application of a ridden horse ethogram to video recordings of 21 horses before and after diagnostic analgesia: Reduction in behaviour scores. Equine Vet. Educ. 2018. [Google Scholar] [CrossRef]
- Dyson, S.; Thomson, K.; Quiney, L.; Bondi, A.; Ellis, A.D. Can veterinarians reliably apply a whole horse ridden ethogram to differentiate nonlame and lame horses based on live horse assessment of behaviour? Equine Vet. Educ. 2019. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S. Saddle fit and management: An investigation of the association with equine thoracolumbar asymmetries, horse and rider health. Equine Vet. J. 2014, 47, 415–421. [Google Scholar] [CrossRef]
- Dyson, S.; Ellis, A.; Quiney, L.; Douglas, J.; Bondi, A.; Harris, P. The Influence of Rider: Horse Bodyweight Ratio on Equine Gait, Behaviour, Response to Thoracolumbar Palpation and Thoracolumbar Dimensions: A Pilot Study. In Proceedings of the 14th International Society of Equitation Science Congress, Rome, Italy, 21–24 September 2018; p. 120. [Google Scholar]
- Dyson, S.; Ellis, A.D.; Guire, R.; Douglas, J.; Bondi, A.; Harris, P. The influence of rider: Horse bodyweight ratio and rider-horse-saddle fit on equine gait and behaviour: A pilot study. Equine Vet. Educ. 2019. [Google Scholar] [CrossRef]
- Roost, L.; Ellis, A.D.; Morris, C.; Bondi, A.; Gandy, E.A.; Harris, P.; Dyson, S. The effects of rider size and saddle fit for horse and rider on forces and pressure distribution under saddles: A pilot study. Equine Vet. Educ. 2019. [Google Scholar] [CrossRef]
- Strunk, R.; Vernon, K.; Blob, R.; Bridges, W.; Skewes, P. Effects of Rider Experience Level on Horse Kinematics and Behavior. J. Equine Vet. Sci. 2018, 68, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S. Can lameness be reliably graded? Equine Vet. J. 2011, 43, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S. Evaluation of poor performance in competition horses: A musculoskeletal perspective. Part 1: Clinical assessment. Equine Vet. Educ. 2015, 28, 284–293. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S. What can we learn from visual and objective assessment of non-lame and lame horses in straight lines, on the lunge and ridden? Equine Vet. Educ. 2018. [Google Scholar] [CrossRef]
- Boado, A.; Nagy, A.; Dyson, S. Ultrasonographic features associated with the lumbosacral or lumbar 5–6 symphyses in 64 horses with lumbosacral-sacroiliac joint region pain (2012–2018). Equine Vet. Educ. 2019. [Google Scholar] [CrossRef]
- Fédération Equestre Internationale. Available online: www.fei.org/fei/regulations/dressage (accessed on 4 March 2020).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cameron, A.C.; Trivedi, P.K. Microeconometrics Using Stata; Stata Press: College Station, TX, USA, 2009. [Google Scholar]
- Terada, K. Comparison of Head Movement and EMG Activity of Muscles between Advanced and Novice Horseback Riders at Different Gaits. J. Equine Sci. 2000, 11, 83–90. [Google Scholar] [CrossRef]
- Lagarde, J.; Kelso, J.A.S.; Peham, C.; Licka, T.F. Coordination dynamics of the horse-rider system. J. Mot. Behav. 2005, 37, 418–424. [Google Scholar] [CrossRef]
- Münz, A.; Eckardt, F.; Witte, K. Horse-rider interaction in dressage riding. Hum. Mov. Sci. 2014, 33, 227–237. [Google Scholar] [CrossRef]
- Münz, A.; Eckardt, F.; Heipertz-Hengst, C.; Peham, C.; Witte, K. A Preliminary Study of an Inertial Sensor-based Method for the Assessment of Human Pelvis Kinematics in Dressage Riding. J. Equine Vet. Sci. 2013, 33, 950–955. [Google Scholar] [CrossRef]
- Schöllhorn, W.I.; Peham, C.; Licka, T.F.; Scheidl, M. A pattern recognition approach for the quantification of horse and rider interactions. Equine Vet. J. 2006, 38, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Licka, T.F.; Kapaun, M.; Peham, C. Influence of rider on lameness in trotting horses. Equine Vet. J. 2004, 36, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Peham, C.; Licka, T.; Kapaun, M.; Scheidl, M. A new method to quantify harmony of the horse-rider system in dressage. Sports Eng. 2001, 4, 95–101. [Google Scholar] [CrossRef]
- Dyson, S.; Martin, C.; Bondi, A.; Ellis, A.D. Ridden horse exercise: The influence of rider skill on behaviour assessed using the Ridden Horse Ethogram. 2020; Manuscript in preparation. [Google Scholar]
- Dyson, S.; Greve, L. Subjective Gait Assessment of 57 Sports Horses in Normal Work: A Comparison of the Response to Flexion Tests, Movement in Hand, on the Lunge, and Ridden. J. Equine Vet. Sci. 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Dyson, S.; Murray, R. Pain associated with the sacroiliac joint region: A clinical study of 74 horses. Equine Vet. J. 2003, 35, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Murray, R. Management of hindlimb proximal suspensory desmopathy by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy: 155 horses (2003–2008). Equine Vet. J. 2012, 44, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Barstow, A.; Dyson, S. Clinical features and diagnosis of sacroiliac joint region pain in 296 horses: 2004–2014. Equine Vet. Educ. 2015, 27, 637–647. [Google Scholar] [CrossRef]
- Egenvall, A.; Lönnell, C.; Roepstorff, L. Orthopaedic health status of riding school horses from eight riding schools—A pilot study. Acta Vet. Scan. 2010, 52, 50. [Google Scholar] [CrossRef]
- Visser, E.K.; Neijenhuis, F.; De Graaf-Roelfsema, E.; Wesselink, H.G.M.; De Boer, J.; Van Wijhe-Kiezebrink, M.C.; Engel, B.; Van Reenen, C.G. Risk factors associated with health disorders in sport and leisure horses in the Netherlands1. J. Anim. Sci. 2014, 92, 844–855. [Google Scholar] [CrossRef]
- Dyson, S. Application of a ridden horse ethogram to horses competing at a 4-star three-day-event: Comparison with cross-country performance. Equine Vet. J. 2019, 51, 11. [Google Scholar]
- Dyson, S.; Ellis, A.D. Application of a Ridden Horse Ethogram to horses competing at 5-star three-day-events: Comparison with performance. 2020; Manuscript submitted. [Google Scholar]
- Merrifield-Jones, M.; Tabor, G.; Williams, J. Inter- and Intra-Rater Reliability of Soft Tissue Palpation Scoring in the Equine Thoracic Epaxial Region. J. Equine Vet. Sci. 2019, 83, 102812. [Google Scholar] [CrossRef] [PubMed]
- Landman, M.A.A.M.; De Blaauw, J.A.; Hofland, L.J.; Van Weeren, P.R. Field study of the prevalence of lameness in horses with back problems. Vet. Rec. 2004, 155, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A.; Norton, S.; Pearman, L.; Dyson, S. Evaluating the suitability of an English saddle for a horse and rider combination. Equine Vet. Educ. 2019. [Google Scholar] [CrossRef]
- Peham, C.; Licka, T.F.; Schobesberger, H.; Meschan, E. Influence of the rider on the variability of the equine gait. Hum. Mov. Sci. 2004, 23, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Ellis, A.; Mullard, J.; Berger, J. Response to Gleerup: Understanding signals that indicate pain in ridden horses. J. Vet. Behav. 2018, 23, 87–90. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S.; Pfau, T. Alterations in thoracolumbosacral movement when pain causing lameness has been improved by diagnostic analgesia. Vet. J. 2017, 224, 55–63. [Google Scholar]
- Persson-Sjodin, E.; Hernlund, E.; Pfau, T.; Andersen, P.H.; Rhodin, M. Influence of seating styles on head and pelvic vertical movement symmetry in horses ridden at trot. PLoS ONE 2018, 13, e0195341. [Google Scholar] [CrossRef]
- Hall, C.; Kay, R.; Yarnell, K. Assessing ridden horse behavior: Professional judgment and physiological measures. J. Vet. Behav. 2014, 9, 22–29. [Google Scholar] [CrossRef]
- Hall, C.; Huws, N.; White, C.; Taylor, E.; Owen, H.; McGreevy, P. Assessment of ridden horse behavior. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 62–73. [Google Scholar] [CrossRef]
| 1. Repeated changes of head position (up/down), not in rhythm with the trot |
| 2. Head tilted or tilting repeatedly |
| 3. Head in front of vertical (>30°) for ≥10 s |
| 4. Head behind vertical (>10°) for ≥10 s |
| 5. Head position changes regularly, tossed or twisted from side to side, corrected constantly |
| 6. Ears rotated back behind vertical or flat (both or one only) ≥5 s; repeatedly lay flat |
| 7. Eye lids closed or half closed for 2–5 s; frequent blinking |
| 8. Sclera exposed repeatedly |
| 9. Intense stare (glazed expression, ‘zoned out’) for ≥5 s |
| 10. Mouth opening ± shutting repeatedly with separation of teeth, for ≥10 s |
| 11. Tongue exposed, protruding or hanging out, and/or moving in and out repeatedly |
| 12. Bit pulled through the mouth on one side (left or right), repeatedly |
| 13. Tail clamped tightly to middle or held to one side |
| 14. Tail swishing large movements: repeatedly up and down/side to side/circular; repeatedly during transitions |
| 15. A rushed gait (frequency of trot steps > 40/15 s); irregular rhythm in trot or canter; repeated changes of speed in trot or canter |
| 16. Gait too slow (frequency of trot steps < 35/15 s); passage-like trot |
| 17. Hindlimbs do not follow tracks of forelimbs but repeatedly deviated to left or right; on three tracks in trot or canter |
| 18. Canter repeated leg changes in front and/or behind; repeated strike off on wrong leg; disunited |
| 19. Spontaneous changes of gait (e.g., breaks from canter to trot, or trot to canter) |
| 20. Stumbles or trips more than once; repeated bilateral hindlimb toe drag |
| 21. Sudden change of direction, against rider’s direction; spooking |
| 22. Reluctance to move forwards (has to be kicked ± verbal encouragement), stops spontaneously |
| 23. Rearing (both forelimbs off the ground) |
| 24. Bucking or kicking backwards (one or both hindlimbs) |
| Ridden Horse Pain Ethogram Behaviour | Prevalence in Lame Horses (n = 44) | Prevalence in Non-Lame Horses (n = 16) | Difference in Prevalence (%) | Fisher’s Exact p-Value (Benjamini–Hochberg Adjusted p-Value *) |
|---|---|---|---|---|
| 1. Repeated changes of head position (up/down) | 24 (54.5%) | 9 (56.3%) | −1.8% | 1.00 (1.00) |
| 2. Head tilted or tilting repeatedly | 33 (75.0%) | 12 (75.0%) | +0.0% | 1.00 (1.00) |
| 3. Head in front of vertical (>30°) for ≥10 s | 23 (52.3%) | 5 (31.3%) | +21.0% | 0.24 (1.00) |
| 4. Head behind vertical for >10 s | 17 (38.6%) | 5 (31.3%) | +7.3% | 0.76 (1.00) |
| 5. Head position changes regularly, tossed or twisted from side to side, corrected constantly | 15 (34.1%) | 8 (50.0%) | −15.9% | 0.37 (0.85) |
| 6. Ears rotated back behind vertical or flat (both or one only) ≥5 s; repeatedly lay flat | 34 (77.3%) | 7 (43.8%) | +33.5% | 0.03 (0.35) |
| 7. Eye lids closed or half closed for 2–5 s; repeated | 5 (11.4%) | 1 (6.3%) | +5.1% | 1.00 (1.00) |
| 8. Sclera (white of eye) exposed | 17 (38.6%) | 6 (37.5%) | +1.1% | 1.00 (1.00) |
| 9. Intense stare for ≥5 s | 36 (81.8%) | 8 (50.0%) | +31.8% | 0.02 (0.46) |
| 10. Mouth opening ± shutting repeatedly with separation of teeth, for ≥10 s | 27 (61.4%) | 7 (43.8%) | +17.6% | 0.25 (0.96) |
| 11. Tongue exposed, protruding or hanging out and/or moving in and out | 18 (40.9%) | 4 (25.0%) | +15.9% | 0.37 (0.77) |
| 12. Bit pulled through the mouth on one side (left or right) | 13 (29.6%) | 3 (18.8%) | +10.8% | 0.52 (0.92) |
| 13. Tail clamped tightly to middle or held to one side | 11 (25.0%) | 4 (25.0%) | +0.0% | 1.00 (1.00) |
| 14. Tail swishing large movements: repeatedly up and down/side to side/circular; during transitions | 23 (52.3%) | 6 (37.5%) | +14.8% | 0.39 (0.75) |
| 15. A rushed gait (frequency of trot steps > 40/15 s); irregular rhythm in trot or canter; repeated changes of speed in trot or canter | 6 (13.6%) | 2 (12.5%) | +1.1% | 1.00 (1.00) |
| 16. Gait too slow (frequency of trot steps <35/15 s); passage-like trot | 4 (9.1%) | 0 (0.0%) | +9.1% | 0.57 (0.94) |
| 17. Hindlimbs do not follow tracks of forelimbs but deviated to left or right; on 3 tracks in trot or canter | 36 (81.8%) | 13 (81.3%) | +0.5% | 1.00 (1.00) |
| 18. Canter repeated leg changes: repeated strike off wrong leg; change of leg in front and/or behind; disunited | 4 (9.1%) | 3 (18.8%) | −9.7% | 0.37 (1.00) |
| 19. Spontaneous changes of gait (e.g., breaks from canter to trot or trot to canter) | 28 (63.6%) | 7 (43.8%) | +19.8% | 0.24 (1.00) |
| 20. Stumbles or trips/catches toe repeatedly | 28 (63.6%) | 5 (31.3%) | +32.3% | 0.04 (0.31) |
| 21. Sudden change of direction, against rider direction; spooking | 4 (9.1%) | 3 (18.8%) | −9.7% | 0.37 (0.95) |
| 22. Reluctant to move forward (has to be kicked ± verbal encouragement), stops spontaneously | 13 (29.6%) | 2 (12.5%) | +17.1% | 0.31 (1.00) |
| 23. Rearing (both forelimbs off the ground) | 0 (0.0%) | 0 (0.0%) | +0.0% | - |
| 24. Bucking or kicking backwards (one or both hindlimbs) | 1 (2.3%) | 0 (0.0%) | +2.3% | 1.00 (1.00) |
| Variable | Coefficient | Robust Standard Error | Incident Rate Ratio (IRR) | IRR 95% CI | Wald p-Value |
|---|---|---|---|---|---|
| Age (continuous) | 0.02 | 0.01 | 1.02 | 0.99, 1.04 | 0.165 |
| Age quartiles | 0.185 | ||||
| 5–9 years | Reference | Reference | |||
| 10–11 years | −0.02 | 0.10 | 0.98 | 0.81, 1.18 | 0.83 |
| 12–14 years | −0.16 | 0.12 | 0.86 | 0.68, 1.07 | 0.18 |
| 15–24 years | 0.12 | 0.11 | 1.13 | 0.91, 1.41 | 0.28 |
| Breed | 0.28 | ||||
| Warmblood | Reference | Reference | |||
| Irish Sports Horse | −0.06 | 0.21 | 0.94 | 0.63, 1.42 | 0.18 |
| Thoroughbred | 0.17 | 0.14 | 1.19 | 0.90, 1.57 | 0.22 |
| Cob | 0.19 | 0.11 | 1.21 | 0.98, 1.50 | 0.08 |
| Warmblood × Thoroughbred | 0.17 | 0.13 | 1.18 | 0.93, 1.51 | 0.79 |
| Other | 0.28 | 0.13 | 1.32 | 1.02, 1.72 | 0.04 |
| Sex | 0.090 | ||||
| Mare | 0.12 | 0.08 | 1.15 | 0.98, 1.34 | |
| Gelding | Reference | Reference | |||
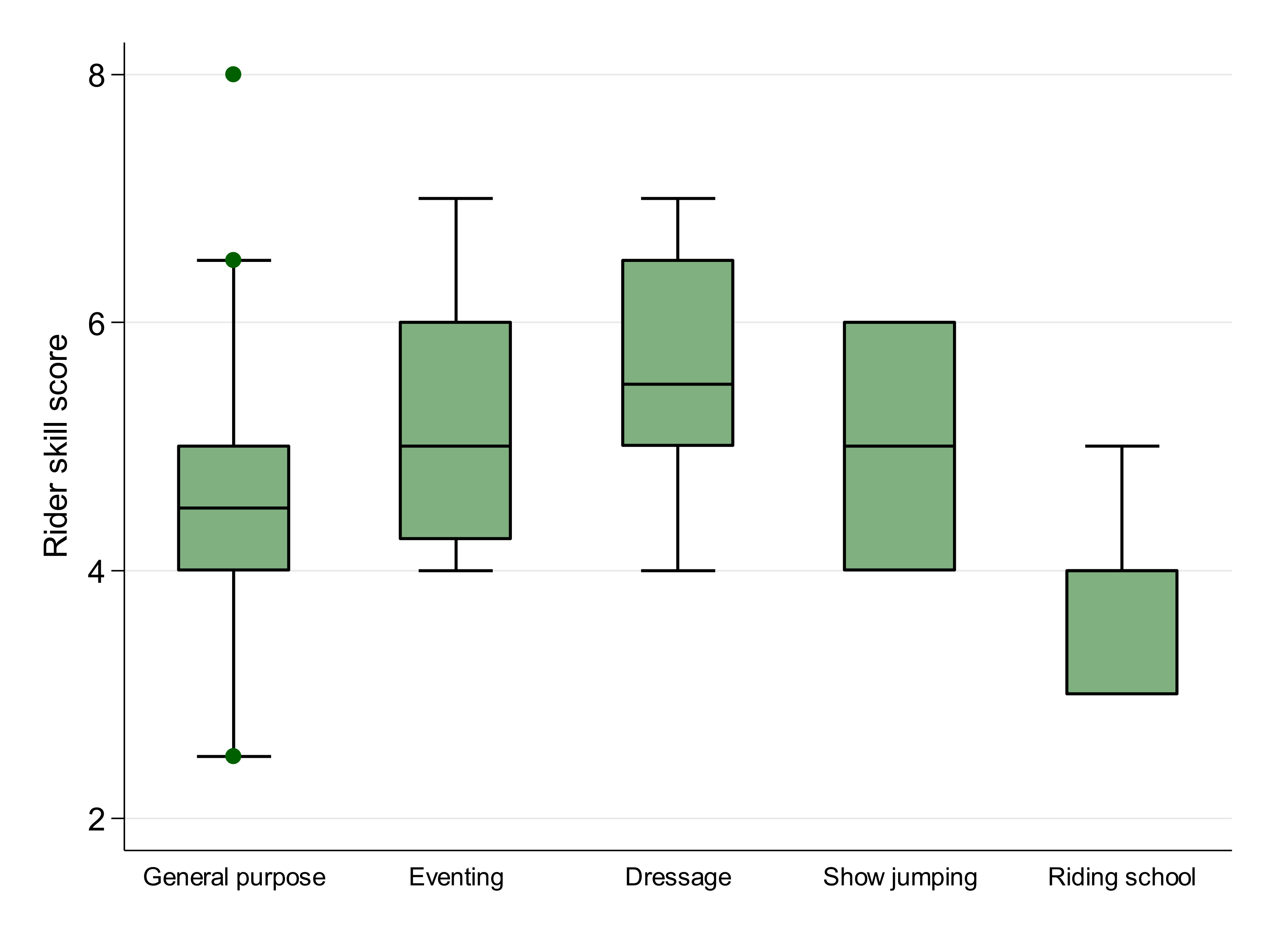
| Work discipline | <0.001 | ||||
| General purpose | Reference | Reference | |||
| Dressage | 0.01 | 0.12 | 1.01 | 0.79, 1.29 | 0.94 |
| Riding School | 0.24 | 0.08 | 1.27 | 1.08, 1.50 | 0.004 |
| Eventing | 0.08 | 0.12 | 1.09 | 0.86, 1.38 | 0.48 |
| Show Jumping | −0.10 | 0.10 | 0.90 | 0.74, 1.10 | 0.32 |
| Lame | 0.027 | ||||
| Yes | 0.24 | 0.11 | 1.27 | 1.03, 1.58 | |
| No | Reference | Reference | |||
| Abnormal canter | 0.119 | ||||
| Yes | 0.13 | 0.08 | 1.13 | 0.97, 1.33 | |
| No | Reference | Reference | |||
| Both lame and abnormal canter | 0.083 | ||||
| Yes | 0.13 | 0.08 | 1.14 | 0.98, 1.33 | |
| No | Reference | Reference | |||
| Pain or increased tension in the thoracolumbosacral epaxial muscles | 0.389 | ||||
| Yes | −0.07 | 0.08 | 0.93 | 0.80, 1.09 | |
| No | Reference | Reference | |||
| Saddle fit likely to negatively affect performance | 0.530 | ||||
| Yes | −0.05 | 0.08 | 0.95 | 0.80, 1.12 | |
| No | Reference | Reference | |||
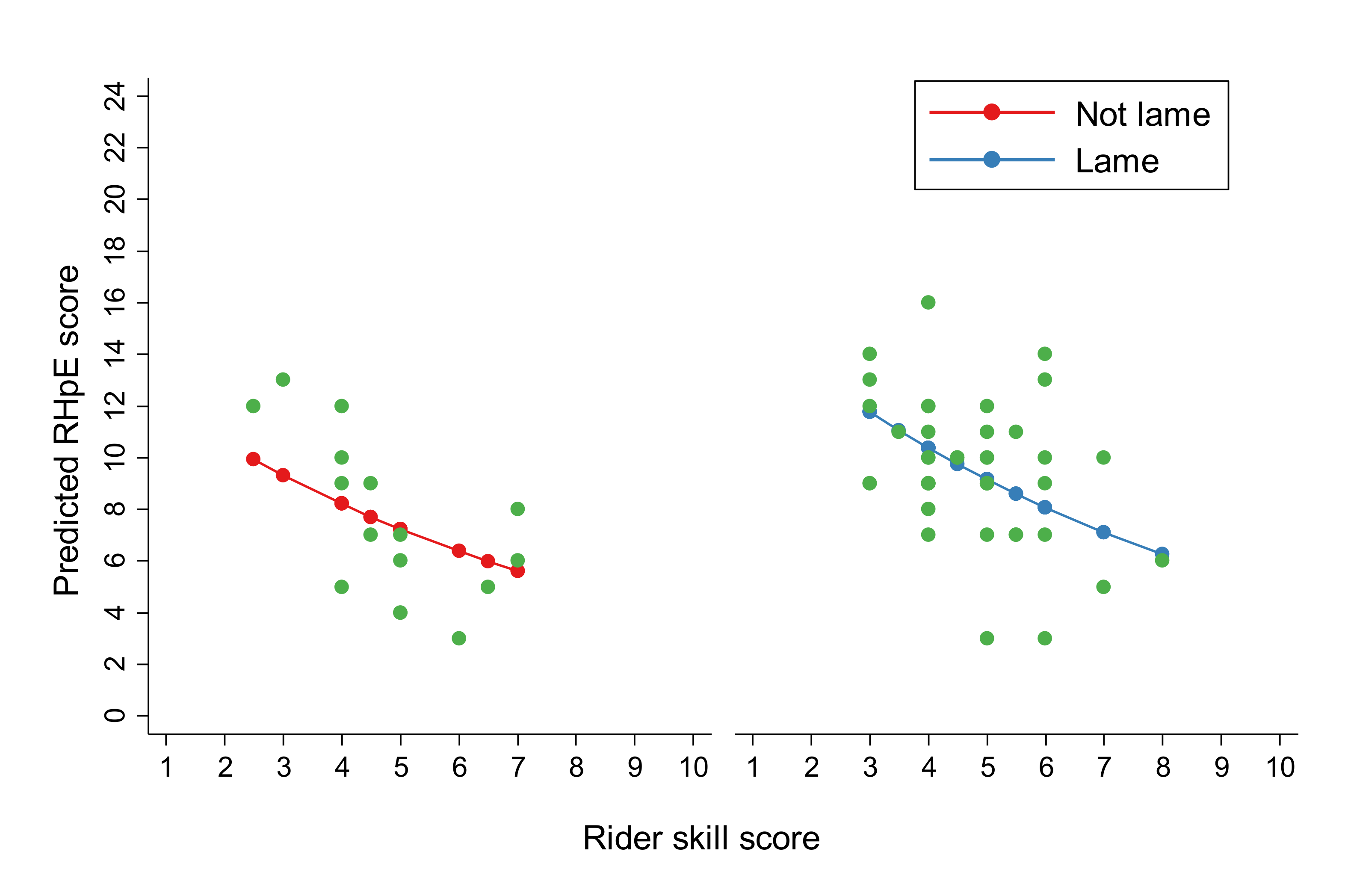
| Rider skill score (continuous) | −0.13 | 0.03 | 0.88 | 0.83, 0.94 | <0.001 |
| Variable | Coefficient | Robust Standard Error | Incident Rate Ratio (IRR) | IRR 95% CI | Wald p-Value |
|---|---|---|---|---|---|
| Rider skill score (continuous) | −0.13 | 0.03 | 0.88 | 0.83, 0.94 | <0.001 |
| Lame | 0.008 | ||||
| Yes | 0.23 | 0.09 | 1.26 | 1.06, 1.50 | |
| No | Reference | Reference |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyson, S.; Pollard, D. Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals 2020, 10, 1044. https://doi.org/10.3390/ani10061044
Dyson S, Pollard D. Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals. 2020; 10(6):1044. https://doi.org/10.3390/ani10061044
Chicago/Turabian StyleDyson, Sue, and Danica Pollard. 2020. "Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses" Animals 10, no. 6: 1044. https://doi.org/10.3390/ani10061044
APA StyleDyson, S., & Pollard, D. (2020). Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals, 10(6), 1044. https://doi.org/10.3390/ani10061044





