Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
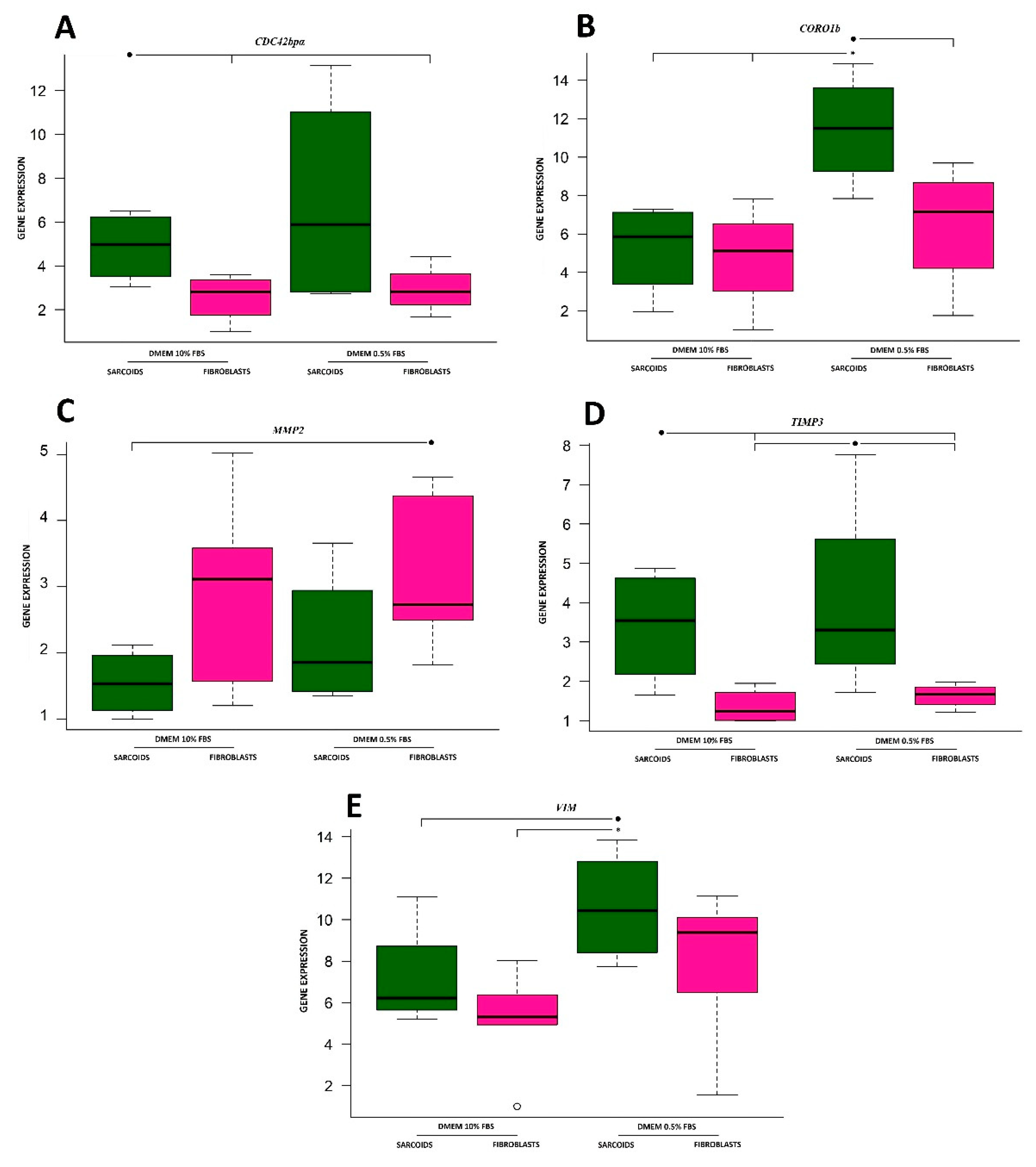
3. Results
3.1. Cell Division Control 42 Binding Protein Alpha
3.2. Coronin
3.3. Matrix Metalloproteinase 2
3.4. Tissue Inhibitor of Metalloproteinases 3
3.5. Vimentin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Knottenbelt, D.C. A Suggested Clinical Classification for the Equine Sarcoid. Clin. Tech. Equine Pr. 2005, 4, 278–295. [Google Scholar] [CrossRef]
- Haralambus, R.; Burgstaller, J.; Klukowska-Rötzler, J.; Steinborn, R.; Buchinger, S.; Gerber, V.; Brandt, S. Intralesional bovine papillomavirus DNA loads reflect severity of equine sarcoid disease. Equine Veter-J. 2010, 42, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Christen, G.; Gerber, V.; Dolf, G.; Burger, D.; Koch, C. Inheritance of equine sarcoid disease in Franches-Montagnes horses. Veter-J. 2014, 199, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Knottenbelt, D.C.; Matthews, J. A Positive Step Forwards in the Diagnosis of Equine Sarcoid. Veter-J. 2001, 161, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.D.; Toth, B.; Baseler, L.J.; Charney, V.A.; Miller, M.A. Lack of Correlation Between Papillomaviral DNA in Surgical Margins and Recurrence of Equine Sarcoids. J. Equine Veter-Sci. 2014, 34, 722–725. [Google Scholar] [CrossRef]
- Nasir, L.; Reid, S. Bovine papillomaviral gene expression in equine sarcoid tumours. Virus Res. 1999, 61, 171–175. [Google Scholar] [CrossRef]
- Yuan, Z.; Gallagher, A.; Gault, E.A.; Campo, M.S.; Nasir, L. Bovine papillomavirus infection in equine sarcoids and in bovine bladder cancers. Veter-J. 2007, 174, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Gault, E.; Gobeil, P.; Nixon, C.; Campo, M.; Nasir, L. Establishment and characterization of equine fibroblast cell lines transformed in vivo and in vitro by BPV-1: Model systems for equine sarcoids. Virology 2008, 373, 352–361. [Google Scholar] [CrossRef]
- Bogaert, L.; Martens, A.; Kast, W.M.; Van Marck, E.; De Cock, H. Bovine papillomavirus DNA can be detected in keratinocytes of equine sarcoid tumors. Veter-Microbiol. 2010, 146, 269–275. [Google Scholar] [CrossRef]
- Gaynor, A.M.; Zhu, K.W.; Affolter, V.K.; Cruz, F.N.D.; Pesavento, P. Localization of Bovine Papillomavirus Nucleic Acid in Equine Sarcoids. Veter-Pathol. 2015, 53, 567–573. [Google Scholar] [CrossRef]
- Rector, A.; Van Ranst, M. Animal papillomaviruses. Virology 2013, 445, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bravo, I.G.; Félez-Sánchez, M. Papillomaviruses. Evol. Med. Public Health 2015, 2015, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Semik-Gurgul, E.; Gurgul, A.; Ząbek, T.; Koch, C.; Mählmann, K.; Ropka-Molik, K.; Bugno-Poniewierska, M. Transcriptome analysis of equine sarcoids. Veter-Comp. Oncol. 2016, 15, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Pawlina-Tyszko, K.; Gurgul, A.; Szmatoła, T.; Koch, C.; Mählmann, K.; Witkowski, M.; Poniewierska, B.; Pawlina-Tyszko, K. Comprehensive characteristics of microRNA expression profile of equine sarcoids. Biochimie 2017, 137, 20–28. [Google Scholar] [CrossRef]
- Pawlina-Tyszko, K.; Gurgul, A.; Szmatoła, T.; Ropka-Molik, K.; Semik-Gurgul, E.; Klukowska-Rötzler, J.; Koch, C.; Mählmann, K.; Poniewierska, B. Genomic landscape of copy number variation and copy neutral loss of heterozygosity events in equine sarcoids reveals increased instability of the sarcoid genome. Biochimie 2017, 140, 122–132. [Google Scholar] [CrossRef]
- Unger, L.; Jagannathan, V.; Pacholewska, A.; Leeb, T.; Gerber, V. Differences in miRNA differential expression in whole blood between horses with sarcoid regression and progression. J. Veter-Intern. Med. 2018, 33, 241–250. [Google Scholar] [CrossRef]
- Unger, L.; Gerber, V.; Pacholewska, A.; Leeb, T.; Jagannathan, V. MicroRNA fingerprints in serum and whole blood of sarcoid-affected horses as potential non-invasive diagnostic biomarkers. Veter-Comp. Oncol. 2018, 17, 107–117. [Google Scholar] [CrossRef]
- Taylor, S.; Haldorson, G. A review of equine sarcoid. Equine Veter-Educ. 2012, 25, 210–216. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. TheHallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Haaksma, C.J.; Eddy, R.J.; Vaughan, M. Fibroblast contraction occurs on release of tension in attached collagen lattices: Dependency on an organized actin cytoskeleton and serum. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 1992, 232, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Teifke, J.P.; Kidney, B.A.; Löhr, C.; Yager, J.A. Detection of papillomavirus-DNA in mesenchymal tumour cells and not in the hyperplastic epithelium of feline sarcoids. Veter-Dermatol. 2003, 14, 47–56. [Google Scholar] [CrossRef]
- Primer3. Available online: http://bioinfo.ut.ee/primer3-0.4.0 (accessed on 17 July 2017).
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- Fife, C.M.; A McCarroll, J.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef] [PubMed]
- Stengel, K.; Zheng, Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell. Signal. 2011, 23, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kenney, S.R.; Muller, C.Y.; Adams, S.; Rutledge, T.; Romero, E.; Murray-Krezan, C.; Prekeris, R.; Sklar, L.A.; Hudson, L.G.; et al. R-Ketorolac Targets Cdc42 and Rac1 and Alters Ovarian Cancer Cell Behaviors Critical for Invasion and Metastasis. Mol. Cancer Ther. 2015, 14, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in Normal Skin and in Pathological Processes. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef]
- Vihinen, P.; Kähäri, V.-M. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int. J. Cancer 2002, 99, 157–166. [Google Scholar] [CrossRef]
- Yuan, Z.; Gobeil, P.A.; Campo, M.S.; Nasir, L. Equine sarcoid fibroblasts over-express matrix metalloproteinases and are invasive. Virology 2010, 396, 143–151. [Google Scholar] [CrossRef]
- Anania, M.C.; Sensi, M.; Radaelli, E.; Miranda, C.; Vizioli, M.G.; Pagliardini, S.; Favini, E.; Cleris, L.; Supino, R.; Formelli, F.; et al. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 2011, 30, 3011–3023. [Google Scholar] [CrossRef]
- Adissu, H.A.; McKerlie, C.; Di Grappa, M.; Waterhouse, P.; Xu, Q.; Fang, H.; Khokha, R.; Wood, G.A. Timp3 loss accelerates tumour invasion and increases prostate inflammation in a mouse model of prostate cancer. Prostate 2015, 75, 1831–1843. [Google Scholar] [CrossRef]
- Williams, H.C.; Martin, A.S.; Adamo, C.M.; Seidel-Rogol, B.; Pounkova, L.; Datla, S.R.; Lassègue, B.; Bear, J.E.; Griendling, K. Role of coronin 1B in PDGF-induced migration of vascular smooth muscle cells. Circ. Res. 2012, 111, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Park, J.-H.; Kim, H.; Lim, H.-J.; Park, H.-Y. Coronin 1B serine 2 phosphorylation by p38α is critical for vascular endothelial growth factor-induced migration of human umbilical vein endothelial cells. Cell. Signal. 2016, 28, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Wee, K.; Budnar, S.; Gomez, G.A.; Yap, A.S.; Michael, M. Coronin 1B supports RhoA signaling at cell-cell junctions through Myosin II. Cell Cycle 2016, 15, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.M.; Bayless, K.J. Vimentin as an Integral Regulator of Cell Adhesion and Endothelial Sprouting. Microcirc. 2014, 21, 333–344. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Product Length (bp) |
|---|---|---|
| CDC42bpα | F:GCTCCATTCAAACGACCACA | 176 |
| R:AAGGATTTGCTGGCCACATC | ||
| CORO1b | F:AGATCGCCCGGTTCTACAAA | 179 |
| R:CAGGGAAATGAGGATGGGGT | ||
| MMP2 | F:TCCCACTTTGATGACGACGA | 182 |
| R:AAGTTGTAGGTGGTGGAGCA | ||
| TIMP3 | F:AAGATGCCCCATGTGCAGTA | 213 |
| R:TGCAGTTACAACCCAGGTGA | ||
| VIM | F:ACAAGTCCAAGTTTGCCGAC | 262 |
| R:CGCGCCATTTCTTTCCTTCAT | ||
| GAPDH | F:TCACCAGGGCTGCTTTTAAC | 156 |
| R:GCCTTTCCGTTGATGACAAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podstawski, P.; Witarski, W.; Szmatoła, T.; Bugno-Poniewierska, M.; Ropka-Molik, K. Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid. Animals 2020, 10, 880. https://doi.org/10.3390/ani10050880
Podstawski P, Witarski W, Szmatoła T, Bugno-Poniewierska M, Ropka-Molik K. Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid. Animals. 2020; 10(5):880. https://doi.org/10.3390/ani10050880
Chicago/Turabian StylePodstawski, Przemysław, Wojciech Witarski, Tomasz Szmatoła, Monika Bugno-Poniewierska, and Katarzyna Ropka-Molik. 2020. "Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid" Animals 10, no. 5: 880. https://doi.org/10.3390/ani10050880
APA StylePodstawski, P., Witarski, W., Szmatoła, T., Bugno-Poniewierska, M., & Ropka-Molik, K. (2020). Mobility and Invasion Related Gene Expression Patterns in Equine Sarcoid. Animals, 10(5), 880. https://doi.org/10.3390/ani10050880





