In Vitro Methods of Assessing Protein Quality for Poultry
Simple Summary
Abstract
1. Introduction
2. Methods of Assessing Protein Quality
2.1. Chemical In Vitro Methods
2.2. pH-Stat/Drop Method
2.3. Closed Enzymatic Methods
2.3.1. Pepsin Assay
2.3.2. Pancreatin
2.3.3. Multi-Enzymatic Assays
3. Factors Influencing Protein Digestion
3.1. Enzyme Specificity
3.2. Protein Structure and Forms
3.3. Enzyme Activity
3.4. Anti Nutritive Agents of Test Samples
3.4.1. Sinapine and Tannins
3.4.2. Protease Inhibitors
3.4.3. Phytate
3.4.4. Effects of Ingredient Processing
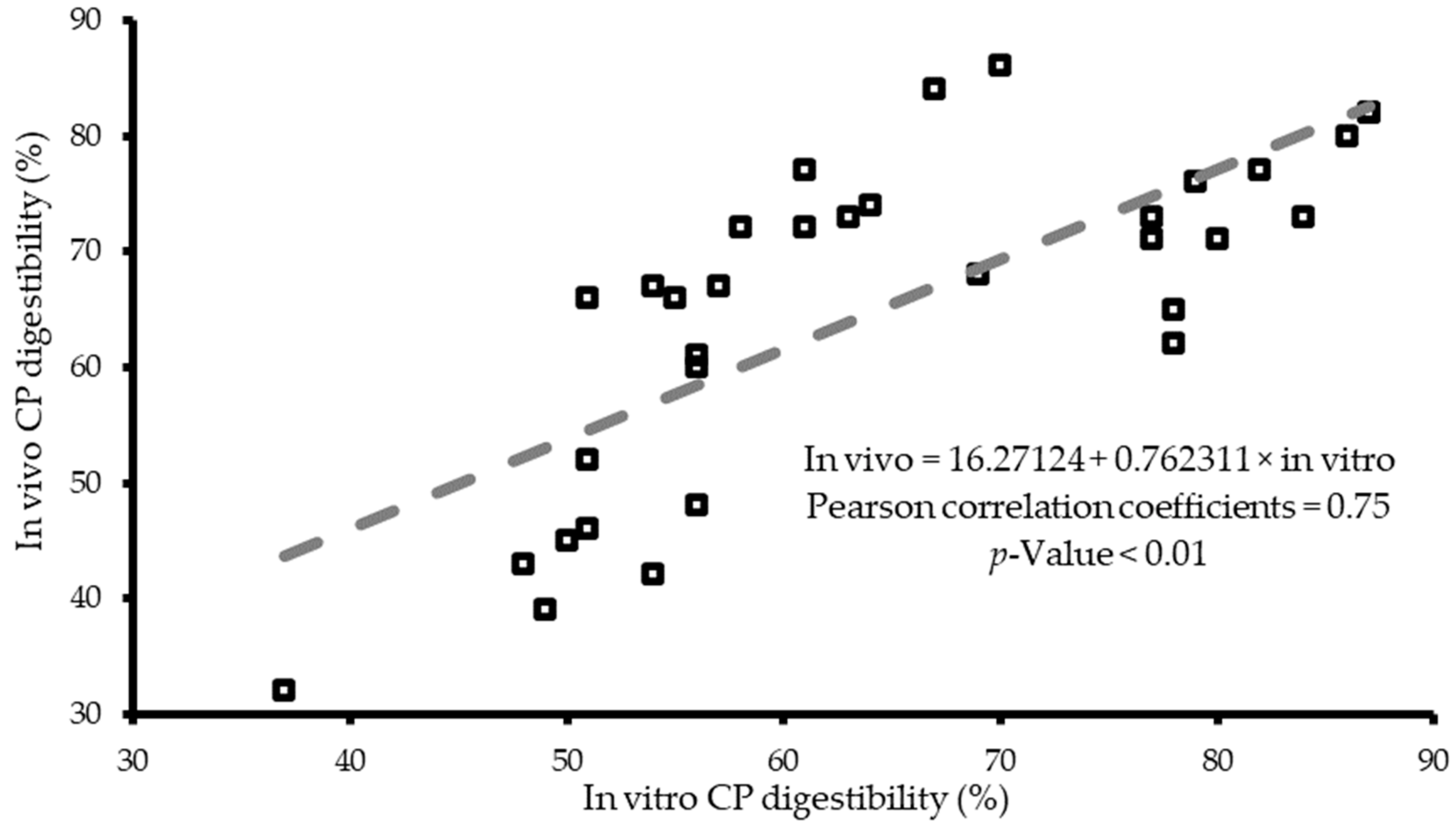
4. In Vitro Digestibility Systems Validation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parsons, C.; Castanon, F.; Han, Y. Protein and amino acid quality of meat and bone meal. Poult. Sci. 1997, 76, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, S.A.; Adeola, O.; Parsons, C.M.; Lilburn, M.S.; Applegate, T.J. Standardized ileal amino acid digestibility of plant feedstuffs in broiler chickens and turkey poults using a nitrogen-free or casein diet. Poult.Sci. 2008, 87, 2535–2548. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Utterback, P.L.; Applegate, T.J.; Parsons, C.M. Comparison of amino acid digestibility of feedstuffs determined with the precision-fed cecectomized rooster assay and the standardized ileal amino acid digestibility assay. Poult. Sci. 2011, 90, 2511–2519. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Bhagavan, N.V. Medical Biochemistry; Jones and Bartlett Publishers: Boston, UK, 1992. [Google Scholar]
- Ravindran, V.; Bryden, W.L. Amino acid availability in poultry—In vitro and in vivo measurements. Aust. J. Agric. Res. 1999, 50, 889–908. [Google Scholar] [CrossRef]
- Lemme, A.; Ravindran, V.; Bryden, W.L. Ileal digestibility of amino acids in feed ingredients for broilers. World’s Poult. Sci. J. 2004, 60, 423–438. [Google Scholar] [CrossRef]
- Batterham, E.S. Availability and utilization of amino acids for growing pigs. Nutr. Res. Rev. 1992, 5, 1–18. [Google Scholar] [CrossRef]
- Boisen, S.; Eggum, B.O. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr. Res. Revi. 1991, 4, 141–162. [Google Scholar] [CrossRef]
- Clunies, M.; Leeson, S. In vitro estimation of dry matter and crude protein digestibility. Poult. Sci. 1984, 63, 89–96. [Google Scholar] [CrossRef]
- Fuller, M.F. Vitro Digestion for Pigs and Poultry; C.A.B. International: Wallingford, UK, 1991; ISBN 0-85198-719-2. [Google Scholar]
- Smith, A.K.; Circle, S.J.; Brother, G.H. Peptization of soybean proteins. The effect of neutral salts on the quantity of nitrogenous constituents extracted from oil-free meal. J. Am. Chem. Soc. 1938, 60, 1316–1320. [Google Scholar] [CrossRef]
- Araba, M.; Dale, N.M. Evaluation of protein solubility as an indicator of over processing soybean meal. Poult. Sci. 1990, 69, 76–83. [Google Scholar] [CrossRef]
- Lee, H.; Garlich, J.D. Effect of overcooked soybean meal on chicken performance and amino acid availability. Poult. Sci. 1992, 71, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Lyman, C.M.; Chang, W.Y.; Couch, J.R. Evaluation of protein quality in cottonseed meals by chick growth and by a chemical index method. J. Nutr. 1953, 49, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.M.; Hashimoto, K.; Wedekind, K.J.; Baker, D.H. Soybean protein solubility in potassium hydroxide: An in vitro test of in vivo protein quality. J. Anim. Sci. 1991, 69, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.R.; Zhang, Y.; Parsons, C.M. Determination of protein solubility in oilseed meals using coomassie blue dye binding. Poult. Sci. 1993, 72, 1925–1930. [Google Scholar] [CrossRef]
- Batal, A.; Douglas, M.; Engram, A.; Parsons, C. Protein dispersibility index as an indicator of adequately processed soybean meal. Poult. Sci. 2000, 79, 1592–1596. [Google Scholar] [CrossRef]
- Newkirk, R.W.; Classen, H.L.; Scott, T.A.; Edney, M.J. The digestibility and content of amino acids in toasted and non-toasted canola meals. Can. J. Anim. Sci. 2003, 83, 131–139. [Google Scholar] [CrossRef]
- Johnson, D.W. Functional properties of oilseed proteins. J. Am. Oil Chem. Soc. 1970, 47, 402–407. [Google Scholar] [CrossRef]
- Veltmann, J.R.; Hansen, B.C.; Tanksley, T.D.; Knabe, D.; Linton, A.S. Comparison of the nutritive value of different heat-treated commercial soybean meals: Utilization by chicks in practical type rations. Poult. Sci. 1986, 65, 1561–1570. [Google Scholar] [CrossRef]
- Clarke, E.; Wiseman, J. Effects of variability in trypsin inhibitor content of soya bean meals on true and apparent ileal digestibility of amino acids and pancreas size in broiler chicks. Anim. Feed Sci. Technol. 2005, 121, 125–138. [Google Scholar] [CrossRef]
- De Coca-Sinova, A.; Valencia, D.G.; Jiménez-Moreno, E.; Lázaro, R.; Mateos, G.G. Apparent ileal digestibility of energy, nitrogen, and amino acids of soybean meals of different origin in broilers. Poult. Sci. 2008, 87, 2613–2623. [Google Scholar] [CrossRef]
- Serrano, M.P.; Valencia, D.G.; Méndez, J.; Mateos, G.G. Influence of feed form and source of soybean meal of the diet on growth performance of broilers from 1 to 42 days of age. 1. Floor pen study. Poult. Sci. 2012, 91, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Calvo, E.; Castrillo, C.; Baucells, M.D.; Guada, J.A. Effect of rendering on protein and fat quality of animal by-products. J. Anim. Physiol. Anim. Nutr. 2010, 94, e154–e163. [Google Scholar] [CrossRef] [PubMed]
- Maga, J.A.; Lorenz, K.; I, O.O. Digestive acceptability of proteins as measured by the initial rate of in vitro proteolysis. J. Food Sci. 1973, 38, 173–174. [Google Scholar] [CrossRef]
- Vavak, D.L.R. A Nutritional Characterization of the Distiller’s Grain Protein Concentrates. Ph.D. Thesis, University of Nebraska—Lincoln, Lincoln, NE, USA, 1975. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Satterlee, L.D.; Kendrick, J.G.; Marshall, H.F.; Jewell, D.K.; Ali, R.A.; Heckman, M.M.; Steinke, H.F.; Larson, P.; Phillips, R.D.; Sarwar, G. In vitro assay for predicting protein efficiency ratio as measured by rat bioassay: Collaborative study Milk, chicken, soy protein, cereals, wheat flour, nutritional quality. J. AOAC Int. 1982, 65, 798–809. [Google Scholar] [CrossRef]
- Pedersen, B.; Eggum, B.O. Prediction of protein digestibility by an in vitro enzymatic pH-stat procedure. Z. Tierphysiol. Tierernähr. Futtermittelkd. 1983, 49, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Rozan, P.; EL Kossori, R.L.; Fanni, J.; Villaume, C.; Mejean, L.; Parmentier, M. Nutritional value of veal bone hydrolysate. J. Food Sci. 1997, 62, 183–189. [Google Scholar] [CrossRef]
- Wang, H.; Faris, R.J.; Wang, T.; Spurlock, M.E.; Gabler, N. Increased in vitro and in vivo digestibility of soy proteins by chemical modification of disulfide bonds. J. Am. Oil Chem. Soc. 2009, 86, 1093–1099. [Google Scholar] [CrossRef]
- Dimes, L.E.; Haard, N.F. Estimation of protein digestibility I. Development of an in vitro method for estimating protein digestibility in salmonids (Salmo gairdneri). Comp. Biochem. Physiol. A Physiol. 1994, 108, 349–362. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Ross, N.W.; Verreth, J.A.J.; Lall, S.P. In vitro pH-Stat protein hydrolysis of feed ingredients for Atlantic cod, Gadus morhua. 1. Development of the method. Aquaculture 2011, 319, 398–406. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Vachon, C.; Jones, J.D.; Savoie, L. Assessment of protein digestibility by in vitro enzymatic hydrolysis with simultaneous dialysis. J. Nutr. 1982, 112, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Gehrt, A.J.; Caldwell, M.J.; Elmslie, W.P. Feed digestibility, chemical method for measuring relative digestibility of animal protein feedstuffs. J. Agric. Food Chem. 1955, 3, 159–162. [Google Scholar] [CrossRef]
- Sheffner, A.L.; Eckfeldt, G.A.; Spector, H. The pepsin-digest-residue (PDR) amino acid index of net protein utilization. J. Nutr. 1956, 60, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Coon, C.N. A comparison of six protein quality assays using commercially available protein meals. Poult. Sci. 1979, 58, 919–927. [Google Scholar] [CrossRef]
- Johnson, J.; Coon, C.N. The use of varying levels of pepsin for pepsin digestion studies with animal proteins. Poult. Sci. 1979, 58, 1271–1273. [Google Scholar] [CrossRef]
- Riesen, W.H.; Clandinin, D.R.; Elvehjem, C.A.; Cravens, W.W. Liberation of essential amino acids from raw, properly heated, and overheated soy bean oil meal. J. Biol. Chem. 1947, 167, 143–150. [Google Scholar] [PubMed]
- Ingram, G.R.; Riesen, W.W.; Cravens, W.W.; Elvehjem, C.A. Evaluating soybean oil meal protein for chick growth by enzymatic release of amino acids. Poult. Sci. 1949, 28, 898–902. [Google Scholar] [CrossRef]
- Anwar, A. Evaluation of proteins by in vitro pancreatin digestion. Poult. Sci. 1962, 41, 1120–1123. [Google Scholar] [CrossRef]
- Altangerel, B.; Sengee, Z.; Kramarova, D.; Rop, O.; Hoza, I. The determination of water-soluble vitamins and in vitro digestibility of selected Czech cheeses. Int. J. Food Sci. Technol. 2011, 46, 1225–1230. [Google Scholar] [CrossRef]
- Bielorai, R.; Harduf, Z.; Iosif, B.; Alumot, E. Apparent amino acid absorption from feather meal by chicks. Brit. J. Nutr. 1983, 49, 395–399. [Google Scholar] [CrossRef]
- Akeson, W.R.; Stahmann, M.A. A pepsin pancreatin digest index of protein quality evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.M.; Connor, M.A.; Booth, A.N.; Bickoff, E.M.; Kohler, G.O. Measurement of digestibility of alfalfa protein concentrates by in vivo and in vitro methods. J. Nutr. 1973, 103, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Cave, N.A. Bioavailability of amino acids in plant feedstuffs determined by in vitro digestion, chick growth assay, and true amino acid availability methods. Poult. Sci. 1988, 67, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Boisen, S.; Fernández, J.A. Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mixtures for pigs by in vitro analyses. Anim. Feed Sci. Technol. 1995, 51, 29–43. [Google Scholar] [CrossRef]
- Saleh, F.; Ohtsuka, A.; Tanaka, T.; Hayashi, K. Effect of enzymes of microbial origin on in vitro digestibilities of dry matter and crude protein in maize. J. Poult. Sci. 2003, 40, 274–281. [Google Scholar] [CrossRef]
- Saleh, F.; Ohtsuka, A.; Tanaka, T.; Hayashi, K. Carbohydrases are digested by proteases present in enzyme preparations during in vitro digestion. J. Poult. Sci. 2004, 41, 229–235. [Google Scholar] [CrossRef]
- Tahir, M.; Saleh, F.; Ohtsuka, A.; Hayashi, K. An effective combination of carbohydrases that enables reduction of dietary protein in broilers: Importance of hemicellulase. Poult. Sci. 2008, 87, 713–718. [Google Scholar] [CrossRef]
- Robbins, R.C. Effect of ratio of enzymes to substrate on amino acid patterns released from proteins in vitro. Int. J. Vitam. Nutr. Res. 1978, 48, 44–53. [Google Scholar]
- Mauron, J.; Mottu, F.; Bujard, E.; Egli, R.H. The availability of lysine, methionine and tryptophan in condensed milk and milk powder. In vitro digestion studies. Arch. Biochem. Biophys. 1955, 59, 433–451. [Google Scholar] [CrossRef]
- Steinhart, H.; Kirchgessner, M. In vitro digestion apparatus for the enzymatic hydrolysis of proteins. Arch. Tierernahr. 1973, 23, 449–459. [Google Scholar] [CrossRef]
- Savoie, L.; Gauthier, S.F. Dialysis cell for the in vitro measurement of protein digestibility. J. Food Sci. 1986, 51, 494–498. [Google Scholar] [CrossRef]
- Moyano, F.J.; Savoie, L. Comparison of in vitro systems of protein digestion using either mammal or fish proteolytic enzymes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 359–368. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Nutritional and antinutritional composition, in vitro amino acid availability, starch digestibility and predicted glycemic index of differentially processed mucuna beans (Mucuna pruriens var. utilis): An under-utilised legume. Food Chem. 2005, 91, 275–286. [Google Scholar] [CrossRef]
- Sáenz de Rodrigáñez, M.A.; Gander, B.; Alaiz, M.; Moyano, F.J. Physico-chemical characterization and in vitro digestibility of commercial feeds used in weaning of marine fish. Aquacult. Nutr. 2011, 17, 429–440. [Google Scholar] [CrossRef]
- Savoie, L.; Galibois, I.; Parent, G.; Charbonneau, R. Sequential release of amino acids and peptides during in vitro digestion of casein and rapeseed proteins. Nutr. Res. 1988, 8, 1319–1326. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Development of an in vitro protein digestibility assay mimicking the chicken digestive tract. Anim. Nutr. 2018, 4, 401–409. [Google Scholar] [CrossRef]
- Appel, W. Chymotrypsin: Molecular and catalytic properties. Clin. Biochem. 1986, 19, 317–322. [Google Scholar] [CrossRef]
- Straumfjord, J.V.; Hummel, J.P. Collagen digestion by dog pancreatic juice. Exp. Biol. Med. 1957, 95, 141–144. [Google Scholar] [CrossRef]
- Becker, P.M.; Yu, P. What makes protein indigestible from tissue-related, cellular, and molecular aspects? Mol. Nutr. Food Res. 2013, 57, 1695–1707. [Google Scholar] [CrossRef]
- Theander, O.; Westerlund, E.; Åman, P.; Graham, H. Plant cell walls and monogastric diets. Anim. Feed Sci. Technol. 1989, 23, 205–225. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, B.; Nyachoti, C.; Campbell, L.; Guenter, W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult. Sci. 2005, 84, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.F.; Vachon, C.; Savoie, L. Enzymatic conditions of an in vitro method to study protein digestion. J. Food Sci. 1986, 51, 960–964. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Chen, S.; Andreasson, E. Update on glucosinolate metabolism and transport. Plant Physiol. Biochem. 2001, 39, 743–758. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. An overview of the phenolics of canola and rapeseed: Chemical, sensory and nutritional significance. J. Am. Oil Chem. Soc. 1992, 69, 917–924. [Google Scholar] [CrossRef]
- Campbell, L.D.; Smith, T.K. Responses of growing chickens to high dietary contents of rapeseed meal. Brit. Poult. Sci. 1979, 20, 231–237. [Google Scholar] [CrossRef]
- Kozlowska, H.; Naczk, M.; Shahidi, F.; Zadernowski, R. Phenolic acids and tannins in rapeseed and canola. In Canola and Rapeseed; Shahidi, F., Ed.; Springer: Boston, MA, USA, 1990; pp. 193–210. ISBN 978-1-4613-6744-4. [Google Scholar]
- Mangan, J.L. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef]
- Sarwar Gilani, G.; Wu Xiao, C.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Brit. J. Nutr. 2012, 108, S315–S332. [Google Scholar] [CrossRef]
- Elkin, R.G.; Freed, M.B.; Hamaker, B.R.; Zhang, Y.; Parsons, C.M. Condensed tannins are only partially responsible for variations in nutrient digestibilities of sorghum grain cultivars. J. Agric. Food Chem. 1996, 44, 848–853. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Applegarth, A.; Furuta, F.; Lepkovsky, S. Response of the Chicken Pancreas to Raw Soybeans: Morphologic Responses, Gross and Microscopic, of the Pancreases of Chickens on Raw and Heated Soybean Diets. Poult. Sci. 1964, 43, 733–739. [Google Scholar] [CrossRef]
- Nelson, T.S.; Ferrara, L.W.; Storer, N.L. Phytate phosphorus content of feed ingredients derived from plants. Poult. Sci. 1968, 47, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, B.L.; De Boland, A.R.; Koirtyohann, S.R. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J. Agric. Food Chem. 1972, 20, 718–723. [Google Scholar] [CrossRef]
- Urbano, G.; López-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The role of phytic acid in legumes: Antinutrient or beneficial function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef]
- Selle, P.H.; Ravindran, V.; Caldwell, A.; Bryden, W.L. Phytate and phytase: Consequences for protein utilisation. Nutr. Res. Rev. 2000, 13, 255–278. [Google Scholar] [CrossRef]
- Li, Z.; Alli, I.; Kermasha, S. In-vitro α-amylase inhibitor activity-phytate relationships in proteins from Phaseolus beans. Food Res. Int. 1993, 26, 195–201. [Google Scholar] [CrossRef]
- Maenz, D.; Classen, H. Phytase activity in the small intestinal brush border membrane of the chicken. Poult. Sci. 1998, 77, 557–563. [Google Scholar] [CrossRef]
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef]
- Doiron, K.; Yu, P.; McKinnon, J.J.; Christensen, D.A. Heat-induced protein structure and subfractions in relation to protein degradation kinetics and intestinal availability in dairy cattle. J. Dairy Sci. 2009, 92, 3319–3330. [Google Scholar] [CrossRef]
- Ljøkjel, K.; Harstad, O.M.; Skrede, A. Effect of heat treatment of soybean meal and fish meal on amino acid digestibility in mink and dairy cows. Anim. Feed Sci. Technol. 2000, 84, 83–95. [Google Scholar] [CrossRef]
- Parsons, C.M.; Hashimoto, K.; Wedekind, K.J.; Han, Y.; Baker, D.H. Effect of overprocessing on availability of amino acids and energy in soybean meal. Poult. Sci. 1992, 71, 133–140. [Google Scholar] [CrossRef]
- Newkirk, R.; Classen, H. The effects of toasting canola meal on body weight, feed conversion efficiency, and mortality in broiler chickens. Poult. Sci. 2002, 81, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Mauron, J. The Maillard reaction in food; a critical review from the nutritional standpoint. Prog. Food Nutr. Sci. 1981, 5, 5–35. [Google Scholar] [PubMed]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agri. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Benzing-Purdie, L.M.; Ripmeester, J.A.; Ratcliffe, C.I. Effects of temperature on maillard reaction products. J. Agric. Food Chem. 1985, 33, 31–33. [Google Scholar] [CrossRef]
- Schroeder, L.J.; Iacobellis, M.; Smith, A.H. Influence of heat on the digestibility of meat proteins. J. Nutr. 1961, 73, 143–150. [Google Scholar] [CrossRef]
- Labuza, T.P.; Ragnarsson, J.O. Kinetic History Effect on Lipid Oxidation of Methyl Linoleate in a Model System. J. Food Sci. 1985, 50, 145–147. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L. Characterization of Protein Sources and Their Effects on Broiler Performance, Digestive Tract Morphology and Caecal Fermentation Metabolites. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2018. [Google Scholar]
- Bryan, D.D.S.L.; Abbott, D.A.; Van Kessel, A.G.; Classen, H.L. In vivo digestion characteristics of protein sources fed to broilers. Poult. Sci. 2019, 98, 3313–3325. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Digestion kinetics of protein sources determined using an in vitro chicken model. Anim. Feed Sci. Technol. 2019, 248, 106–113. [Google Scholar] [CrossRef]

| Enzymes | Bond Cleave | Reference |
|---|---|---|
| Pepsin | N-terminal of aromatic amino acids phenylalanine, tryptophan and tyrosine | [4,8] |
| Trypsin | Lysyl or arginyl peptide bond to expose lysine or arginine | [10] |
| Chymotrypsin | Aromatic or large hydrophobic amino acid residues such as tyrosine, phenylalanine, tryptophan, leucyl, methionyl, asparaginyl, and glutamyl | [4,8,60] |
| Elastase | Glycine and alanine of elastin | [4,8] |
| Carboxypeptidase A | Peptide bond adjacent to the C-terminal end of a polypeptide chain, | [4,8] |
| Carboxypeptidase B | Basic amino acids from the C-terminal end of polypeptide chains | [8] |
| Collagenase | Alpha peptides and hydrogen bonds in the superhelix of tropocollagen and collagen | [61] |
| Item | Regression Coefficients | ANOVA | In Vitro Digestible CP | |||
|---|---|---|---|---|---|---|
| Intercept | In Vitro Digestible CP | R2 | MSE | Correlation Coefficients | P-Value | |
| Aspartic acid | 3.27 | 0.83 | 0.35 | 252.55 | 0.59 | <0.01 |
| Estimate SE | 13.68 | 0.21 | - | - | - | - |
| Estimate p-Value | 0.81 | <0.01 | - | - | - | - |
| Threonine | 27.35 | 0.55 | 0.35 | 115.73 | 0.59 | <0.01 |
| Estimate SE | 9.25 | 0.14 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Serine | 38.29 | 0.43 | 0.18 | 168.08 | 0.43 | 0.02 |
| Estimate SE | 11.15 | 0.17 | - | - | - | - |
| Estimate p-Value | <0.01 | 0.02 | - | - | - | - |
| Glutamic acid | 22.19 | 0.75 | 0.50 | 112.11 | 0.71 | <0.01 |
| Estimate SE | 9.11 | 0.14 | - | - | - | - |
| Estimate p-Value | 0.02 | <0.01 | - | - | - | - |
| Proline | 13.72 | 0.75 | 0.35 | 206.60 | 0.59 | <0.01 |
| Estimate SE | 12.36 | 0.19 | - | - | - | - |
| Estimate p-Value | 0.28 | <0.01 | - | - | - | - |
| Glycine | 41.58 | 0.40 | 0.25 | 94.69 | 0.50 | <0.01 |
| Estimate SE | 8.37 | 0.13 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Alanine | 35.01 | 0.56 | 0.39 | 95.38 | 0.63 | <0.01 |
| Estimate SE | 8.40 | 0.13 | - | - | - | - |
| Estimate p-Value | <0.01 | 0.56 | - | - | - | - |
| Cysteine | 26.18 | 0.41 | 0.09 | 326.99 | 0.30 | 0.09 |
| Estimate SE | 15.55 | 0.23 | - | - | - | - |
| Estimate p-Value | 0.10 | 0.09 | - | - | - | - |
| Valine | 42.74 | 0.40 | 0.21 | 113.32 | 0.46 | <0.01 |
| Estimate SE | 9.16 | 0.14 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Methionine | 32.256 | 0.63 | 0.45 | 97.07 | 0.67 | <0.01 |
| Estimate SE | 8.47 | 0.13 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Isoleucine | 43.74 | 0.44 | 0.26 | 110.68 | 0.51 | <0.01 |
| Estimate SE | 9.05 | 0.14 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Leucine | 35.38 | 0.56 | 0.35 | 113.76 | 0.59 | <0.01 |
| Estimate SE | 9.17 | 0.14 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Tyrosine | 28.97 | 0.63 | 0.39 | 121.17 | 0.62 | <0.01 |
| Estimate SE | 9.47 | 0.14 | - | - | - | - |
| Estimate P-Value | <0.01 | <0.01 | - | - | - | - |
| Phenylalanine | 39.97 | 0.5 | 0.29 | 120.73 | 0.54 | <0.01 |
| Estimate SE | 9.45 | 0.14 | - | - | - | - |
| Estimate P-Value | <0.01 | <0.01 | - | - | - | - |
| Lysine | 34.44 | 0.57 | 0.50 | 62.50 | 0.71 | <0.01 |
| Estimate SE | 6.80 | 0.10 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
| Histidine | 12.17 | 0.84 | 0.48 | 150.04 | 0.70 | <0.01 |
| Estimate SE | 10.54 | 0.16 | - | - | - | - |
| Estimate p-Value | 0.26 | <0.01 | - | - | - | - |
| Arginine | 33.27 | 0.63 | 0.40 | 119.31 | 0.63 | <0.01 |
| Estimate SE | 9.39 | 0.14 | - | - | - | - |
| Estimate p-Value | <0.01 | <0.01 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryan, D.D.S.L.; Classen, H.L. In Vitro Methods of Assessing Protein Quality for Poultry. Animals 2020, 10, 551. https://doi.org/10.3390/ani10040551
Bryan DDSL, Classen HL. In Vitro Methods of Assessing Protein Quality for Poultry. Animals. 2020; 10(4):551. https://doi.org/10.3390/ani10040551
Chicago/Turabian StyleBryan, Dervan D.S.L., and Henry L. Classen. 2020. "In Vitro Methods of Assessing Protein Quality for Poultry" Animals 10, no. 4: 551. https://doi.org/10.3390/ani10040551
APA StyleBryan, D. D. S. L., & Classen, H. L. (2020). In Vitro Methods of Assessing Protein Quality for Poultry. Animals, 10(4), 551. https://doi.org/10.3390/ani10040551





