Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Husbandry Conditions and Animals
2.2. Blood Sampling
2.3. Cortisol Assay
2.4. Statistical Analysis
2.5. Ethical Approval
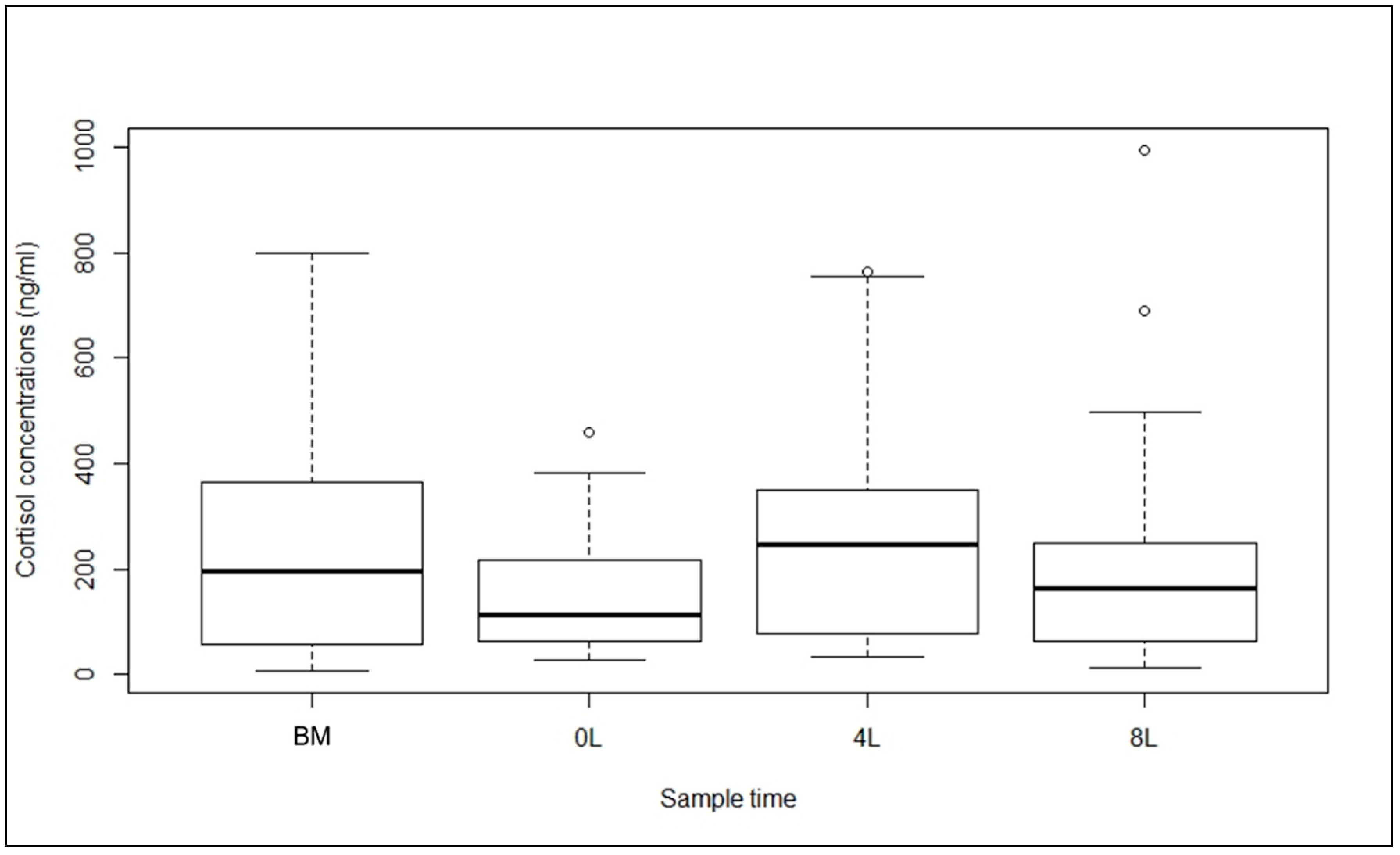
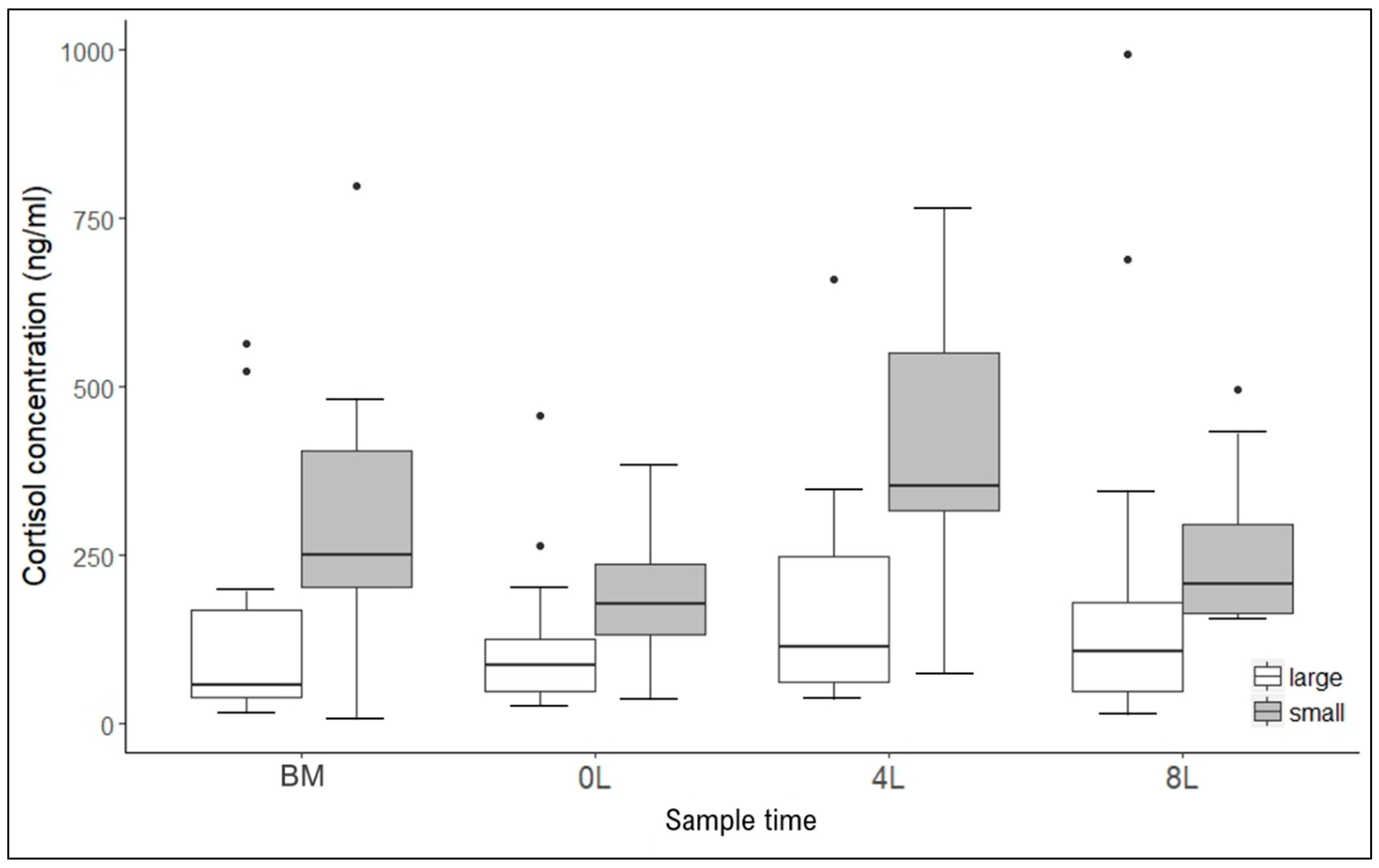
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gittleman, J.L.; Thompson, S.D. Energy allocation in mammalian reproduction. Am. Zool. 1988, 2828, 863–875. [Google Scholar] [CrossRef]
- Oftedal, O.T.; Gittleman, J.L. Patterns of energy output during reproduction in carnivores. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Springer Nature: New York, NY, USA, 1989; pp. 355–378. [Google Scholar]
- Kenagy, G.J.; Masman, D.; Sharbaugh, S.M.; Nagy, K.A. Energy expenditure during lactation in relation to litter size in free-living golden-mantled ground squirrels. J. Anim. Ecol. 1990, 5959, 73–88. [Google Scholar] [CrossRef]
- Arnbom, T.; Fedak, M.A.; Boyd, I.L. Factors affecting maternal expenditure in Southern elephant seals during lactation. Ecology 1997, 7878, 471–483. [Google Scholar] [CrossRef]
- Deacon, F.; Nel, P.J.; Bercovitch, F.B. Concurrent pregnancy and lactation in wild giraffes (Giraffa camelopardalis). Afr. Zool. 2015, 5050, 331–334. [Google Scholar] [CrossRef]
- Klug, H.; Alonzo, S.H.; Bonsall, M.B. Theoretical foundations of parental care. In The Evolution of Parental Care; Royle, N.J., Smiseth, P.T., Kölliker, M., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 21–39. [Google Scholar]
- Hau, M.; Ricklefs, R.E.; Wikelski, M.; Lee, K.A.; Brawn, J.D. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 2010, 277, 3203–3212. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 4343, 2–15. [Google Scholar] [CrossRef]
- Naidenko, S.V.; Ivanov, E.A.; Lukarevskii, V.S.; Hernandez-Blanco, J.A.; Sorokin, P.A.; Litvinov, M.N.; Kotlyar, A.K.; Rozhnov, V.V. Activity of the hypothalamo-pituitary-adrenals axis in the Siberian tiger (Panthera tigris altaica) in captivity and in the wild, and its dynamics throughout the year. Biol. Bull. 2011, 38, 301–305. [Google Scholar] [CrossRef]
- Naidenko, S.V.; Berezhnoi, M.A.; Kumar, V.; Umapathy, G. Comparison of tigers’ fecal glucocorticoids level in two extreme habitats. PLoS ONE 2019, 14, e0214447. [Google Scholar] [CrossRef]
- Pavlova, E.V.; Naidenko, S.V. Noninvasive monitoring of glucocorticoids in feces of the Amur leopard cat (Prionailurus bengalensis euptilura). Zool. Zh. 2008, 87, 1375–1381. (In Russian) [Google Scholar]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A.; Douglas, A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008, 20, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.W.; Liebl, A.L.; Owen, J.C.; Martin, L.B.; Betts, M.G. Baseline corticosterone is positively related to juvenile survival in a migrant passerine bird. Funct. Ecol. 2012, 26, 1127–1134. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, L.; Troisi, A.; Verstegen, J.P.; Menchetti, L.; Elad Ngonput, A.; Boiti, C.; Canello, S.; Zelli, R.; Polisca, A. Serum concentration dynamic of energy homeostasis hormones, leptin, insulin, thyroid hormones, and cortisol throughout canine pregnancy and lactation. Theriogenology 2017, 97, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.M.; Monson, D.H.; Tinker, M.T.; Staedler, M.M.; Crocker, D.E. Lactation and resource limitation affect stress responses, thyroid hormones, immune function, and antioxidant capacity of sea otters (Enhydra lutris). Ecol. Evol. 2018, 8, 8433–8447. [Google Scholar] [CrossRef] [PubMed]
- Bonier, F.; Moore, I.T.; Robertson, R.J. The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol. Lett. 2011, 7, 944–946. [Google Scholar] [CrossRef]
- Guinet, C.; Servera, N.; Mangin, S.; Georges, J.Y.; Lacroix, A. Change in plasma cortisol and metabolites during the attendance period ashore in fasting lactating Subantarctic fur seals. Comp. Biochem. Physiol. A 2004, 137, 523–531. [Google Scholar] [CrossRef]
- Kenagy, G.J.; Place, N.J. Seasonal changes in plasma glucocorticosteroids of free-living female yellow-pine chipmunks: Effects of reproduction and capture and handling. Gen. Comp. Endocrinol. 2000, 117, 189–199. [Google Scholar] [CrossRef]
- Montiglio, P.O.; Garant, D.; Pelletier, F.; Réale, D. Intra-individual variability in fecal cortisol metabolites varies with lifetime exploration and reproductive life history in eastern chipmunks (Tamias striatus). Behav. Ecol. Sociobiol. 2015, 69, 1–11. [Google Scholar] [CrossRef]
- Deag, J.M.; Lawrence, C.E.; Manning, A. The consequences of differences in litter size for the nursing cat and her kittens. J. Zool. 1987, 213, 153–179. [Google Scholar] [CrossRef]
- Mendl, M. The effects of litter size variation on mother-offspring relationships and behavioural and physical development in several mammalian species (principally rodents). J. Zool. 1988, 215, 15–34. [Google Scholar] [CrossRef]
- Tardif, S.D.; Power, M.; Oftedal, O.T.; Power, R.A.; Layne, D.G. Lactation, maternal behavior and infant growth in common marmoset monkeys (Callithrix jacchus): Effects of maternal size and litter size. Behav. Ecol. Sociobiol. 2001, 51, 17–25. [Google Scholar]
- Neuhaus, P. Weight comparisons and litter size manipulation in Columbian ground squirrels (Spermophilus columbianus) show evidence of costs of reproduction. Behav. Ecol. Sociobiol. 2000, 48, 75–83. [Google Scholar] [CrossRef]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Del Rosso, L.; Mendoza, S.P.; Capitanio, J.P. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Golet, G.H.; Schmutz, J.A.; Irons, D.B.; Estes, J.A. Determinants of reproductive costs in the long-lived black-legged kittiwake: A multiyear experiment. Ecol. Monogr. 2004, 74, 353–372. [Google Scholar] [CrossRef]
- Welcker, J.; Speakman, J.R.; Elliott, K.H.; Hatch, S.A.; Kitaysky, A.S. Resting and daily energy expenditures during reproduction are adjusted in opposite directions in free-living birds. Funct. Ecol. 2015, 29, 250–258. [Google Scholar] [CrossRef]
- Bahr, N.I.; Pryce, C.R.; Döbeli, M.; Martin, R.D. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol. Behav. 1998, 64, 429–437. [Google Scholar] [CrossRef]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 2003, 997, 136–149. [Google Scholar] [CrossRef]
- Wigger, A.; Lörscher, P.; Oehler, I.; Keck, M.E.; Naruo, T.; Neumann, I.D. Nonresponsiveness of the rat hypothalamo-pituitary-adrenocortical axis to parturition-related events: Inhibitory action of endogenous opioids. Endocrinology 1999, 140, 2843–2849. [Google Scholar] [CrossRef]
- Liberg, O. Courtship behavior and sexual selection of the domestic cat. Appl. Anim. Ethol. 1983, 10, 117–132. [Google Scholar] [CrossRef]
- Turner, D.C.; Bateson, P. The Domestic Cat: The Biology of Its Behavior, 3rd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Martin, P. An experimental study of weaning in the domestic cat. Behaviour 1986, 99, 221–249. [Google Scholar] [CrossRef]
- Leyhausen, P. Cat Behaviour. The Predatory and Social Behaviour of Domestic and Wild Cats; Garland STPM Press: Boca Raton, FL, USA, 1979. [Google Scholar]
- Loveridge, G.G. Body weight changes and energy intake of cats during gestation and lactation. Anim. Technol. 1986, 37, 7–15. [Google Scholar]
- Alekseeva, G.S. Correlation of Maternal Behavior and Physiological Status of Domestic Cat Females (Felis catus) with Their Offspring Development. Ph.D. Thesis, A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia, 2017. [Google Scholar]
- Michael, R.P. Observations upon the sexual behaviour of the domestic cat (Felis catus L.) under laboratory conditions. Behaviour 1961, 18, 1–24. [Google Scholar] [CrossRef]
- Hurni, H. Day length and breeding in the domestic cat. Lab. Anim. 1981, 15, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Nutter, F.B.; Levine, J.F.; Stoskopf, M.K. Reproductive capacity of free-roaming domestic cats and kitten survival rate. J. Am. Vet. Med. Assoc. 2004, 225, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, G.S.; Naidenko, S.V. Changes of body weight and steroid hormones level during offspring growth in domestic cat females (Felis catus; Felidae, Mammalia). Povolzh. Ecol. J. 2014, 4, 436–443. (In Russian) [Google Scholar]
- Loshchagina, J.; Tsvey, A.; Naidenko, S. Baseline and stress-induced corticosterone levels are higher during spring than autumn migration in European robins. Horm. Behav. 2018, 98, 96–102. [Google Scholar] [CrossRef]
- Romero, L.M.; Romero, R.C. Corticosterone responses in wild birds: The importance of rapid initial sampling. Condor 2002, 104, 129–135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2018. Available online: https://cran.r-project.org (accessed on 10 March 2020).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Nature: New York, NY, USA, 2009. [Google Scholar]
- Bates, D.; Martin, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Buchanan, K.; Burt de Perera, T.; Carere, C.; Carter, T.; Hailey, A.; Hubrecht, R.; Jennings, D.; Metcalfe, N.; Pitcher, T.; Peron, F. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [Google Scholar]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D. Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog. Brain Res. 2001, 133, 143–152. [Google Scholar] [PubMed]
- Concannon, P.W.; Butler, W.R.; Hansel, W.; Knight, P.J.; Hamilton, J.M. Parturition and lactation in the bitch: Serum progesterone, cortisol and prolactin. Biol. Reprod. 1978, 19, 1113–1118. [Google Scholar] [CrossRef]
- Dantzer, B.; McAdam, A.G.; Palme, R.; Fletcher, Q.E.; Boutin, S.; Humphries, M.M.; Boonstra, R. Fecal cortisol metabolite levels in free-ranging North American red squirrels: Assay validation and the effects of reproductive condition. Gen. Comp. Endocrinol. 2010, 167, 279–286. [Google Scholar] [CrossRef]
- Broussard, D.R.; Risch, T.S.; Dobson, F.S.; Murie, J.O. Senescence and age-related reproduction of female Columbian ground squirrels. J. Anim. Ecol. 2003, 72, 212–219. [Google Scholar] [CrossRef]
- Martin, J.G.A.; Festa-Bianchet, M. Age-independent and age-dependent decreases in reproduction of females. Ecol. Lett. 2011, 14, 576–581. [Google Scholar] [CrossRef]
- Lunn, N.J.; Boyd, I.L.; Croxall, J.P. Reproductive performance of female Antarctic fur seals: The influence of age, breeding experience, environmental variation and individual quality. J. Anim. Ecol. 1994, 63, 827–840. [Google Scholar] [CrossRef]
- Weladji, R.B.; Holand, Ø.; Gaillard, J.M.; Yoccoz, N.G.; Mysterud, A.; Nieminen, M.; Stenseth, N.C. Age-specific changes in different components of reproductive output in female reindeer: Terminal allocation or senescence? Oecologia 2010, 162, 261–271. [Google Scholar] [CrossRef]
- Erofeeva, M.N.; Naidenko, S.V. Spatial organization of felids populations and some traits of their reproductive strategies. Zh. Obsch. Biol. 2011, 72, 284–297. (In Russian) [Google Scholar]
- Trivers, R. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Aldine: Atherton, CA, USA, 1972; pp. 136–179. [Google Scholar]
- Stearns, S.C. Trade-offs in life-history evolution. Funct. Ecol. 1989, 3, 259–268. [Google Scholar] [CrossRef]
- Nicolás, L.; Martínez-Gómez, M.; Hudson, R.; Bautista, A. Littermate presence enhances motor development, weight gain and competitive ability in newborn and juvenile domestic rabbits. Dev. Psychobiol. 2011, 53, 37–46. [Google Scholar] [CrossRef] [PubMed]
| Year of Study | Female Code | Maternal Experience | Number of Litter | Number of Kittens per Litter | Litter Size |
|---|---|---|---|---|---|
| 2011 | MK | primiparous | 1 | 3 | small |
| 2012 | BN | multiparous | 3 | 4 | large |
| GR | multiparous | 2 | 4 | large | |
| MR | multiparous | 3 | 7 | large | |
| MS | multiparous | 2 | 5 | large | |
| MK | multiparous | 2 | 2 | small | |
| 2013 | BN | multiparous | 4 | 6 | large |
| GM | multiparous | 4 | 4 | large | |
| GR | multiparous | 3 | 3 | small | |
| MR | multiparous | 4 | 2 | small | |
| MS | multiparous | 3 | 1 | small | |
| MK | multiparous | 3 | 3 | small | |
| OR | primiparous | 1 | 5 | large | |
| PZ | multiparous | 3 | 4 | large | |
| 2014 | FK | primiparous | 1 | 3 | small |
| GM | multiparous | 5 | 4 | large | |
| GR | multiparous | 4 | 3 | small | |
| OR | multiparous | 2 | 1 | small | |
| PK | primiparous | 1 | 4 | large | |
| 2015 | GM | multiparous | 6 | 3 | small |
| GR | multiparous | 5 | 5 | large | |
| MK | multiparous | 4 | 5 | large | |
| OR | multiparous | 3 | 4 | large | |
| 2018 | FK | multiparous | 3 | 5 | large |
| PZ | multiparous | 6 | 5 | large | |
| PK | multiparous | 3 | 4 | large | |
| VR | primiparous | 1 | 2 | small |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, G.S.; Loshchagina, J.A.; Erofeeva, M.N.; Naidenko, S.V. Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals 2020, 10, 903. https://doi.org/10.3390/ani10050903
Alekseeva GS, Loshchagina JA, Erofeeva MN, Naidenko SV. Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals. 2020; 10(5):903. https://doi.org/10.3390/ani10050903
Chicago/Turabian StyleAlekseeva, Galina S., Julia A. Loshchagina, Mariya N. Erofeeva, and Sergey V. Naidenko. 2020. "Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats" Animals 10, no. 5: 903. https://doi.org/10.3390/ani10050903
APA StyleAlekseeva, G. S., Loshchagina, J. A., Erofeeva, M. N., & Naidenko, S. V. (2020). Stressed by Maternity: Changes of Cortisol Level in Lactating Domestic Cats. Animals, 10(5), 903. https://doi.org/10.3390/ani10050903





