Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
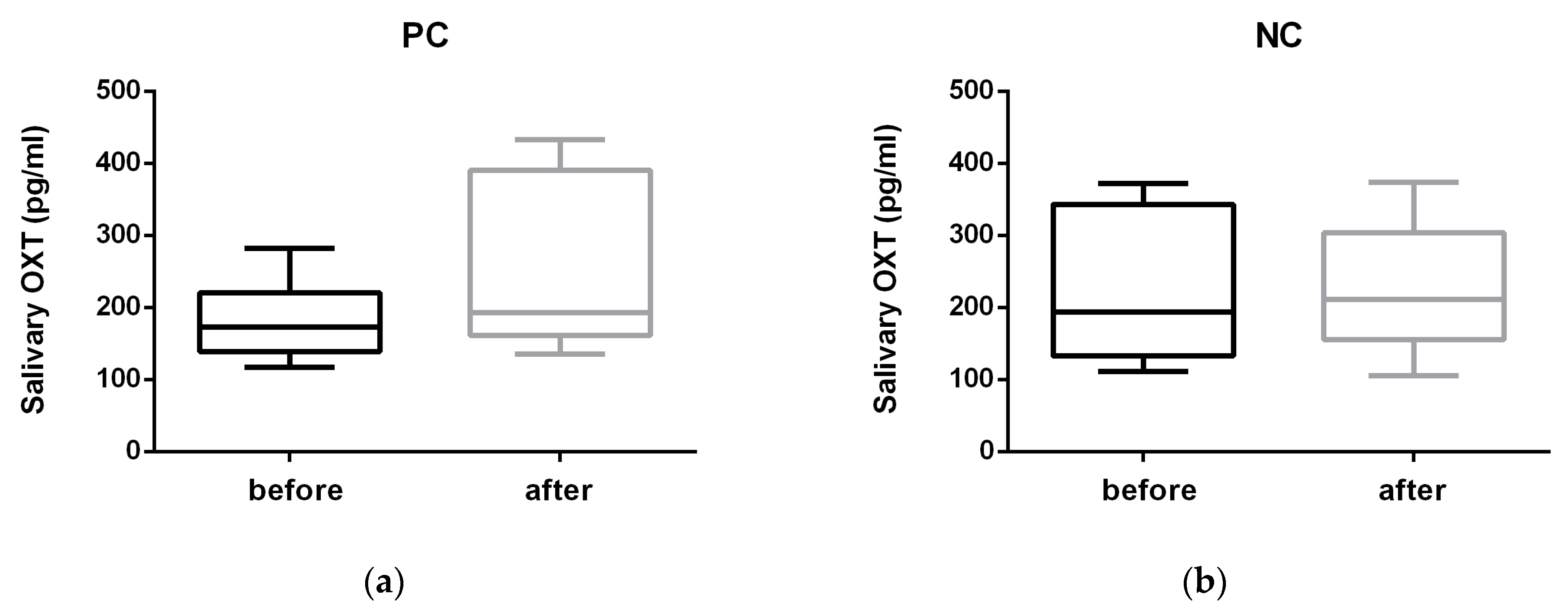
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, D.M.; Sue Carter, C.; Walton, D.M. Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol. Biochem. Behav. 1990, 37, 63–69. [Google Scholar] [CrossRef]
- Insel, T.R.; Shapiro, L.E. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. 1992, 89, 5981–5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.R.; Insel, T.R.; Harbaugh, C.R.; Carter, C.S. Oxytocin Administered Centrally Facilitates Formation of a Partner Preference in Female Prairie Voles (Microtus ochrogaster). J. Neuroendocrinol. 1994, 6, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.M. Oxytocin and rodent sociosexual responses: From behavior to gene expression. Neurosci. Biobehav. Rev. 1995, 19, 315–324. [Google Scholar] [CrossRef]
- Insel, T.R.; Hulihan, T.J. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 1995, 109, 782. [Google Scholar] [CrossRef]
- Olazabal, D.E.; Young, L.J. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 2006, 141, 559–568. [Google Scholar] [CrossRef]
- Ross, H.E.; Cole, C.D.; Smith, Y.; Neumann, I.D.; Landgraf, R.; Murphy, A.Z.; Young, L.J. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 2009, 162, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Bosch, O.J.; Dabrowska, J.; Modi, M.E.; Johnson, Z.V.; Keebaugh, A.C.; Barrett, C.E.; Ahern, T.H.; Guo, J.; Grinevich, V.; Rainnie, D.G. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 2016, 64, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Barrett, C.E.; Arambula, S.E.; Young, L.J. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl. Psychiatry 2015, 5, e606. [Google Scholar] [CrossRef] [Green Version]
- Tabbaa, M.; Paedae, B.; Liu, Y.; Wang, Z. Neuropeptide regulation of social attachment: The prairie vole model. Compr. Physiol. 2011, 7, 81–104. [Google Scholar]
- Rincon, A.V.; Deschner, T.; Schülke, O.; Ostner, J. Oxytocin increases after affiliative interactions in male Barbary macaques. Horm. Behav. 2020, 119, 104661. [Google Scholar] [CrossRef] [PubMed]
- Crockford, C.; Wittig, R.M.; Langergraber, K.; Ziegler, T.E.; Zuberbühler, K.; Deschner, T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preis, A.; Samuni, L.; Mielke, A.; Deschner, T.; Crockford, C.; Wittig, R.M. Urinary oxytocin levels in relation to post-conflict affiliations in wild male chimpanzees (Pan troglodytes verus). Horm. Behav. 2018, 105, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Benítez, M.E.; Sosnowski, M.J.; Tomeo, O.B.; Brosnan, S.F. Urinary oxytocin in capuchin monkeys: Validation and the influence of social behavior. Am. J. Primatol. 2018, 80, e22877. [Google Scholar] [CrossRef] [PubMed]
- Wittig, R.M.; Crockford, C.; Deschner, T.; Langergraber, K.E.; Ziegler, T.E.; Zuberbühler, K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133096. [Google Scholar] [CrossRef] [Green Version]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.-J.; Liu, J.; Li, J.; Kwong, K.; Koh, M.; Sukijthamapan, P.; Guo, J.J.; Sun, Z.J.; Song, Y. Probiotics and oxytocin nasal spray as neuro-social-behavioral interventions for patients with autism spectrum disorders: A pilot randomized controlled trial protocol. Pilot Feasibility Stud. 2020, 6, 20. [Google Scholar] [CrossRef]
- Gazzano, A.; Ogi, A.; Torracca, B.; Mariti, C.; Casini, L. Plasma tryptophan/large neutral amino acids ratio in domestic dogs is affected by a single meal with high carbohydrates level. Animals 2018, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Marshall-Pescini, S.; Schaebs, F.S.; Gaugg, A.; Meinert, A.; Deschner, T.; Range, F. The role of oxytocin in the dog–owner relationship. Animals 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Kis, A.; Ciobica, A.; Topál, J. The effect of oxytocin on human-directed social behaviour in dogs (Canis familiaris). Horm. Behav. 2017, 94, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odendaal, J.S.J.; Meintjes, R.A. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 2003, 165, 296–301. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary oxytocin as a noninvasive biomarker of positive emotion in dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Romero, T.; Nagasawa, M.; Mogi, K.; Hasegawa, T.; Kikusui, T. Oxytocin promotes social bonding in dogs. Proc. Natl. Acad. Sci. USA 2014, 111, 9085–9090. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef]
- Handlin, L.; Hydbring-Sandberg, E.; Nilsson, A.; Ejdebäck, M.; Jansson, A.; Uvnäs-Moberg, K. Short-Term Interaction between Dogs and Their Owners: Effects on Oxytocin, Cortisol, Insulin and Heart Rate—An Exploratory Study. Anthrozoos 2011, 24, 301–315. [Google Scholar] [CrossRef]
- Handlin, L.; Nilsson, A.; Ejdebäck, M.; Hydbring-Sandberg, E.; Uvnäs-Moberg, K. Associations between the psychological characteristics of the human–dog relationship and oxytocin and cortisol levels. Anthrozoos 2012, 25, 215–228. [Google Scholar] [CrossRef]
- Rehn, T.; Handlin, L.; Uvnäs-Moberg, K.; Keeling, L.J. Dogs’ endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiol. Behav. 2014, 124, 45–53. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of affiliative human-animal interaction on dog salivary and plasma oxytocin and vasopressin. Front. Psychol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Jonas, W.; Johansson, L.M.; Nissen, E.; Ejdebäck, M.; Ransjö-Arvidson, A.B.; Uvnäs-Moberg, K. Effects of intrapartum oxytocin administration and epidural analgesia on the concentration of plasma oxytocin and prolactin, in response to suckling during the second day postpartum. Breastfeed. Med. 2009, 4, 71–82. [Google Scholar] [CrossRef]
- Brandtzaeg, O.K.; Johnsen, E.; Roberg-Larsen, H.; Seip, K.F.; MacLean, E.L.; Gesquiere, L.R.; Leknes, S.; Lundanes, E.; Wilson, S.R. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Promises and pitfalls of hormone research in human-animal interaction. In How Animals Affect Us: Examining the Influence of Human-Animal Interaction on Child Development and Human Health; Peggy McCardle James, A., Griffin Valerie Maholmes, S.M., Eds.; American Psychological Association (APA), School of Life Sciences, University of Skövde: Skövde, Sweden, 2011; ISBN 978-1-4338-0865-4. [Google Scholar]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.; Levy, K.; Martin, W.L.; Carter, C.S. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods 2018, 293, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulon, M.; Nowak, R.; Andanson, S.; Ravel, C.; Marnet, P.G.; Boissy, A.; Boivin, X. Human-lamb bonding: Oxytocin, cortisol and behavioural responses of lambs to human contacts and social separation. Psychoneuroendocrinology 2013, 38, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Onaka, T.; Takayanagi, Y. Role of oxytocin in the control of stress and food intake. J. Neuroendocrinol. 2019, 31, e12700. [Google Scholar] [CrossRef]
- Buttner, A.P. Neurobiological underpinnings of dogs’ human-like social competence: How interactions between stress response systems and oxytocin mediate dogs’ social skills. Neurosci. Biobehav. Rev. 2016, 71, 198–214. [Google Scholar] [CrossRef]
- Marazziti, D.; Dell’Osso, B.; Baroni, S.; Mungai, F.; Catena, M.; Rucci, P.; Albanese, F.; Giannaccini, G.; Betti, L.; Fabbrini, L. A relationship between oxytocin and anxiety of romantic attachment. Clin. Pract. Epidemiol. Ment. Heal. 2006, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Tabak, B.A.; McCullough, M.E.; Szeto, A.; Mendez, A.J.; McCabe, P.M. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology 2011, 36, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Torres, N.; Martins, D.; Santos, A.J.; Prata, D.; Veríssimo, M. How do hypothalamic nonapeptides shape youth’s sociality? A systematic review on oxytocin, vasopressin and human socio-emotional development. Neurosci. Biobehav. Rev. 2018, 90, 309–331. [Google Scholar] [CrossRef]
- McQuaid, R.J.; McInnis, O.A.; Paric, A.; Al-Yawer, F.; Matheson, K.; Anisman, H. Relations between plasma oxytocin and cortisol: The stress buffering role of social support. Neurobiol. Stress 2016, 3, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Ng, Z.Y.; Pierce, B.J.; Otto, C.M.; Buechner-Maxwell, V.A.; Siracusa, C.; Werre, S.R. The effect of dog–human interaction on cortisol and behavior in registered animal-assisted activity dogs. Appl. Anim. Behav. Sci. 2014, 159, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Buttner, A.P.; Thompson, B.; Strasser, R.; Santo, J. Evidence for a synchronization of hormonal states between humans and dogs during competition. Physiol. Behav. 2015, 147, 54–62. [Google Scholar] [CrossRef]
- Mariti, C.; Carlone, B.; Protti, M.; Diverio, S.; Gazzano, A. Effects of petting before a brief separation from the owner on dog behavior and physiology: A pilot study. J. Vet. Behav. 2018, 27, 41–46. [Google Scholar] [CrossRef]
- Mongillo, P.; Pitteri, E.; Carnier, P.; Gabai, G.; Adamelli, S.; Marinelli, L. Does the attachment system towards owners change in aged dogs? Physiol. Behav. 2013, 120, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Guardini, G.; Mariti, C.; Bowen, J.; Fatjó, J.; Ruzzante, S.; Martorell, A.; Sighieri, C.; Gazzano, A. Influence of morning maternal care on the behavioural responses of 8-week-old Beagle puppies to new environmental and social stimuli. Appl. Anim. Behav. Sci. 2016, 181, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Tod, E.; Brander, D.; Waran, N. Efficacy of dog appeasing pheromone in reducing stress and fear related behaviour in shelter dogs. Appl. Anim. Behav. Sci. 2005, 93, 295–308. [Google Scholar] [CrossRef]
- Mariti, C.; Ricci, E.; Carlone, B.; Moore, J.L.; Sighieri, C.; Gazzano, A. Dog attachment to man: A comparison between pet and working dogs. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 135–145. [Google Scholar] [CrossRef]
- Prato-Previde, E.; Custance, D.M.; Spiezio, C.; Sabatini, F. Is the dog—Human relationship an attachment bond ? An observational study using Ainsworth’ s strange situation. Behaviour 2003, 140, 225–254. [Google Scholar] [CrossRef]
- Palestrini, C.; Previde, E.P.; Spiezio, C.; Verga, M. Heart rate and behavioural responses of dogs in the Ainsworth’s Strange Situation: A pilot study. Appl. Anim. Behav. Sci. 2005, 94, 75–88. [Google Scholar] [CrossRef]
- Mariti, C.; Carlone, B.; Ricci, E.; Sighieri, C.; Gazzano, A. Intraspecific attachment in adult domestic dogs (Canis familiaris): Preliminary results. Appl. Anim. Behav. Sci. 2014, 152, 64–72. [Google Scholar] [CrossRef]
- Parthasarathy, V.; Crowell-Davis, S.L. Relationship between attachment to owners and separation anxiety in pet dogs (Canis lupus familiaris). J. Vet. Behav. Clin. Appl. Res. 2006, 1, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Dreschel, N.A.; Granger, D.A. Physiological and behavioral reactivity to stress in thunderstorm-phobic dogs and their caregivers. Appl. Anim. Behav. Sci. 2005, 95, 153–168. [Google Scholar] [CrossRef]
- Owczarczak-Garstecka, S.C.; Burman, O.H.P. Can sleep and resting behaviours be used as indicators of welfare in shelter dogs (Canis lupus familiaris)? PLoS One 2016, 11, e0163620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.A.R.A.; de Vries, H.W.; Mol, J.A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Rooney, N.; Gaines, S.; Hiby, E. A practitioner’s guide to working dog welfare. J. Vet. Behav. 2009, 4, 127–134. [Google Scholar] [CrossRef]
- Kotrschal, K.; Schöberl, I.; Bauer, B.; Thibeaut, A.M.; Wedl, M. Dyadic relationships and operational performance of male and female owners and their male dogs. Behav. Processes 2009, 81, 383–391. [Google Scholar] [CrossRef]
- Cozzi, A.; Mariti, C.; Ogi, A.; Sighieri, C.; Gazzano, A. Behavioral modification in sheltered dogs. Dog Behav. 2016, 2, 1–12. [Google Scholar]
- Scaglia, E.; Cannas, S.; Minero, M.; Frank, D.; Bassi, A.; Palestrini, C. Video analysis of adult dogs when left home alone. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 412–417. [Google Scholar] [CrossRef]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Domest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K.; Nilsson, A.; Gustafson, L.L.; Hydbring-Sandberg, E.; Handlin, L. Oxytocin and cortisol levels in dog owners and their dogs are associated with behavioral patterns: An exploratory study. Front. Psychol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski Jr, G.W.; Mattingly, B.A.; Pedreiro, A. Under pressure: The effects of stress on positive and negative relationship behaviors. J. Soc. Psychol. 2014, 154, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, F.; Ripamonti, A.; Garoni, E.C.; Stradiotti, S.; Albertini, M. Measuring social synchrony and stress in the handler-dog dyad during animal-assisted activities: A pilot study. J. Vet. Behav. Clin. Appl. Res. 2017, 21, 45–52. [Google Scholar] [CrossRef]
- Brown, D. Personality and gender influences on human relationships with horses and dogs. In Pet Connection: Its Influence on Our Health and Quality of Life; Anderson, R.K., Hart, B.L., Hart, L.A., Eds.; Cent to Study Human-Animal Relat and Environ, Univ of Minnesota: Minneapolis, MN, USA, 1984. [Google Scholar]
- Prato-Previde, E.; Fallani, G.; Valsecchi, P. Gender differences in owners interacting with pet dogs: An observational study. Ethology 2006, 112, 64–73. [Google Scholar] [CrossRef]
- Javor, A.; Riedl, R.; Kindermann, H.; Brandstätter, W.; Ransmayr, G.; Gabriel, M. Correlation of plasma and salivary oxytocin in healthy younq men - Experimental evidence. Neuroendocrinol. Lett. 2014, 35, 470–473. [Google Scholar]
- Grewen, K.M.; Davenport, R.E.; Light, K.C. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology 2010, 47, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Feldman, R.; Gordon, I.; Schneiderman, I.; Weisman, O.; Zagoory-Sharon, O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology 2010, 35, 1133–1141. [Google Scholar] [CrossRef]
- Feldman, R.; Gordon, I.; Zagoory-Sharon, O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev. Sci. 2011, 14, 752–761. [Google Scholar] [CrossRef]
- Hernandez, C.E.; Thierfelder, T.; Svennersten-Sjaunja, K.; Berg, C.; Orihuela, A.; Lidfors, L. Time lag between peak concentrations of plasma and salivary cortisol following a stressful procedure in dairy cattle. Acta Vet. Scand. 2014, 56, 61. [Google Scholar] [CrossRef] [Green Version]
- Cimarelli, G.; Turcsán, B.; Bánlaki, Z.; Range, F.; Virányi, Z. Dog Owners’ Interaction Styles: Their Components and Associations with Reactions of Pet Dogs to a Social Threat. Front. Psychol. 2016, 7, 1979. [Google Scholar] [CrossRef] [Green Version]
| ETHOGRAM | ||
|---|---|---|
| Behavior | Definition | References |
| Lying (SE) | Ventral/lateral lying on ground with all four legs resting and in contact with ground | [46] |
| Sitting (SE) | Hindquarters on ground with front two legs being used for support | [46,47] |
| Standing (SE) | All four paws on ground and legs upright and extended, supporting body | [46,47] |
| Exploration (SE) | Activity directed towards physical aspects of the environment, including sniffing, close visual inspection, distal visual inspection, and gentle oral examination, such as licking | [48,49,50] |
| Locomotion (SE) | Walking, running, pacing around without exploring the environment or playing | [46] |
| Attention oriented to the door (SE) | Staring fixedly at the door, either when close to it or from a distance | [48] |
| Behaviors oriented to the door (SE) | All active behaviors resulting in physical contact with the door, including scratching the door with the paws, jumping on the door, pulling on the door handle with the forelegs or mouth | [48,51] |
| Barking (SE) | Sharp explosive vocalization | [46,51] |
| Howling (SE) | Low-pitched, long-duration vocalization | [46,52] |
| Whining/Yelping (SE) | Whine: High-pitched vocalization Yelp: loud (relative to whining), high-pitched vocalization | [46] |
| Growling (SE) | Deep threatening rumble, with or without exposed teeth | [46] |
| Panting (SE) | Mouth open, tongue can be outside of mouth, quick and shallow breathing (inhalations/exhalations visible) | [53,54] |
| Paw lifting (PE) | A forepaw is lifted to a position of approximately 45° | [55] |
| Body shaking (SE) | The dog shakes his/her body | [46] * |
| Nose licking (SE) | Tongue extends upwards to cover nose, before retracting into mouth | [46,47] |
| Yawn (PE) | Mouth widely opened for a period of a few seconds, then closed | [46,47] |
| Tongue out (PE) | Licking the lips or the nose, and also keeping the tongue out even if it is not licking any part of the snout | [55,56,57] |
| OTHER BEHAVIORS | Any activity not included in the behavioral catalogue, such as self-grooming, digging, or circling | [46,55] |
| OBSERVED BEHAVIORS | ||||
|---|---|---|---|---|
| Behavior | Frequency min-max | Median | Duration min-max (%) | Median (%) |
| Lying | 0–2 | 1 | 0.0–81.5 | 51.5 |
| Sitting | 0–1 | 1 | 0.0–64.1 | 6.8 |
| Standing | 1–13 | 3.5 | 5.8–77.2 | 27.7 |
| Exploration | 1–4 | 3 | 0.8–10.4 | 1.8 |
| Locomotion | 0–13 | 2 | 0.0–72.0 | 3.2 |
| Attention oriented to the door | 2–24 | 13.5 | 5.3–78.7 | 46.0 |
| Behaviors oriented to the door | 0–2 | 0 | 0.0–1.1 | 0.0 |
| Barking | 0–3 | 0 | 0.0–0.6 | 0.0 |
| Whining/Yelping | 0–32 | 6.5 | 0.0–57.7 | 5.7 |
| Body shaking | 0–1 | 0 | 0.0–0.6 | 0.0 |
| SALIVARY ΔOXT AND CORTISOL DURING NC | ||||||
|---|---|---|---|---|---|---|
| Trainer | Dog № Sex | ΔOT (pg/mL) | CORTISOL (µg/dL) | ΔOXT Rank | CORTISOL Rank | |
| A | 1 | F | 77.5 | 0.233 | 8 | 8 |
| A | 2 | F | 15.85 | 0.116 | 4 | 4 |
| B | 3 | M | 20.77 | 0.164 | 5 | 6 |
| B | 4 | M | −45.83 | 0.067 | 2 | 1 |
| C | 5 | F | 37.87 | 0.114 | 6 | 3 |
| C | 6 | M | −31.86 | 0.129 | 3 | 5 |
| D | 7 | M | 66.36 | 0.176 | 7 | 7 |
| D | 8 | F | −162.19 | 0.111 | 1 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogi, A.; Mariti, C.; Baragli, P.; Sergi, V.; Gazzano, A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals 2020, 10, 708. https://doi.org/10.3390/ani10040708
Ogi A, Mariti C, Baragli P, Sergi V, Gazzano A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals. 2020; 10(4):708. https://doi.org/10.3390/ani10040708
Chicago/Turabian StyleOgi, Asahi, Chiara Mariti, Paolo Baragli, Valeria Sergi, and Angelo Gazzano. 2020. "Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results" Animals 10, no. 4: 708. https://doi.org/10.3390/ani10040708
APA StyleOgi, A., Mariti, C., Baragli, P., Sergi, V., & Gazzano, A. (2020). Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals, 10(4), 708. https://doi.org/10.3390/ani10040708






