Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fenugreek Seed Gum Characterisation
2.2. In Vitro Digestive Behaviour
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coudert, P.; Lebas, F.; Licois, D. A new pathology devastates the breedings. The profession is mobilized (published in French). Cuniculture 1997, 24, 225–229. [Google Scholar]
- Rosell, J.M.; de la Fuente, L.F.; Badiola, J.I.; Fernández de Luco, D.; Casal, J.; Saco, M. Study of urgent visits to commercial rabbit farms in Spain and Portugal during 1997–2007. World Rabbit Sci. 2009, 17, 127–136. [Google Scholar] [CrossRef]
- Gidenne, T.; Carabaño, R.; García, J.; De Blas, C. Fibre digestion. In Nutrition of the Rabbit; De Blas, C., Wiseman, J., Eds.; CABI Publishing: Wallingford, UK, 2010; pp. 66–82. [Google Scholar]
- Gidenne, T.; Arveux, P.; Madec, O. The effect of the quality of dietary lignocellulose on digestion, zootechnical performance and health of the growing rabbit. Anim. Sci. 2001, 73, 97–104. [Google Scholar] [CrossRef]
- Carabaño, R.; Villamide, M.J.; García, J.; Nicodemus, N.; Llorente, A.; Chamorro, S.; Menoyo, D.; García-Rebollar, P.; García-Ruiz, A.I.; De Blas, J.C. New concepts and objectives for protein-amino acid nutrition in rabbits: A review. World Rabbit Sci. 2009, 17, 1–14. [Google Scholar] [CrossRef]
- Trocino, A.; García, J.; Carabaño, R.; Xiccato, G. A meta-analysis of the role of soluble fiber in diets for growing rabbits. World Rabbit Sci. 2013, 21, 1–15. [Google Scholar] [CrossRef]
- Falcão-E-Cunha, L.; Castro-Solla, L.; Maertens, L.; Marounek, M.; Pinheiro, V.; Freire, J.; Mourão, J.L. Alternatives to antibiotic growth promoters in rabbit feeding. A review. World Rabbit Sci. 2007, 15, 127–140. [Google Scholar]
- Peeters, J.E.; Maertens, L.; Geeroms, R. Influence of galacto-oligosaccharides in zootechnical performance, cecal biochemistry and experimental Colibacillosis o103/8+ in weanling rabbits. J. Appl. Rabbit Res. 1992, 15, 1129–1136. [Google Scholar]
- Gidenne, T. Effect of fibre level reduction and gluco-oligosaccharide addition on the growth performance and caecal fermentation in the growing rabbit. Anim. Feed Sci. Technol. 1995, 56, 253–263. [Google Scholar] [CrossRef]
- Aguilar, J.C.; Roca, T.; Sanz, E. Fructo-oligosaccharides in rabbit diet. Study of efficiency in suckling and fattening periods. In Proceedings of the 6th World Rabbit Congress, Toulouse, France, 9–12 July 1996; pp. 73–77. [Google Scholar]
- Lebas, F. Effects of fruct-oligo-saccharides origin on rabbit’s growth performance in 2 seasons. In Proceedings of the 6th World Rabbit Congres, Toulouse, France, 9–12 July 1996; pp. 211–215. [Google Scholar]
- Mourão, J.L.; Alves, A.; Pinheiro, V. Effects of fructooligosaccharides on performances of growing rabbits. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004; pp. 915–921. [Google Scholar]
- Morisse, J.P.; Maurice, R.; Boilletot, E.; Cotte, J.P. Assessment of the activity of a fructo oligossaccharides on different caecal parameters in rabbit experimentally infected with E. Coli 0.103. Ann. Zootech. 1992, 42, 81–87. [Google Scholar] [CrossRef]
- Maertens, L.; Aerts, J.; De Boever, J. Degradation of dietary oligofructose and inulin in the gastrointestinal tract and the effects on pH and VFA. World Rabbit Sci. 2004, 12, 235–246. [Google Scholar]
- Mourão, J.L.; Pinheiro, V.; Alves, A.; Guedes, C.M.; Pinto, L.; Saavedra, M.J.; Spring, P.; Kocher, A. Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation of fattening rabbits. Anim. Feed Sci. Technol. 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Jiang, J.X.; Zhu, L.W.; Zhang, W.M.; Sun, R.C. Characterization of Galactomannan Gum from Fenugreek (Trigonella foenum-graecum) seeds and its rheological properties. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 1145–1154. [Google Scholar] [CrossRef]
- Kakani, R. Prebiotic Properties of Yeast Cell Wall Mannanoligosaccharides and Guar Gum Galactomannans in Starting Broilers. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2013. [Google Scholar]
- Wang, J.-T.; Li, D.F.; Gong, L.M.; Cheng, G. Effect of galactomannan-oligosaccharides on the growth performance and immune function in broilers. Chin. J Anim. Sci. 2003, 39, 5–6. [Google Scholar]
- Van Nevel, C.J.; Decuypere, J.A.; Dierick, N.A.; Koen Molly, K. Incorporation of galactomannans in the diet of newly weaned piglets: Effect on bacteriological and some morphological characteristics of the small intestine. Arch. Anim. Nutr. 2005, 59, 123–138. [Google Scholar] [CrossRef]
- Dakia, P.A.; Blecker, C.; Roberta, C.; Watheleta, B.; Paquota, M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hydrocolloids 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Sivakumar, A.; Sankaran, N.; Kirankumar, B.; Furqan, A. Galactomannan from Trigonella Foenum Graecum seed: Prebiotic application and its fermentation by the probiotic Bacillus coagulans strain MTCC 5856. Food Sci. Nutr. 2018, 6, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carmona, J.; Blas, E.; Pascual, J.J.; Maertens, L.; Gidenne, T.; Xiccato, G.; García, J. Recommendations and guidelines for applied nutrition experiments in rabbits. World Rabbit Sci. 2005, 13, 209–228. [Google Scholar] [CrossRef]
- Zemzmi, J.; Mabrouki, S.; Abdouli, H.; Najar, T. Preliminary characterization of fenugreek seed gum for use as prebiotic in rabbits’ nutrition. In Proceedings of the 20th International Symposium on Housing and Diseases of Rabbits, Fur Providing Animals and Pet Animals, Celle, Germany, 17–18 May 2017; pp. 179–186. [Google Scholar]
- Narayana, K.; Narsinga-Rao, M.S. Functional properties of raw and heat processed winged bean (Psophocarpus tetragonolobus) flour. J. Food Sci. 1982, 42, 534–538. [Google Scholar] [CrossRef]
- Torio, M.A.O.; Saez, J.; Merca, F.E. Physicochemical characterization of galactomannan from sugar palm (Arenga Saccharifera Labill.) endosperm at different stages of nut maturity. Philipp. J. Sci. 2006, 135, 19–30. [Google Scholar]
- Marten, G.C.; Barnes, R.F. Prediction of energy digestibility of forages with in vitro rumen fermentation and fungal enzyme systems. In Standardization of Analytical Methodology for Feeds, Proceedings of the International Workshop on Standardization of Analytical Methodology for Feeds, Ottawa, ON, Canada, 12–14 March 1979; Pigden, W.J., Balch, C.C., Graham, M., Eds.; International Development Research Centre: Ottawa, ON, Canada, 1980; pp. 61–71. [Google Scholar]
- Schofield, P.; Pitt, R.E.; Pell, A.N. Kinetics of fiber digestion from in vitro gas production. J. Anim. Sci. 1994, 72, 2980–2991. [Google Scholar] [CrossRef]
- De Blas, C.; Mateos, C.C. Feed Formulation. In Nutrition of the rabbit; De Blas, C., Wiseman, J., Eds.; CABI Publishing: Wallingford, UK, 2010; pp. 222–232. [Google Scholar]
- Ramos, M.; Carabaño, R. Nutritive evaluation of rabbit diets by an in vitro method. In Proceedings of the 6th World Rabbit Congress, Toulouse, France, 9–12 July 1996; pp. 277–282. [Google Scholar]
- Fernández-Carmona, J.; Jover, J.; Gutiérrez, J. Características físicas de piensos extrusionados y aceptabilidad por cangrejo rojo (Procambarus clarkii). Archivos de Zootecnia 1993, 42, 219–228. [Google Scholar]
- Gibbs, R.D. Chemotaxonomy of Flowering Plants; McGill Queen’s University Press: Montreal, QC, Canada; London, UK, 1974; Volume 1. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal Plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods; Chapman and Hall Ltd.: London, UK, 1973; pp. 49–188. [Google Scholar]
- Evans, W.C. Trease Evans Pharmacognosy; International Edition E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Amabye, T.G.; Bezabh, A.M.; Mekonen, F. Phytochemical and antimicrobial potentials leaves extract of Eucalyptus Globulus oil from Maichew Tigray Ethiopia. Int. J. Complement. Alt. Med. 2016, 2, 56–59. [Google Scholar]
- Kumar, A.; Ilavarasn, R.; Jayachandran, T.; Decaraman, M.; Aravindhan, P.; Padmanaban, N.; Krishnan, M.R.V. Phytochemical investigation on a tropical plant. Pak. J. Nutr. 2009, 8, 83–85. [Google Scholar] [CrossRef]
- Rizk, A.M.; AM, R.; Al Nagdy, S.A.; El Missiry, M.M. Constituents of plant growing in Qatar. Fitoterapia 1982, 52, 35–42. [Google Scholar]
- Plummer, D.T. Practical Biochemistry; McGraw Hill Book Company: London, UK, 1971. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Office Analysis Chemistry: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Batey, I.L. Starch Analysis Using Thermostable Alpha-Amylases. Starch/Stärke 1982, 34, 125–128. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Robertson, J.B.; Van Soest, P.J. The detergent system analysis and its application to human foods in the analysis of dietary fiber food. In The Analysis of Dietary Fiber Foods; James, W.P.T., Theander, J., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 123–157. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bosh, L.; Alegría, A.; Farre, R. Application of the 6-aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) reagent to determination of amino acids in infant foods. J. AOAC Int. 2006, 78, 736–744. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide (Release 9.2); SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Deshpande, P.; Mohan, V.; Thakurdesai, P. Preclinical safety evaluation of low molecular weight galactomannans based standardized fenugreek seeds extract. EXCLI J. 2016, 15, 446. [Google Scholar]
- Mc Cleary, B.V. Carob and guar galactomannans. Method Enzymol. 1988, 160, 523–527. [Google Scholar]
- Chaires-Martínez, L.; Salazar-Montoya, J.A.; Ramos-Ramirez, E.G. Physicochemical and function characterization of the galactomannan obtained from mesquite seeds (Prosopis Pallida). Eur. Food Res. Technol. 2008, 227, 1669–1679. [Google Scholar] [CrossRef]
- Nour, A.A.M.; Magboul, B.I. Chemical and amino acid composition of fenugreek seeds grown in Sudan. Food Chem. 1986, 22, 1–5. [Google Scholar] [CrossRef]
- Schofield, P. Gas production methods. In Farm Animal Metabolism and Nutrition; D’Mello, J.P.F., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 209–232. [Google Scholar]
- Piquer, O.; Casado, C.; Biglia, S.; Fernández, C.; Blas, E.; Pascual, J.J. In vitro gas production kinetics of whole citrus fruits. J. Anim. Feed Sci. 2009, 18, 743–757. [Google Scholar] [CrossRef]
- Abad-Guamán, R.; Larrea-Dávalos, J.A.; Carabaño, R.; García, J.; Carro, M.D. Influence of inoculum type (ileal, caecal and faecal) on the in vitro fermentation of different sources of carbohydrates in rabbits. World Rabbit Sci. 2018, 26, 227–240. [Google Scholar] [CrossRef]
- Ocasio-Vega, C.; Abad-Guamán, R.; Delgado, R.; Carabaño, R.; Dolores Carro, M.; García, J. Effect of cellobiose supplementation and dietary soluble fibre content on in vitro caecal fermentation of carbohydrate-rich substrates in rabbits. Arch. Anim. Nutr. 2018, 72, 1477–2817. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Conde, M.S.; Pérez de Rozas, A.; Badiola, I.; Pérez-Alba, L.; de Blas, C.; Carabaño, R.; García, J. Effect of neutral detergent soluble fibre on digestion, intestinal microbiota and performance in twenty five day old weaned rabbits. Livest. Sci. 2009, 125, 192–198. [Google Scholar] [CrossRef]
- Trocino, A.; Fragkiadakis, M.; Majolini, D.; Tazzoli, M.; Radaelli, G.; Xiccato, G. Soluble fibre, starch and protein level in diets for growing rabbits: Effects on digestive efficiency and productive traits. Anim. Feed Sci. Technol. 2013, 180, 73–82. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics: The Concept Revisited. J. Nutr. 2007, 137, 830–837. [Google Scholar] [CrossRef]
- Gidenne, T. Dietary fibres in the nutrition of the growing rabbit and recommendations to preserve digestive health: A review. Animal 2015, 9, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Marounek, M.; Vovk, S.J.; Benda, V. Fermentation patterns in rabbit caecal cultures supplied with plant polysaccharides and lactate. Acta Veterinaria Brno 1997, 67, 9–13. [Google Scholar] [CrossRef]
- García, J.; Gidenne, T.; Falcão-E-Cunha, L.; de Blas, C. Identification of the main factors that influence caecal fermentation traits in growing rabbits. Anim. Res. 2002, 51, 165–173. [Google Scholar] [CrossRef]
- Van Soest, P.J. Mathematical applications: Digestibility. In Nutritional Ecology of the Ruminant, 2nd ed.; Comstock Publishing Associates: Ithaca, NY, USA, 1994; pp. 354–370. [Google Scholar]
- Volek, Z.; Marounek, M. Dried chicory root (Cichorium intybus L.) as a natural fructan source in rabbit diet: Effects on growth performance, digestion and caecal and carcass traits. World Rabbit Sci. 2011, 19, 143–150. [Google Scholar] [CrossRef]
- Owusu-Asiedu, A.J.F.J.; Patience, J.F.; Laarveld, B.; Van Kessel, A.G.; Simmins, P.H.; Zijlstra, R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006, 84, 843–852. [Google Scholar] [CrossRef]
- Dartois, A.; Singh, J.; Kaur, L.; Singh, H. Influence of guar gum on the in vitro starch digestibility—Rheological and microstructural characteristics. Food Biophys. 2010, 5, 149–160. [Google Scholar] [CrossRef]
| Ingredients and Chemical Composition | Basal Diet a | Experimental Diets | |
|---|---|---|---|
| SF | IF | ||
| Ingredients | |||
| Wheat bran | 247 | 221 | 221 |
| Sunflower meal | 212 | 190 | 190 |
| Beet pulp | 108 | 197 | 97 |
| Grape seeds | 0 | 0 | 100 |
| Alfalfa hay | 100 | 90 | 90 |
| Wheat straw | 80 | 72 | 72 |
| Corn flour | 80 | 72 | 72 |
| Corn germ cake | 49 | 44 | 44 |
| Molasses | 40 | 36 | 36 |
| Rice bran | 30 | 27 | 27 |
| Corn grain | 25 | 22 | 22 |
| Calcium carbonate | 11 | 11 | 11 |
| Sodium chloride | 3 | 3 | 3 |
| Premix b | 15 | 15 | 15 |
| Chemical composition | |||
| Dry matter (DM, g/kg) | 898 | 896 | 895 |
| Ash | 70.0 | 70.1 | 66.4 |
| Crude protein | 176 | 168 | 169 |
| Neutral detergent fibre | 397 | 401 | 415 |
| Acid detergent fibre | 197 | 204 | 231 |
| Lignin acid detergent | 29.7 | 27.7 | 66.1 |
| Total dietary fibre | - | 489 | 474 |
| Soluble fibre | - | 88.1 | 59.0 |
| Chemical Composition | (g/kg DM) |
|---|---|
| Dry matter (DM) (g/kg) | 913 |
| Ashes | 12.7 |
| Crude protein (CP) | 223 |
| Sugars | 630 |
| Sugar composition (g/kg sugars): | |
| Galactose | 515 |
| Mannose | 485 |
| Fructose | <1 |
| Glucose | <1 |
| Lactose | <1 |
| Maltotriose | <1 |
| Galactose/mannose ratio | 1.1 |
| Amino acid composition (g/kg CP): | |
| Alanine | 25.9 |
| Arginine | 76.3 |
| Aspartate | 107 |
| Cysteine | 13.4 |
| Glutamate | 172 |
| Glycine | 27.9 |
| Histidine | 22.3 |
| Isoleucine | 47.9 |
| Leucine | 64.1 |
| Lysine | 57.9 |
| Methionine | 9.10 |
| Phenylalanine | 33.1 |
| Proline | 32.9 |
| Serine | 39.4 |
| Threonine | 23.1 |
| Tyrosine | 15.2 |
| Valine | 32.1 |
| Gas Production Kinetics a | Urea Addition (g/mL) | |||
|---|---|---|---|---|
| 0 | 0.5 | SEM | p-Value | |
| Vf (mL) | 24.46 | 24.92 | 1.597 | 0.8545 |
| k (h−1) | 0.152 | 0.126 | 0.006 | 0.0318 |
| Lag (h) | 6.118 | 4.077 | 0.245 | 0.0047 |
| µm (mL/h) | 3.722 | 3.119 | 0.130 | 0.0340 |
| tµm (h) | 9.411 | 8.063 | 0.127 | 0.0019 |
| Traits | Mean | Contrasts 1 | Linear Effect of FSG Inclusion | |||
|---|---|---|---|---|---|---|
| SF Versus IF | 0 Versus 1000 | All Diets | SF Diets | IF Diets | ||
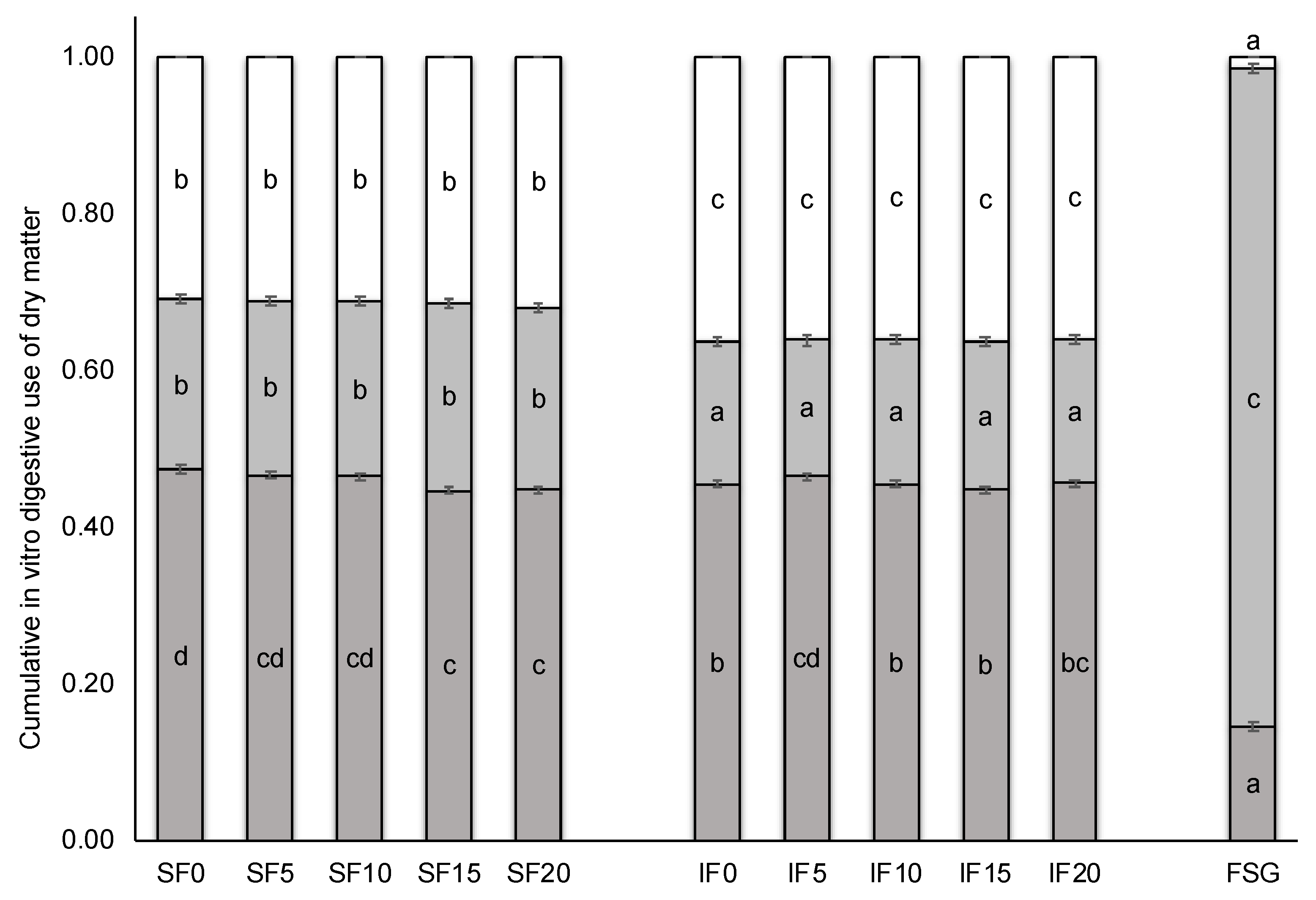
| Indigestible fraction (g/kg) | 560.8 | −4.05 ± 2.68 | −319.5 ± 6.1 *** | 0.86 ± 0.02 *** | 1.43 ± 0.27 *** | 0.29 ± 0.27 |
| Indigestible fraction composition (g/kg): | ||||||
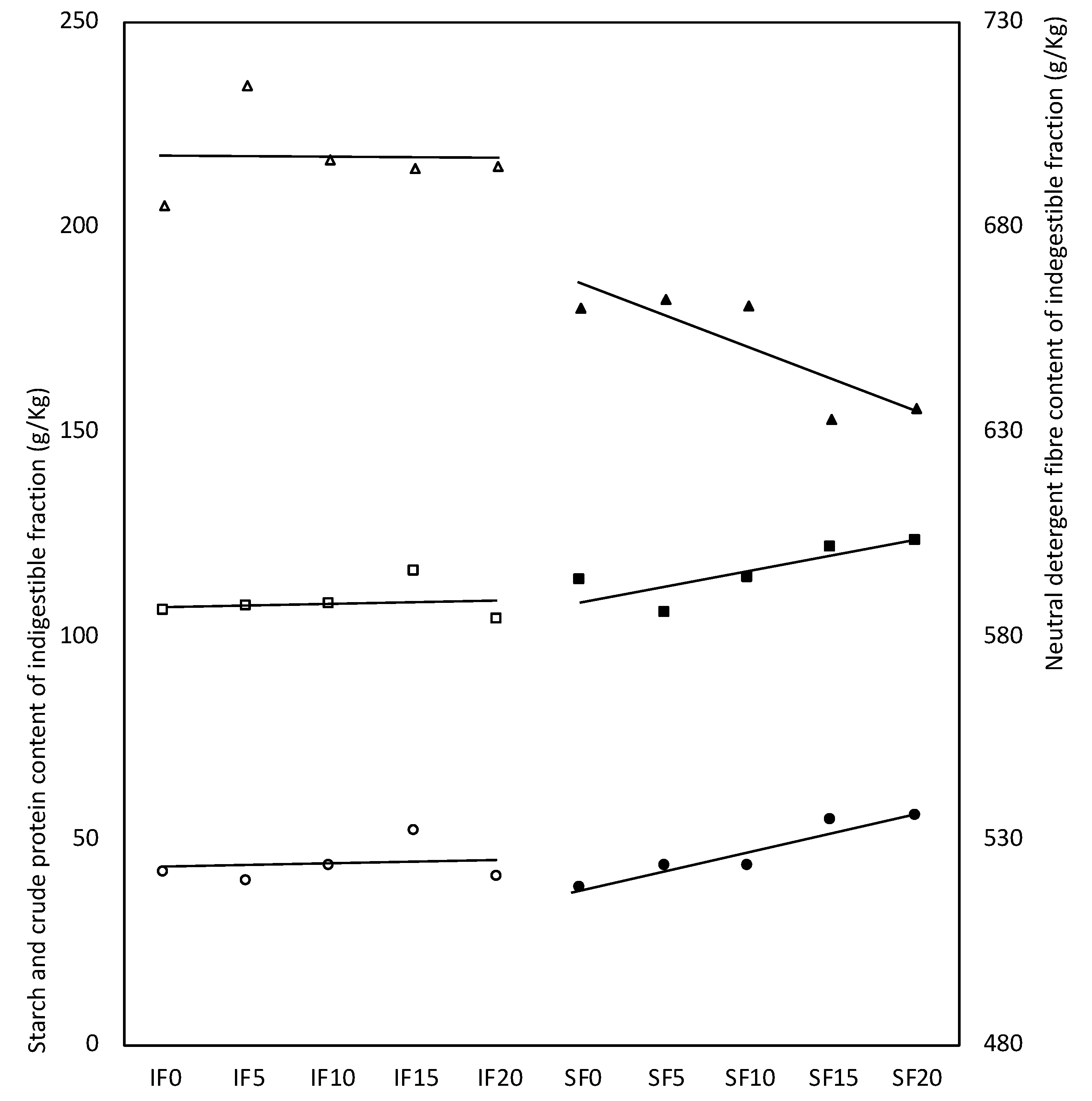
| Crude protein | 116.5 | 7.45 ± 1.60 *** | −54.5 ± 3.1 *** | 0.39 ± 0.11 ** | 0.70 ± 0.16 ** | 0.01 ± 0.16 |
| Neutral detergent fibre | 673.6 | −46.70 ± 2.22 *** | −0.79 ± 0.15 *** | −1.57 ± 0.22 *** | −0.01 ± 0.22 | |
| Acid detergent fibre | 381.9 | −64.27 ± 3.96 *** | −0.41 ± 0.28 | −1.08 ± 0.40 * | 0.25 ± 0.40 | |
| Lignin acid detergent | 101.2 | −74.75 ± 3.30 *** | 0.13 ± 0.23 | −0.16 ± 0.33 | 0.42 ± 0.33 | |
| Hemicellulose | 291.7 | 17.57 ± 3.36 *** | −0.38 ± 0.24 | −0.49 ± 0.34 | −0.26 ± 0.34 | |
| Cellulose | 280.8 | 10.48 ± 2.03 *** | −0.55 ± 0.14 ** | −0.92 ± 0.20 ** | −0.17 ± 0.20 | |
| Starch | 41.5 | −3.49 ± 2.20 | 39.1 ± 4.3 *** | 0.56 ± 0.16 ** | 0.93 ± 0.22 *** | 0.20 ± 0.22 |
| Traits | Mean | Contrasts 1 | Linear Effect of FSG Inclusion | |||
|---|---|---|---|---|---|---|
| SF versus IF | 0 versus 1000 | All Diets | SF Diets | IF Diets | ||
| Unfermented fraction (g/kg) | 566.9 | −83.1 ± 3.7 *** | 610.8 ± 7.1 *** | −0.6 ± 0.3 * | −0.7 ± 0.4 | −0.6 ± 0.4 |
| Gas pressure (mbar) | 0.62 | 0.15 ± 0.02 *** | −0.61 ± 0.03 *** | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| pH | 6.18 | −0.12 ± 0.02 *** | 0.68 ± 0.03 *** | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Total VFA (mmol/L) | 19.49 | 5.72 ± 1.46 *** | −27.32 ± 3.31 *** | 0.03 ± 0.10 | −0.04 ± 0.15 | 0.11 ± 0.15 |
| VFA profile (%): | ||||||
| Acetate | 63.25 | 1.94 ± 1.64 | 4.28 ± 3.72 | −0.05 ± 0.12 | −0.11 ± 0.16 | 0.01 ± 0.16 |
| Propionate | 11.63 | 0.21 ± 0.38 | −3.08 ± 0.87 ** | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.04 ± 0.04 |
| Butyrate | 21.84 | −2.29 ± 1.04 * | −2.12 ± 2.35 | −0.02 ± 0.07 | 0.01 ± 0.10 | −0.06 ± 0.10 |
| Iso-butyrate | 0.37 | −0.07 ± 0.03 * | −0.15 ± 0.06 * | 0.00 ± 0.00 | 0.00 ± 0.01 | 0.00 ± 0.00 |
| Valerate | 0.70 | 0.02 ± 0.04 | 0.26 ± 0.09 * | 0.01 ± 0.00 * | 0.01 ± 0.00 * | 0.00 ± 0.00 |
| Isovalerate | 0.31 | −0.09 ± 0.04 * | −0.49 ± 0.09 *** | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 |
| Caproate | 1.83 | 0.27 ± 0.17 | 1.23 ± 0.39 ** | 0.01 ± 0.01 | 0.03 ± 0.02 | −0.00 ± 0.02 |
| Heptanoate | 0.09 | 0.01 ± 0.02 | 0.06 ± 0.04 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| N-NH3 (mmol/L) | 28.89 | −15.39 ± 3.09 *** | 65.15 ± 5.89 *** | 0.33 ± 0.22 | 0.67 ± 0.33 | −0.00 ± 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemzmi, J.; Ródenas, L.; Blas, E.; Najar, T.; Pascual, J.J. Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition. Animals 2020, 10, 1041. https://doi.org/10.3390/ani10061041
Zemzmi J, Ródenas L, Blas E, Najar T, Pascual JJ. Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition. Animals. 2020; 10(6):1041. https://doi.org/10.3390/ani10061041
Chicago/Turabian StyleZemzmi, Jihed, Luis Ródenas, Enrique Blas, Taha Najar, and Juan José Pascual. 2020. "Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition" Animals 10, no. 6: 1041. https://doi.org/10.3390/ani10061041
APA StyleZemzmi, J., Ródenas, L., Blas, E., Najar, T., & Pascual, J. J. (2020). Characterisation and In Vitro Evaluation of Fenugreek (Trigonella foenum-graecum) Seed Gum as a Potential Prebiotic in Growing Rabbit Nutrition. Animals, 10(6), 1041. https://doi.org/10.3390/ani10061041






