Molecular Detection of Arthropod-Borne Pathogens in Eurasian Badgers (Meles meles) from the United Kingdom
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Post Mortem Examination
2.2. Molecular Analysis
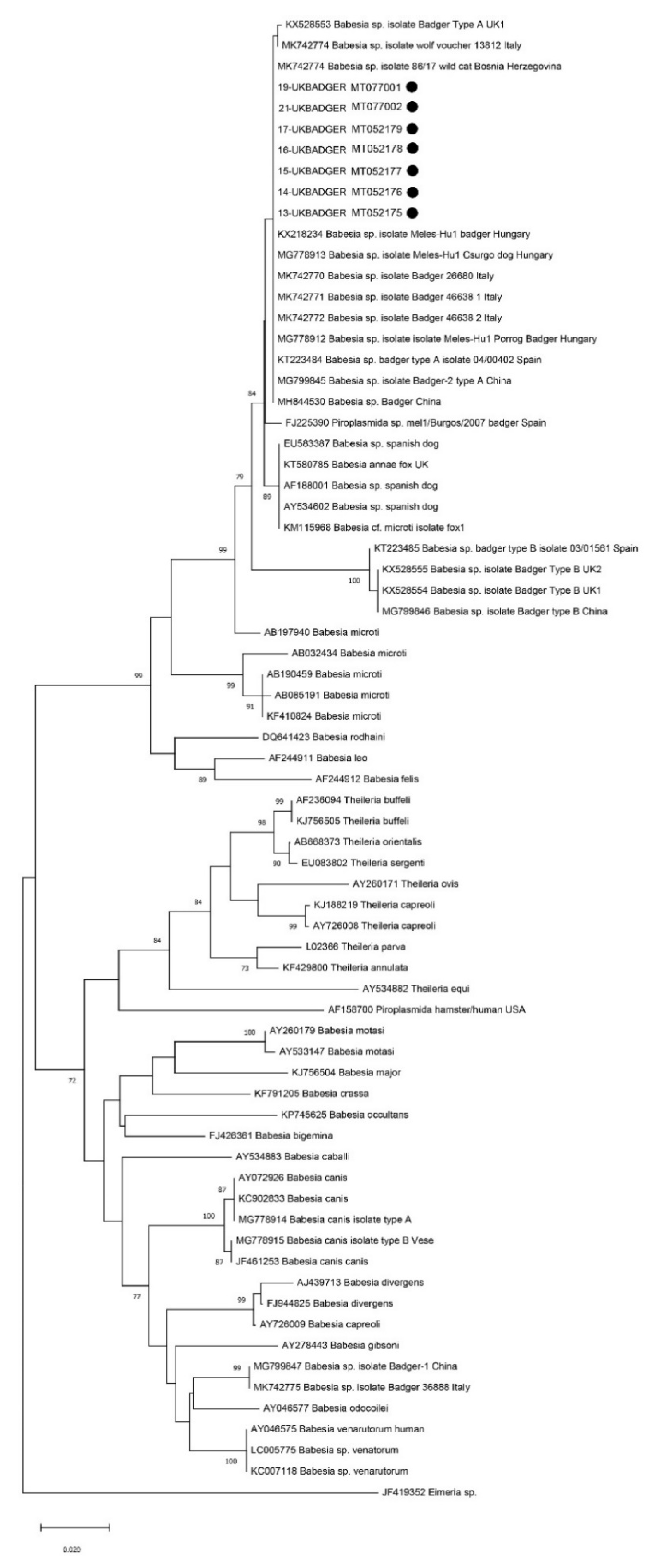
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health 2017, 16, 112. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Vaux, A.G.; Cull, B.; Gillingham, E.; Leach, S. Assessment of the public health threats posed by vector-borne disease in the United Kingdom (UK). Int. J. Environ. Res. Public Health 2018, 15, 2145. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trend Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Alvarado-Rybak, M.; Solano-Gallego, L.; Millán, J. A review of piroplasmid infections in wild carnivores worldwide: Importance for domestic animal health and wildlife conservation. Parasites Vectors 2016, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Gehrt, S.D.; Riley, S.P.D.; Cypher, B.L. Urban Carnivores, Ecology, Conflict, and Conservation; John Hopkins University Press: Baltimore, MD, USA, 2010. [Google Scholar]
- Millán, J.; Proboste, T.; De Mera, I.G.F.; Chirife, A.D.; De La Fuente, J.; Altet, L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human–wildlife interface. Ticks Tick Borne Dis. 2016, 7, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Piza Roca, C.; La Haye, M.J.J.; Jongejans, E. Environmental drivers of the distribution and density of the European badger (Meles meles): A review. Lutra 2014, 57, 87–109. [Google Scholar]
- Hornok, S.; Trauttwein, K.; Takács, N.; Hodžić, A.; Duscher, G.G.; Kontschán, J. Molecular analysis of Ixodes rugicollis, Candidatus Neoehrlichia sp.(FU98) and a novel Babesia genotype from a European badger (Meles meles). Ticks Tick Borne Dis. 2017, 8, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.M.; Wilson, C.; Innes, E.A.; Katzer, F. Detection of Babesia DNA in blood and spleen samples from Eurasian badgers (Meles meles) in Scotland. Parasitology 2017, 144, 1203–1210. [Google Scholar] [CrossRef]
- Smith, G.C.; Wilkinson, D. Modelling disease spread in a novel host: Rabies in the European badger Meles meles. J. Appl. Ecol. 2002, 39, 865–874. [Google Scholar] [CrossRef]
- Sandoval Barron, E.; Swift, B.; Chantrey, J.; Christley, R.; Gardner, R.; Jewell, C.; McGrath, I.; Mitchell, A.; O’Cathail, C.; Prosser, A.; et al. A study of tuberculosis in road traffic-killed badgers on the edge of the British bovine TB epidemic area. Sci. Rep. 2018, 8, 17206. [Google Scholar] [CrossRef] [PubMed]
- Hancox, M. Parasites and infectious diseases of the Eurasian badger (Meles meles L.): A review. Mammal Rev. 1980, 10, 151–162. [Google Scholar] [CrossRef]
- Gloor, S.; Bontadina, F.; Hegglin, D.; Deplazes, P.; Breitenmoser, U. The rise of urban fox populations in Switzerland. Mamm. Biol. 2001, 66, 155–164. [Google Scholar]
- Judge, J.; Wilson, G.J.; Macarthur, R.; Delahay, R.J.; McDonald, R.A. Density and abundance of badger social groups in England and Wales in 2011–2013. Sci. Rep. 2014, 4, 3809. [Google Scholar] [CrossRef] [PubMed]
- Barandika, J.F.; Espí, A.; Oporto, B.; Del Cerro, A.; Barral, M.; Povedano, I.; García-Pérez, A.L.; Hurtado, A. Occurrence and genetic diversity of piroplasms and other apicomplexa in wild carnivores. Parasitol. Open 2016, 2, e6. [Google Scholar] [CrossRef]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A world emerging. Infect Genet Evol 2012, 12, 1788–1809. [Google Scholar] [CrossRef]
- Veronesi, F.; Ravagnan, S.; Cerquetella, M.; Carli, E.; Olivieri, E.; Santoro, A.; Pesaro, S.; Berardi, S.; Rossi, G.; Ragni, B.; et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 2016, 7, 853–858. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasites Vectors 2015, 8, 75. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—A widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Berri, M.; Rekiki, A.; Boumedine, K.S.; Rodolakis, A. Simultaneous differential detection of Chlamydophila abortus, Chlamydophila pecorum and Coxiella burnetii from aborted ruminant’s clinical samples using multiplex PCR. BMC Microbiol. 2009, 9, 130. [Google Scholar] [CrossRef]
- Ellis, J.; Oyston, P.C.; Green, M.; Titball, R.W. Tularemia. Clin. Microbiol. Rev. 2002, 15, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Cote, M.; Le Rhun, D.; Lecuelle, B.; Levin, M.L.; Vayssier-Taussat, M.; Bonnet, S.I. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl. Trop. Dis. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R., III; Wormser, G.P. Bartonella spp. transmission by ticks not established. Emerg. Infect. Dis. 2010, 16, 379. [Google Scholar] [CrossRef]
- De Sousa, K.C.M.; Do Amaral, R.B.; Herrera, H.M.; Santos, F.M.; Macedo, G.C.; De Andrade Pinto, P.C.E.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Genetic Diversity of Bartonella spp. in wild mammals and ectoparasites in Brazilian Pantanal. Microb. Ecol. 2018, 76, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Rocchigiani, G.; Nardoni, S.; Bertelloni, F.; Vasta, V.; Papini, R.A.; Verin, R.; Poli, A.; Mancianti, F. Molecular detection of tick-borne pathogens in wild red foxes (Vulpes vulpes) from Central Italy. Acta Trop. 2017, 172, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT; Nucleic acids symposium series; Oxford Academic: London, UK, 1999; Volume 41, No. 41; pp. 95–98. [Google Scholar]
- Hodžić, A.; Alić, A.; Duscher, G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick Borne Dis. 2018, 9, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.E.; Mugford, A.R.; Humm, K.R.; Kellett-Gregory, L.M. Ehrlichia canis infection in a dog with no history of travel outside the United Kingdom. J. Small Anim. Pract. 2013, 54, 425–427. [Google Scholar] [CrossRef]
- Gürcan, Ş. Epidemiology of tularemia. Balkan Med. J. 2014, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Garcı́a-Pérez, A.L.; Oporto, B.; Espí, A.; Del Cerro, A.; Barral, M.; Povedano, I.; Barandika, J.; Hurtado, A. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick Borne Dis. 2016, 7, 264–269. [Google Scholar] [CrossRef]
- Battisti, E.; Zanet, S.; Khalili, S.; Trisciuoglio, A.; Hertel, B.; Ferroglio, E. Molecular survey on vector-borne pathogens in alpine wild carnivorans. Front. Vet. Sci. 2020, 7, 1. [Google Scholar] [CrossRef]
- Halsby, K.D.; Kirkbride, H.; Walsh, A.L.; Okereke, E.; Brooks, T.; Donati, M.; Morgan, D. The epidemiology of Q fever in England and Wales 2000–2015. Vet. Sci. 2017, 4, 28. [Google Scholar] [CrossRef]
- Thomas, D.R.; Treweek, L.; Salmon, R.L.; Kench, S.M.; Coleman, T.J.; Meadows, D.; Morgan-Capner, P.; Caul, E.O. The risk of acquiring Q fever on farms: A seroepidemiological study. Occup. Environ. Med. 1995, 52, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Valergakis, G.E.; Russell, C.; Grogono-Thomas, R.; Eisler, M.C.; Bradley, A.J. Coxiella burnetii in bulk tank milk of dairy cattle in south-west England. Vet. Rec. 2012, 171, 156. [Google Scholar] [CrossRef] [PubMed]
- Meerburg, B.G.; Reusken, C.B. The role of wild rodents in spread and transmission of Coxiella burnetii needs further elucidation. Wildl. Res. 2011, 38, 617–625. [Google Scholar] [CrossRef]
- Smith, F.D.; Wall, L.E.R. Prevalence of Babesia and Anaplasma in ticks infesting dogs in Great Britain. Vet. Parasitol. 2013, 198, 18–23. [Google Scholar] [CrossRef]
- Gerrikagoitia, X.; Gil, H.; García-Esteban, C.; Anda, P.; Juste, R.A.; Barral, M. Presence of Bartonella species in wild carnivores of northern Spain. Appl. Environ. Microbiol. 2012, 78, 885–888. [Google Scholar] [CrossRef]
- Vieira-Damiani, G.; De Paiva Diniz, P.P.V.; Pitassi, L.H.U.; Sowy, S.; Scorpio, D.G.; Lania, B.G.; Drummond, M.R.; Soares, T.C.B.; Barjas-Castro, M.D.L.; Breitschwerdt, E.B.; et al. Bartonella clarridgeiae bacteremia detected in an asymptomatic blood donor. J. Clin. Microbiol. 2015, 53, 352–356. [Google Scholar] [CrossRef]
- Soares, H.S.; Marcili, A.; Barbieri, A.R.; Minervino, A.H.; Moreira, T.R.; Gennari, S.M.; Labruna, M.B. Novel piroplasmid and Hepatozoon organisms infecting the wildlife of two regions of the Brazilian Amazon. Int. J. Parasitol. Parasites Wildl. 2017, 6, 115–121. [Google Scholar] [CrossRef]
- Young, K.M.; Corrin, T.; Wilhelm, B.; Uhland, C.; Greig, J.; Mascarenhas, M.; Waddell, L.A. Zoonotic Babesia: A scoping review of the global evidence. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Bartley, P.M.; Hamilton, C.; Wilson, C.; Innes, E.A.; Katzer, F. Detection of Babesia annae DNA in lung exudate samples from red foxes (Vulpes vulpes) in Great Britain. Parasites Vectors 2016, 9, 84. [Google Scholar] [CrossRef]
- Gimenez, C.; Casado, N.; Criado-Fornelio, Á.; De Miguel, F.Á.; Dominguez-Peñafiel, G. A molecular survey of Piroplasmida and Hepatozoon isolated from domestic and wild animals in Burgos (northern Spain). Vet. Parasitol. 2009, 162, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Horváth, G.; Takács, N.; Kontschán, J.; Sz˝oke, K.; Farkas, R. Molecular identification of badger-associated Babesia sp. DNA in dogs: Updated phylogeny of piroplasms infecting Caniformia. Parasit Vectors 2018, 11, 235. [Google Scholar] [CrossRef]
- Peirce, M.A.; Neal, C. Piroplasmosis in British badgers (Meles meles). Vet. Rec. 1974, 94, 493–494. [Google Scholar] [CrossRef]
- Gallagher, J.; Nelson, J. Cause of ill health and natural death in badgers in Gloucestershire. Tuberculosis 1979, 10, 14–16. [Google Scholar]
- Macdonald, D.W.; Anwar, M.; Newman, C.; Woodroffe, R.; Johnson, P.J. Inter-annual differences in the age-related prevalences of Babesia and Trypanosoma parasites of European badgers (Meles meles). J. Zool. 1999, 247, 65–70. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, H.J.; Jeong, Y.I.; Cho, S.H.; Lee, W.J.; Kim, J.T.; Lee, S.E. Serological and molecular detection of Toxoplasma gondii and Babesia microti in the blood of rescued wild animals in Gangwon-do (Province), Korea. Korean J. Parasitol. 2017, 55, 207. [Google Scholar] [CrossRef]
- Santoro, M.; Auriemma, C.; Lucibelli, M.G.; Borriello, G.; D’Alessio, N.; Sgroi, G.; Veneziano, V.; Galliero, G.; Fusco, G. Molecular Detection of Babesia spp. (Apicomplexa: Piroplasma) in Free-Ranging Canids andMustelids from Southern Italy. Front. Vet. Sci. 2019, 6, 269. [Google Scholar] [CrossRef]
| N | Sex | Age Class | Weight (kg) | BCS | Carcass Status | PCR—Piroplasmid Blood Samples | PCR—Piroplasmid Heart Samples |
|---|---|---|---|---|---|---|---|
| 1 | F | Young | 7.6 | 4/5 | Good | n. a. | Neg |
| 2 | F | Adult | 11.6 | 4/5 | Good | n. a. | Neg |
| 3 | F | Adult | 12.8 | 3/5 | Good | n. a. | Pos |
| 4 | M | Adult | 12 | 3/5 | Mildly autolytic | n. a. | Pos |
| 5 | F | Juvenile | 6.7 | 4/5 | Moderately autolytic | n. a. | Neg |
| 6 | F | Adult | 12.8 | 4/5 | Good | n. a. | Neg |
| 7 | F | Adult | 14.1 | 4/5 | Good | n. a. | Neg |
| 8 | M | Adult | 15.8 | 3/5 | Autolytic | n. a. | Neg |
| 9 | M | Adult | 10.1 | 4/5 | Good | n. a. | Neg |
| 10 | F | Adult | 14.3 | 3/5 | Autolytic | n. a. | Neg |
| 11 | M | Adult | 10.3 | 4/5 | Good | Pos | Pos |
| 12 | M | Juvenile | 10.5 | 3/5 | Very good | Pos | Pos |
| 13 | M | Juvenile | 9.8 | 3/5 | Good | Pos | Pos |
| 14 | M | Adult | 11.4 | 3/5 | Good | Pos | Pos |
| 15 | F | Juvenile | 9.4 | 3/5 | Good | Pos | Pos |
| 16 | F | Adult | 9.4 | 4/5 | Good | Neg | Neg |
| 17 | F | Adult | 11.6 | 3/5 | Good | Pos | Pos |
| 18 | M | Juvenile | 8.3 | 2/5 | Autolytic | Pos | Pos |
| References | Geographical Area | Species | N | Positive/Examined (Prevalence %) | Babesia sp. Found |
|---|---|---|---|---|---|
| Battisti et al. 2020 [32] | Italy | Badger (M. meles) | 45 | 41/45 (91.1%) | Badger-associated Babesia spp. type A and B; B. capreoli, Babesia sp. DO23163 |
| Santoro et al. 2019 [49] | Italy | Badger (M. meles) | 13 | 7/13 (53.8%) | Two sequence types: badger-associated Babesia spp. from MK742770 to MK742773 and MK742775 |
| Wolf (Canis lupus) | 13 | 1/13 (7.7%) | Badger-associated Babesia spp. (MK742774) | ||
| Hodžić et al. 2018 [28] | Bosnia and Herzegovina | European wild cat (Felis silvestris silvestris) | 18 | 5.5% | Badger-associated Babesia sp. (MF614153) |
| Bartley et al. 2017 [10] | UK (Scotland) | Badger (M. meles) | 64 | overall: 70.2%; Type A: 12·8% spleen and 38.3% blood; Type B: 19.1% spleen and 46.8% blood. Both type A and B sequences 10.6% spleen and 31.9% blood | Babesia Badger type A UK1 (KX528553), Badger type B UK1 (KX528554) and Badger type B UK2 (KX528555) additional sequences registered under accession nr. From KY250472 to KY250477 * |
| Hornok et al. 2017 [9] | Hungary | Badger (M. meles) | 1 | case report (n = 1) | Babesia sp. Meles-Hu1 (KX218234) |
| Ixodes rugicollis | 3 | 1/3 (33.3%) | Babesia sp. Meles-Hu1 | ||
| Hornok et al. 2018 [44] | Hungary | Badger (M. meles) | 5 | 5/5 (100%); | Babesia sp. Meles-Hu1 (MG778912) |
| Dogs | 90 | 6/90 (6.7%); | Babesia sp. Meles-Hu1 (MG778913) | ||
| Ixodes canisuga | 27 | 18/27 (66.7%) | Babesia sp. Meles-Hu1 | ||
| Hong et al. 2017 [48] | Korea | Badgers | 3 | 2/3 (66.7%) | Babesia microti |
| Barandika et al. 2016 [16] | Spain | Badger (M. meles) | 122 | 64/122 (52.2%) | Babesia sp. badger type A (KT223484) and B (KT223485) |
| Millán et al. 2016 [7] | Spain | Badger (M. meles) | 3 | 0 | - |
| Gimenez et al. 2009 [43] | Spain | Badger (M. meles) | 5 | 1/5 (20%) | Piroplasmida sp. mel1/Burgos/2007 (FJ225390) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardone, L.; Ebani, V.V.; Verin, R.; Nardoni, S.; Consolazione, A.; Bennett, M.; Mancianti, F. Molecular Detection of Arthropod-Borne Pathogens in Eurasian Badgers (Meles meles) from the United Kingdom. Animals 2020, 10, 446. https://doi.org/10.3390/ani10030446
Guardone L, Ebani VV, Verin R, Nardoni S, Consolazione A, Bennett M, Mancianti F. Molecular Detection of Arthropod-Borne Pathogens in Eurasian Badgers (Meles meles) from the United Kingdom. Animals. 2020; 10(3):446. https://doi.org/10.3390/ani10030446
Chicago/Turabian StyleGuardone, Lisa, Valentina Virginia Ebani, Ranieri Verin, Simona Nardoni, Antonio Consolazione, Malcolm Bennett, and Francesca Mancianti. 2020. "Molecular Detection of Arthropod-Borne Pathogens in Eurasian Badgers (Meles meles) from the United Kingdom" Animals 10, no. 3: 446. https://doi.org/10.3390/ani10030446
APA StyleGuardone, L., Ebani, V. V., Verin, R., Nardoni, S., Consolazione, A., Bennett, M., & Mancianti, F. (2020). Molecular Detection of Arthropod-Borne Pathogens in Eurasian Badgers (Meles meles) from the United Kingdom. Animals, 10(3), 446. https://doi.org/10.3390/ani10030446







