GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Sample Preparation
2.2. COCs Processing
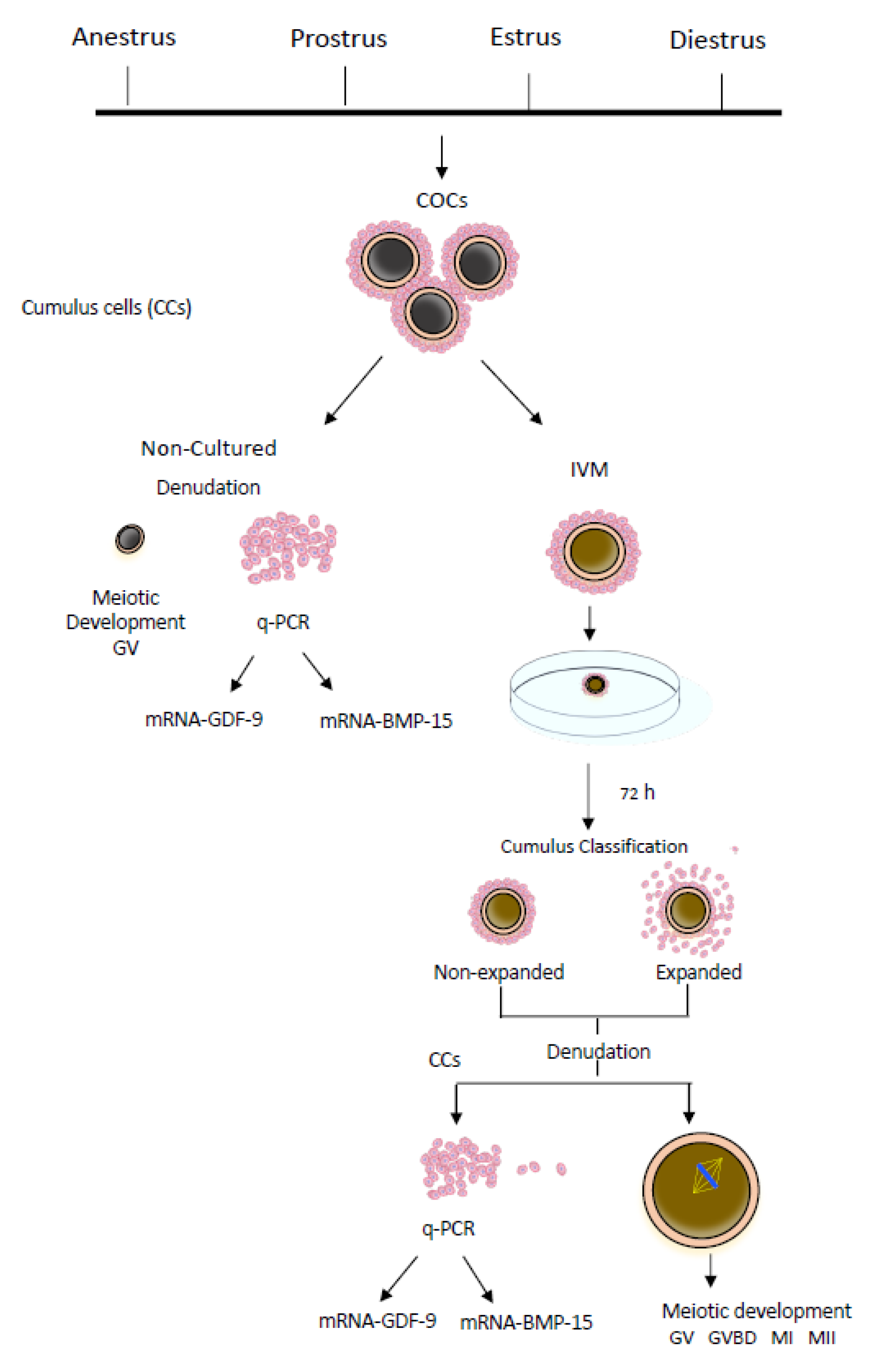
2.3. Experimental Design
2.3.1. Noncultured COCs
2.3.2. COCs Subjected to In Vitro Maturation (IVM)
2.4. Total RNA Extraction and Reverse Transcription Analysis by Real-Time Polymerase Chain Reaction (RT-qPCR)
2.5. Statistical Analysis
3. Results
3.1. Estrous Cycle Determination
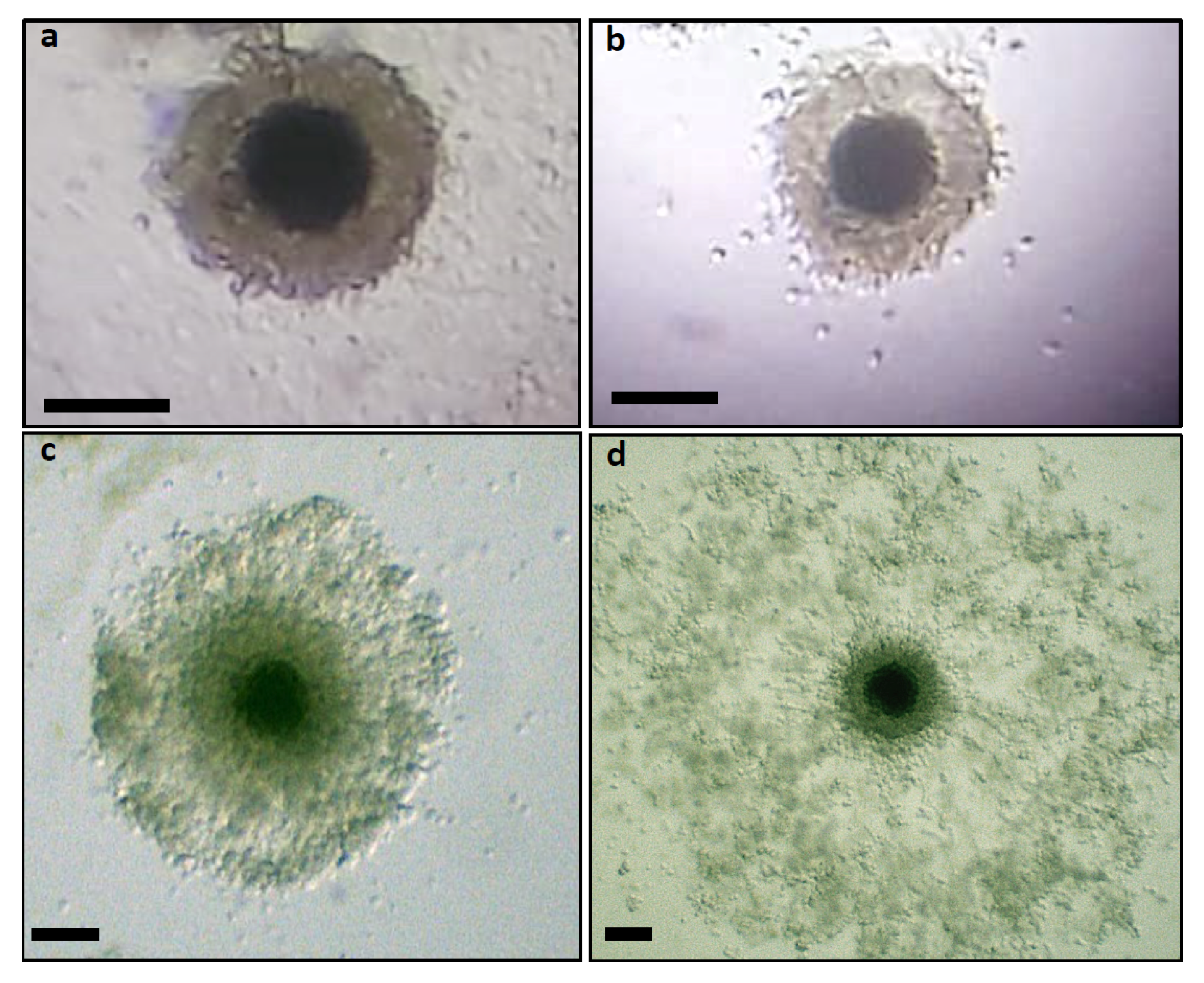
3.2. Expansion of Cumulus Cells
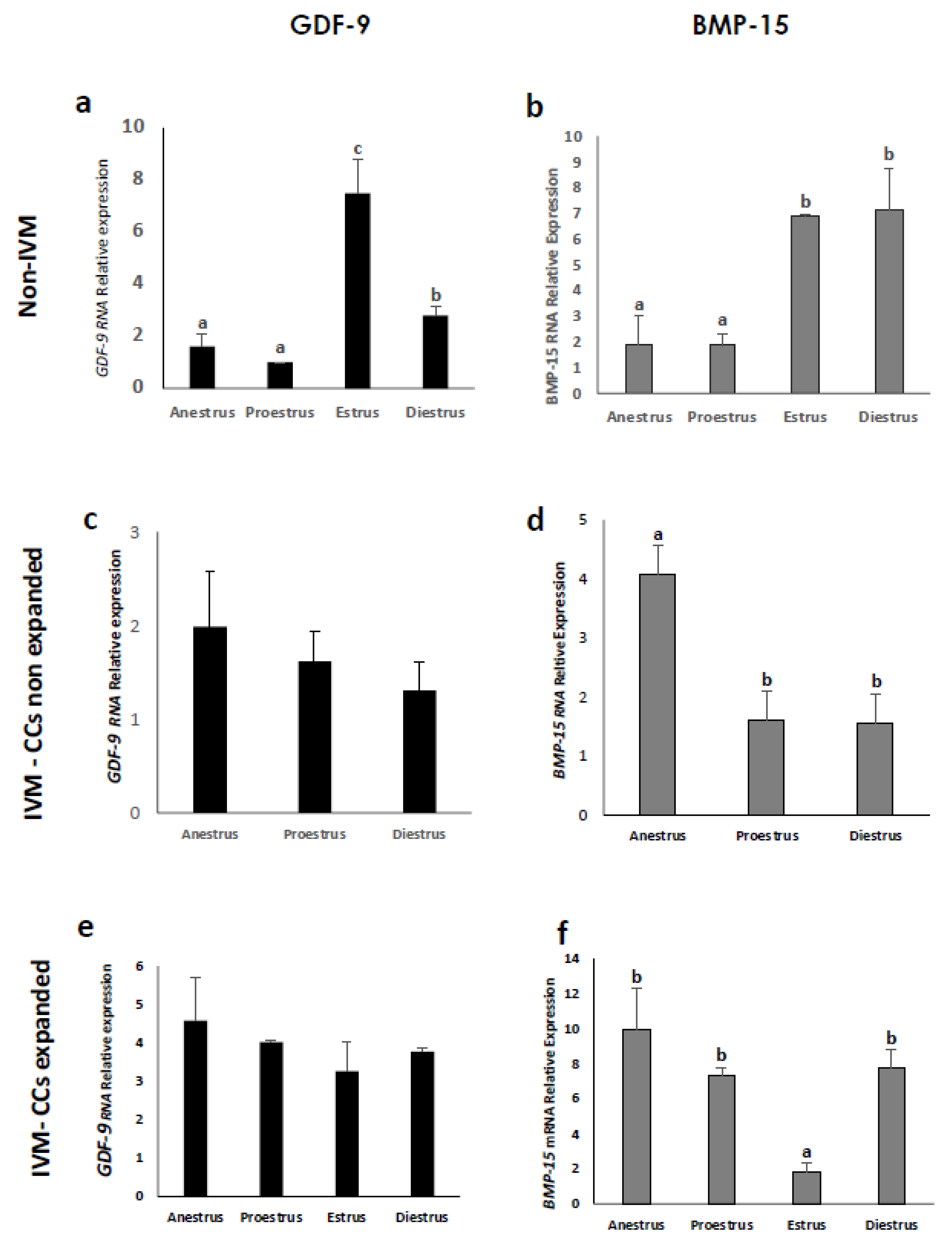
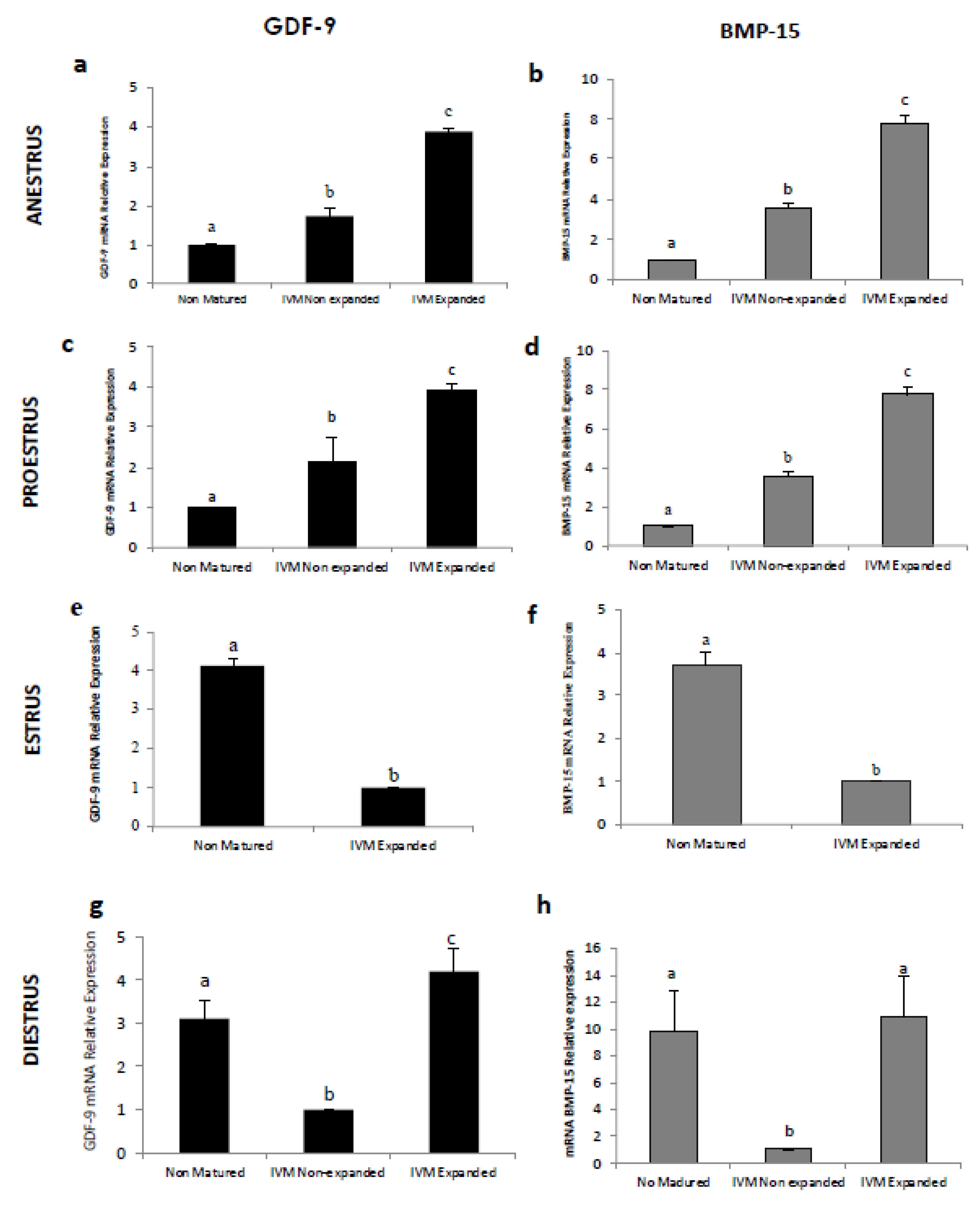
3.3. Expression of Cumulus Expansion-Related Transcripts by Cumulus Cells
3.4. Meiotic Growth and Cumulus Expansion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Wells, D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. Mol. Hum. Reprod. 2010, 16, 715–725. [Google Scholar] [CrossRef]
- Kimura, N.; Totsukawa, K.; Sato, E. Significance of mammalian cumulus-oocyte complex Matrix in Oocyte meiotic maturation: Review of the synthetic control and possible roles of hyaluronan (HA) and HA-binding protein. J. Mamm. Ova Res. 2006, 23, 36–51. [Google Scholar] [CrossRef]
- Kidde, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Li, R.; Norman, R.J.; Armstrong, D.T.; Gilchrist, R.B. Oocyte-secreted factors determine functional differences between bovine mural granulosa cells and cumulus cells. Biol. Reprod. 2000, 63, 839–845. [Google Scholar] [CrossRef]
- Su, Y.Q.; Sugiura, K.; Wigglesworth, K.; O’Brien, M.J.; Affourtit, J.P.; Pangas, S.A.; Eppig, J.J. Oocyte regulation of metabolic co- operativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 2008, 135, 111–121. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ritter, L.J.; Armstrong, D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82–83, 431–446. [Google Scholar] [CrossRef]
- Eppig, J.J. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001, 122, 829–838. [Google Scholar] [CrossRef]
- Belli, M.; Shimasaki, S. Molecular aspects and clinical Relevance of GDF9 and BMP15 in ovarian function. Vitam Horm. 2018, 107, 317–348. [Google Scholar]
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 2–5. [Google Scholar] [CrossRef]
- Haas, C.S.; Rovani, M.T.; Oliveira, F.C.; Vieira, A.D.; Bordignon, V.; Gonçalves, P.B.; Ferreira, R.; Gasperin, B.G. Expression of growth and differentiation factor 9 and cognate receptor s during final follicular growth in cattle. Anim. Reprod. 2015, 13, 756–761. [Google Scholar] [CrossRef]
- Maupeu, D.; Palomino, J.; De los Reyes, M. Immunohistochemistry localization of growth differentiation factor 9 (GDF-9) and bone morphogenetic protein 15 (BMP-15) in canine follicles throughout estrus cycle. Reprod. Fertil. Dev. 2014, 27, 182. [Google Scholar] [CrossRef]
- De los Reyes, M.; Parraguez, V.H.; Rojas, C.; Palomino, J. Expression of growth differentiation factor 9 (GDF-9) during in vitro maturation in canine oocytes. Theriogenology 2013, 80, 587–596. [Google Scholar] [CrossRef]
- Fernandez, T.; Palomino, J.; Parraguez, V.H.; Peralta, O.A.; De los Reyes, M. Differential expression of GDF-9 and BMP- 15 during follicular development in canine ovaries evaluated by flow cytometry. Anim. Reprod. Sci. 2016, 167, 59–67. [Google Scholar] [CrossRef]
- Garcia, P.; Aspee, K.; Ramirez, G.; Dettleff, P.; Palomino, J.; Peralta, O.A.; Parraguez, V.H.; De los Reyes, M. Influence of growth differentiation factor 9 and bone morphogenetic protein 15 on in vitro maturation of canine oocytes. Reprod. Dom. Anim. 2019, 54, 373–380. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 15, 29–33. [Google Scholar] [CrossRef]
- Russel, D.L.; Gilchrist, R.B.; Brown, H.M.; Thomsom, J.G. Bidirectional communication between cumulus cells and the oocyte: Old hands and new players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Zeng, H.T.; Richani, D.; Sutton-McDowall, M.L.; Ren, Z.; Smitz, J.E.; Stokes, Y.; Gilchrist, R.B.; Thompson, J.G. Prematuration with cyclic adenosine monophosphate modulators alters cumulus cell and oocyte metabolism and enhances developmental competence of in vitro- matured mouse oocytes. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef]
- Elvin, J.A.; Clarck, A.T.; Wang, P.; Wolfman, N.M.; Matzuk, M.M. Paracrine Actions of Growth Differentiation Factor-9 in the mammalian ovary. Mol. Endocrinol. 1999, 13, 1035–1048. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Up 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Gen. 2018, 35, 1741–1750. [Google Scholar] [CrossRef]
- Hussein, T.S.; Thompson, J.G.; Gilchrist, R.B. Oocyte-secreted factors enhance oocyte developmental competence. Dev. Biol. 2006, 296, 514–521. [Google Scholar] [CrossRef]
- Reynaud, K.; Fontbonne, A.; Marseloo, N.; Thoumire, S.; Chebrout, M.; de Lesegno, C.V.; Chastant-Maillard, S. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005, 130, 193–201. [Google Scholar] [CrossRef]
- Viaris de Lesegno, C.; Reynaud, K.; Pechoux, C.; Thoumire, S.; Chastant-Maillard, S. Ultrastructure of canine oocytes during in vivo maturation. Mol. Reprod. Dev. 2008, 75, 115–125. [Google Scholar] [CrossRef]
- Brugger, N.; Otzdorff, C.; Walter, B.; Hoffmann, B.; Braun, J. Quantitative Determination of Progesterone (P4) in Canine Blood Serum Using an Enzyme-linked Fluorescence Assay. Repro Dom Anim. 2011, 46, 870–873. [Google Scholar] [CrossRef]
- Palomino, J.; De los Reyes, M. Temporal expression of GDF-9 and BMP-15 in canine ovarian follicles. Theriogenology 2016, 86, 1541–1549. [Google Scholar] [CrossRef]
- De los Reyes, M.; Palomino, J.; Parraguez, V.H.; Hidalgo, M.; Saffie, P. Mitochondrial distribution and meiotic progression in canine oocytes during in vivo and in vitro maturation. Theriogenology 2011, 75, 346–353. [Google Scholar] [CrossRef]
- Cho, S.J.; Lee, K.L.; Kim, Y.G.; Kim, D.H.; Yoo, J.G.; Yang, B.C.; Kong, I.K. Differential gene-expression profiles from canine cumu- lus cells of ovulated versus in vitro-matured oocytes. Reprod. Fertil. Dev. 2016, 28, 278–285. [Google Scholar] [CrossRef]
- Prochazka, R.; Nemcova, L.; Nagyova, E.; Kanka, J. Expression of Growth Differentiation Factor 9 Messenger RNA in Porcine Growing and Preovulatory Ovarian Follicles. Biol. Reprod. 2004, 71, 1290–1295. [Google Scholar] [CrossRef]
- Hosoe, M.; Kaneyam, K.; Ushizawa, K.; Hayashi, K.; Takahashi, T. Quantitative analysis of bone morphogenetic protein 15 (BMP15) and growth differentiation factor 9 (GDF9) gene expression in calf and adult bovine ovaries. Reprod. Biol. Endocrin. 2011, 9, 33. [Google Scholar] [CrossRef]
- Concannon, P.W. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef]
- Peng, X.R.; Hsueh, A.J.; LaPolt, P.S.; Bjersing, L.; Ny, T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 1991, 129, 3200–3207. [Google Scholar] [CrossRef]
- Wen, X.H.; Feng, H.L.; Sun, Q.Y. In vitro maturation of follicular oocytes of the silver fox (Vulpes fulva). Theriogenology 1994, 41, 333. [Google Scholar] [CrossRef]
- De los Reyes, M.; de Lange, J.; Miranda, P.; Palomino, J.; Barros, C. Effect of human chorionic gonadotrophin supplementation during different culture periods on in vitro maturation of canine oocytes. Theriogenology 2005, 64, 1–11. [Google Scholar] [CrossRef]
- Juengel, J.L.; Hudson, N.L.; Heath, D.A.; Smith, P.; Reader, K.L.; Lawrence, S.B.; Groome, N.P. Growth differentiation factor 9 and bone morphogenetic protein 15 are essential for ovarian follicular development in sheep. Biol. Reprod. 2002, 67, 1777–1789. [Google Scholar] [CrossRef]
- Assidi, M.; Dieleman, S.J.; Sirard, M.A. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: Potential early markers of oocyte competence. Reproduction 2010, 140, 835–852. [Google Scholar] [CrossRef]
- Mattioli, M.; Barboni, B. Signal transduction mechanism for LH in the cumulus–oocyte complex. Mol. Cell Endocrinol. 2000, 161, 19–23. [Google Scholar] [CrossRef]
- Kim, M.K.; Fibrianto, Y.H.; Oh, H.J.; Jang, G.; Kim, H.J.; Lee, K.S.; Kang, S.K.; Lee, B.C.; Hwang, W.S. Effects of estradiol-17β and progesterone supplementation on in vitro nuclear maturation of canine oocytes. Theriogenology 2005, 63, 1342–1353. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.Q.; Ou, S.B.; Zhang, N.F.; Ren, L.; Wei, L.; Zhang, Q.X.; Yang, D.Z. Increased GDF9 and BMP15 mRNA levels in cumulus granulosa cells correlate with oocyte maturation, fertilization, and embryo quality in humans. Reprod. Biol. Endocrinol. 2014, 12, 1–9. [Google Scholar] [CrossRef]
- Pant, D.; Reynolds, L.P.; Luther, J.S.; Borowicz, P.P.; Stenbak, T.M.; Bilski, J.J.; Weigl, R.M.; Lopes, F.; Petry, K.; Johnson, M.L.; et al. Expression of connexin 43 and gap junctional intercellular communication in the cumulus–oocyte complex in sheep. Reproduction 2005, 129, 191–200. [Google Scholar] [CrossRef]
- Sanchez, F.; Adriaenssens, T.; Romero, S.; Smitz, J. Quantification of oocyte-specific transcripts in follicle-enclosed oocytes during antral development and maturation in vitro. Mol. Hum. Reprod. 2009, 15, 539–550. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Qiao, J.; Chen, Y.J.; Liu, P.; Li, J.; Yan, J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 261–267. [Google Scholar] [CrossRef]
- Sugiura, K.; Su, Y.Q.; Li, Q.; Wigglesworth, K.; Matzuk, M.M.; Eppig, J.J. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol. Endocrinol. 2010, 24, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Casasola, A.; Martínez-Armendáriz, K.E.; Itzá-Ortiz, M.F.; Escárcega-Ávila, A.M.; Pérez-Eguía, E.; Filipiak, Y.; Larocca, C.; Carrera-Chávez, J.M. Effect of presence of corpora lutea on cumulus expansion of in vitro matured bovine oocytes selected by trypan blue and brilliant cresyl blue tests. J. Appl. Anim. Res. 2018, 46, 967–972. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wigglesworth, K.; Eppig, J.J. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev. Biol. 2007, 305, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Nevoral, J.; Orsák, M.; Petr, J.; Dvořakova, M.; Weingartová, I.; Vyskočilova, A.; Aámostna, K.; Krejčová, T.; Jílek, F. Cumulus cell expansion, its role in oocyte biology and perspectives of measurement: A review. Sci. Agric. Bohem. 2014, 45, 12–225. [Google Scholar] [CrossRef]
- Adriaenssens, T.; Wathlet, S.; Segers, I.; Verheyen, G.; De Vos, A.; Van der Elst, J.; Coucke, W.; Devroey, P.; Smitz, J. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum. Reprod. 2010, 25, 1259–1270. [Google Scholar] [CrossRef]
- Rodrigues, B.A.; Rodrigues, J.L. Cumulus cell features and nuclear chromatin configuration of in vitro matured canine COCs and the influence of in vivo serum progesterone concentrations of ovary donors. Zygote 2009, 17, 79–91. [Google Scholar] [CrossRef]
| Gene | Oligos (5’–3’) | Amplicon (bp) | Accession Number | References |
|---|---|---|---|---|
| ACTB | F: ATTGTCATGGACTCTGGGGATG | 191 | AF021873.2 | [25] |
| R: TCCTTGATGTCACGCACGAT | ||||
| GDF-9 | F: TACCCCCATCCCTGCTTTTA | 155 | NM001168013.1 | [27] |
| R: TCCACCTTCAGTCGATTCCT | ||||
| BMP-15 | F: CCCTGCCCCTGATTCGGGAG | 82 | XM003640274.3 | [25] |
| R: CCGCAAAGGATGCCCAAGGAC |
| Oocytes Meiotic Stage (%) | ||||||
|---|---|---|---|---|---|---|
| Cycle | Exp | GV | GVBD | MI | MII | N |
| Anestrus | + | 0 a | 37 a,b | 53 a,b | 10 a,b | 51 |
| − | 7 b | 37 a,b | 48 a,b | 7 a | 54 | |
| Proestrus | + | 0 a | 60 c | 36 a | 4 a | 53 |
| − | 9 b | 21 a | 57 a,b | 13 a,b | 56 | |
| Estrus | + | 6 b | 21 a | 56 a,b | 18 b | 34 |
| − | 5 b | 26 a | 68 b | 0 a | 19 | |
| Diestrus | + | 4 b | 50 b,c | 39 a | 7 a | 46 |
| − | 5 b | 22 a | 56 a,b | 17 b | 59 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, G.; Palomino, J.; Aspee, K.; De los Reyes, M. GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process. Animals 2020, 10, 462. https://doi.org/10.3390/ani10030462
Ramirez G, Palomino J, Aspee K, De los Reyes M. GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process. Animals. 2020; 10(3):462. https://doi.org/10.3390/ani10030462
Chicago/Turabian StyleRamirez, George, Jaime Palomino, Karla Aspee, and Monica De los Reyes. 2020. "GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process" Animals 10, no. 3: 462. https://doi.org/10.3390/ani10030462
APA StyleRamirez, G., Palomino, J., Aspee, K., & De los Reyes, M. (2020). GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process. Animals, 10(3), 462. https://doi.org/10.3390/ani10030462







