The Effect of Topical Anaesthesia on the Cortisol Responses of Calves Undergoing Dehorning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Design and Treatments
2.3. Topical Anaesthetic Formulation
2.4. Blood Collection and Analysis
2.5. Statistical Analysis
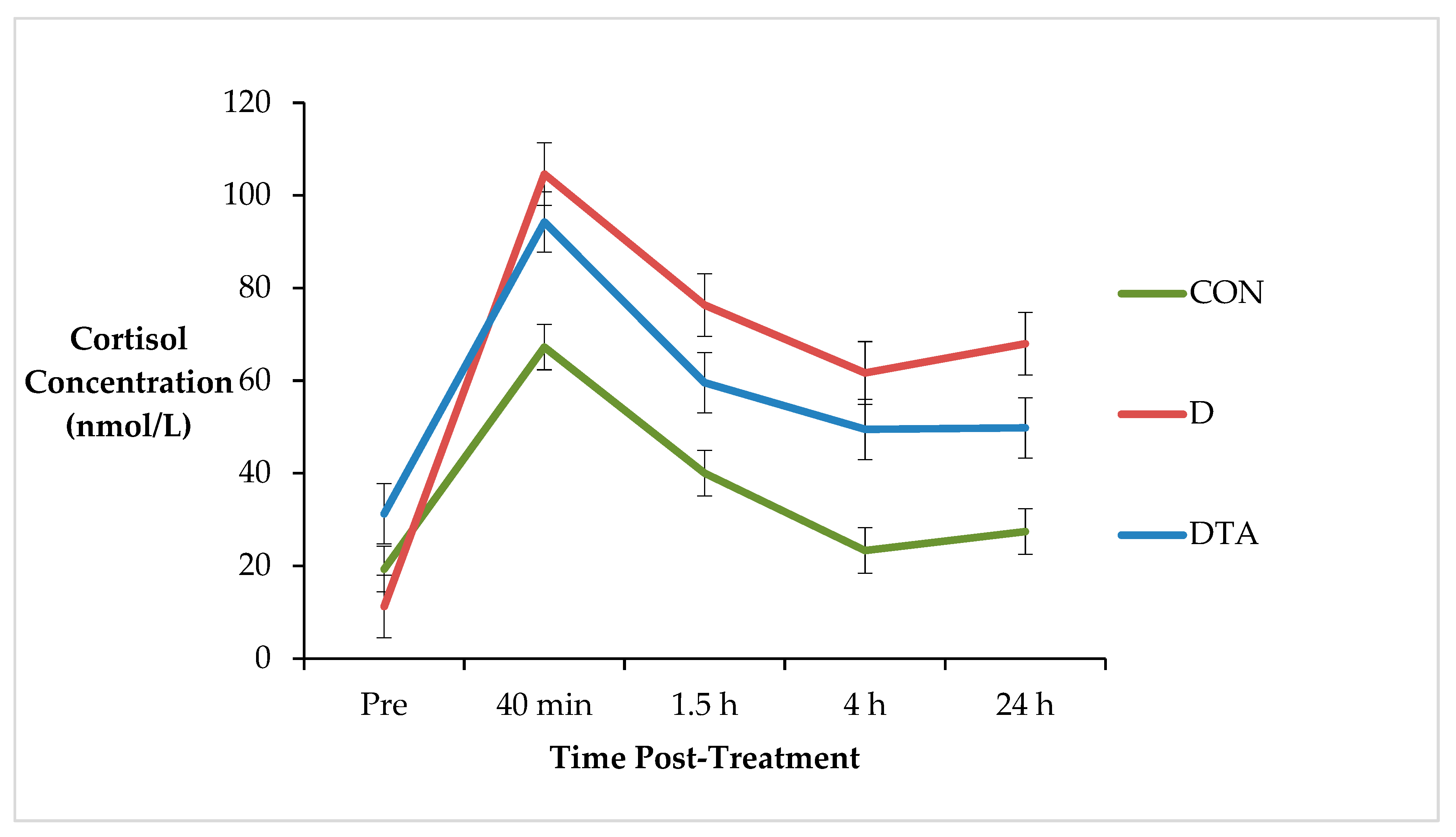
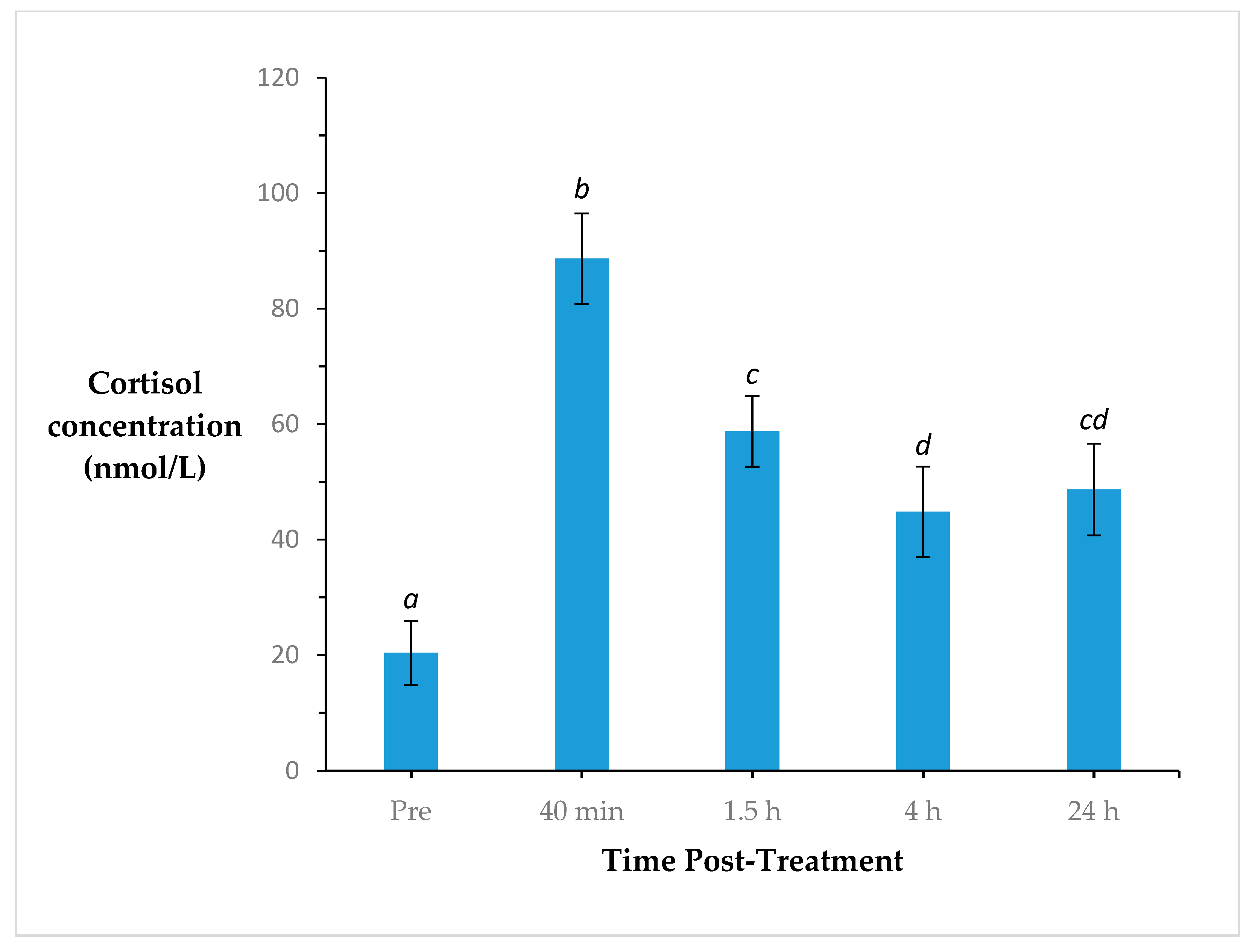
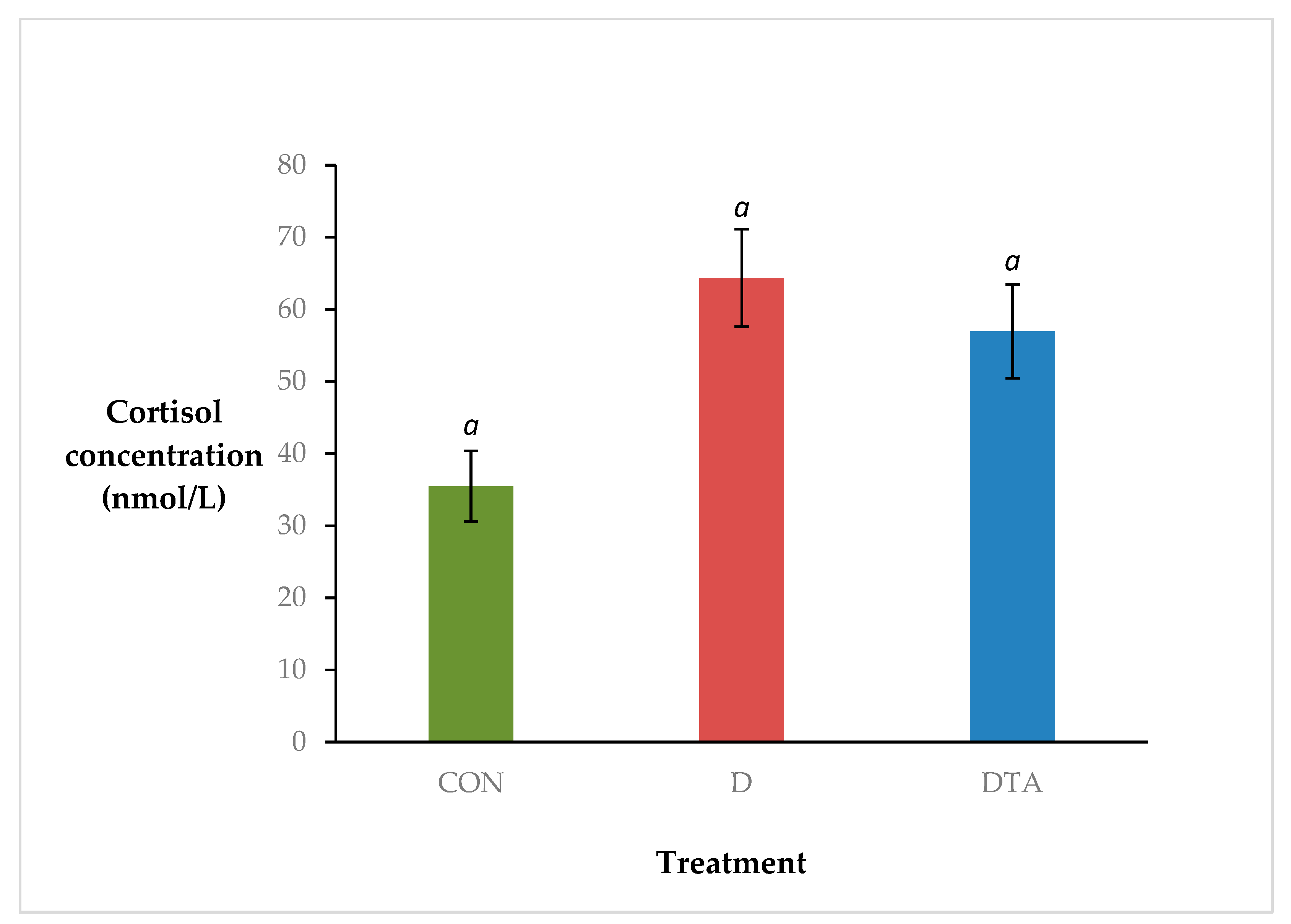
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marshall, B.L. Bruising in cattle presented for slaughter. N. Zeal. Vet. J. 1977, 25, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.J.; Mellor, D.J. Dehorning and disbudding distress and its alleviation in calves. Vet. J. 2005, 169, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Stookey, J.M.; Goonewardene, L.A. A comparison of production traits and welfare implications between horned and polled beef bulls. Can. J. Anim. Sci. 1996, 76, 1–5. [Google Scholar] [CrossRef]
- Prayaga, K.C. Genetic options to replace dehorning in beef cattle—A review. Aust. J. Agric. Res. 2007, 58, 1–8. [Google Scholar] [CrossRef]
- Schafberg, R.; Swalve, H.H. The history of breeding for polled cattle. Livest. Sci. 2015, 179, 54–70. [Google Scholar] [CrossRef]
- Windig, J.J.; Hoving-Bolink, R.A.; Veerkamp, R.F. Breeding for polledness in Holstein cattle. Livest. Sci. 2015, 179, 96–101. [Google Scholar] [CrossRef]
- Stafford, K.J.; Mellor, D.J. Addressing the pain associated with disbudding and dehorning in cattle. Appl. Anim. Behav. Sci. 2011, 135, 226–231. [Google Scholar] [CrossRef]
- Janzen, E. Dehorning. Available online: http://www.beefresearch.ca/research-topic.cfm/dehorning-69 (accessed on 21 January 2020).
- Sylvester, S.P.; Mellor, D.J.; Stafford, K.J.; Bruce, R.A.; Ward, R.N. Acute cortisol responses of calves to scoop dehorning using local anaesthesia and/or cautery of the wound. Aust. Vet. J. 1998, 76, 118–122. [Google Scholar] [CrossRef]
- Sylvester, S.P.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N. Acute cortisol responses of calves to four methods of dehorning by amputation. Aust. Vet. J. 1998, 76, 123–126. [Google Scholar] [CrossRef]
- Sylvester, S.P.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N. Behavioural responses of calves to amputation dehorning with and without local anaesthesia. Aust. Vet. J. 2004, 82, 697–700. [Google Scholar] [CrossRef]
- McMeekan, C.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N.; Gregory, N. Effects of a local anaesthetic and a non-steroidal anti-inflammatory analgesic on the behavioural responses of calves to dehorning. N. Zeal. Vet. J. 1999, 47, 92–96. [Google Scholar] [CrossRef]
- McMeekan, C.M.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N.; Gregory, N.G. Effects of regional analgesia and/or a non-steroidal anti-inflammatory analgesic on the acute cortisol response to dehorning in calves. Res. Vet. Sci. 1998, 64, 147–150. [Google Scholar] [CrossRef]
- Gottardo, F.; Nalon, E.; Contiero, B.; Normando, S.; Dalvit, P.; Cozzi, G. The dehorning of dairy calves: Practices and opinions of 639 farmers. J. Dairy Sci. 2011, 94, 5724–5734. [Google Scholar] [CrossRef] [PubMed]
- Misch, L.J.; Duffield, T.F.; Millman, S.T.; Lissemore, K.D. An investigation into the practices of dairy producers and veterinarians in dehorning dairy calves in Ontario. Can. Vet. J. Rev. Vet. Can. 2007, 48, 1249–1254. [Google Scholar]
- Model Code of Practice for the Welfare of Animals: Cattle; CSIRO Publishing: Collingwood, Australia, 2005.
- Cozzi, G.; Gottardo, F.; Brscic, M.; Contiero, B.; Irrgang, N.; Knierim, U.; Pentelescu, O.; Windig, J.J.; Mirabito, L.; Eveillard, F.K.; et al. Dehorning of cattle in the EU Member States: A quantitative survey of the current practices. Livest. Sci. 2015, 179, 4–11. [Google Scholar] [CrossRef]
- Reddy, P. Animal Welfare (Care and Procedures) Regulations; LI 2018/50; Government of New Zealand: Wellington, New Zealand, 2018.
- Roberts, C.D.; Windsor, P.A. Innovative pain management solutions in animals may provide improved wound pain reduction during debridement in humans: An opinion informed by veterinary literature. Int. Wound J. 2019, 16, 968–973. [Google Scholar] [CrossRef]
- Lomax, S.; Dickson, H.; Sheil, M.; Windsor, P.A. Topical anaesthesia alleviates short-term pain of castration and tail docking in lambs. Aust. Vet. J. 2010, 88, 67–74. [Google Scholar] [CrossRef]
- Lomax, S.; Sheil, M.; Windsor, P.A. Impact of topical anaesthesia on pain alleviation and wound healing in lambs after mulesing. Aust. Vet. J. 2008, 86, 159–168. [Google Scholar] [CrossRef]
- Espinoza, C.; Lomax, S.; Windsor, P. The effect of a topical anesthetic on the sensitivity of calf dehorning wounds. J. Dairy Sci. 2013, 96, 2894–2902. [Google Scholar] [CrossRef]
- Espinoza, C.A.; McCarthy, D.; White, P.J.; Windsor, P.A.; Lomax, S.H. Evaluating the efficacy of a topical anaesthetic formulation and ketoprofen, alone and in combination, on the pain sensitivity of dehorning wounds in Holstein-Friesian calves. Anim. Prod. Sci. 2016, 56, 1512–1519. [Google Scholar] [CrossRef]
- Molony, V.; Kent, J.E. Assessment of acute pain in farm animals using behavioral and physiological measurements. J. Anim. Sci. 1997, 75, 266–272. [Google Scholar] [CrossRef] [PubMed]
- McMeekan, C.M.; Mellor, D.J.; Stafford, K.J.; Bruce, R.A.; Ward, R.N.; Gregory, N.G. Effects of local anaesthesia of 4 to 8 hours’ duration on the acute cortisol response to scoop dehorning in calves. Aust. Vet. J. 1998, 76, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.J.; Mellor, D.J.; Todd, S.E.; Ward, R.N.; McMeekan, C.M. The effect of different combinations of lignocaine, ketoprofen, xylazine and tolazoline on the acute cortisol response to dehorning in calves. N. Zeal. Vet. J. 2003, 51, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.J.; Mellor, D.J. The assessment of pain in cattle: A review. Cab Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006, 1, 10. [Google Scholar] [CrossRef]
- Mellor, D.J.; Stafford, K.J. Acute castration and/or tailing distress and its alleviation in lambs. N. Zeal. Vet. J. 2000, 48, 33–43. [Google Scholar] [CrossRef]
- Mellor, D.J.; Stafford, K.J. Interpretation of cortisol responses in calf disbudding studies. N. Zeal. Vet. J. 1997, 45, 126–127. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Mellor, D.J.; Stafford, K.J.; Gregory, N.G.; Bruce, R.A.; Ward, R.N. Effect of local anaesthetic combined with wound cauterisation on the cortisol response to dehorning in calves. Aust. Vet. J. 2002, 80, 165–167. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Mellow, D.J.; Stafford, K.J.; Gregory, N.G.; Bruce, R.A.; Ward, R.N. Cortisol responses to dehorning of calves given a 5-h local anaesthetic regimen plus phenylbutazone, ketoprofen, or adrenocorticotropic hormone prior to dehorning. Res. Vet. Sci. 2002, 73, 115–123. [Google Scholar] [CrossRef]
- National Health; Medical Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013.
- Brennan, T.J.; Vandermeulen, E.P.; Gebhart, G.F. Characterization of a rat model of incisional pain. Pain 1996, 64, 493–501. [Google Scholar] [CrossRef]
- Keizer, D.; van Wijhe, M.; Post, W.J.; Uges, D.R.A.; Wierda, J. Assessment of the clinical relevance of quantitative sensory testing with Von Frey monofilaments in patients with allodynia and neuropathic pain. A pilot study. Eur. J. Anaesth. 2007, 24, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Stilwell, G.G.; de Carvalho, R.C.; Lima, M.S.; Broom, D.M. The effect of duration of manual restraint during blood sampling on plasma cortisol levels in calves. Anim. Welf. 2008, 17, 383–385. [Google Scholar]
- Fox, S.M.; Mellor, D.J.; Firth, E.C.; Hodge, H.; Lawoko, C.R.O. Changes in plasma-cortisol concentrations before, during and after analgesia, anesthesia and anesthesia plus ovariohysterectomy in bitches. Res. Vet. Sci. 1994, 57, 110–118. [Google Scholar] [CrossRef]
- Hughan, S.C.; Loose, J.M.; Caddy, D.J.; Canny, B.J.; Tilbrook, A.J.; Young, I.R. Combined xylazine and ketamine as an analgesic regimen in sheep. Aust. Vet. J. 2001, 79, 207–211. [Google Scholar] [CrossRef] [PubMed]
- McMeekan, C.M.; Mellor, D.J.; Stafford, K.J.; Bruce, R.A.; Ward, R.N.; Gregory, N.G. Effects of shallow scoop and deep scoop dehorning on plasma cortisol concentrations in calves. N. Zeal. Vet. J. 1997, 45, 72–74. [Google Scholar] [CrossRef]
- Petrie, N.J.; Mellor, D.J.; Stafford, K.J.; Bruce, R.A.; Ward, R.N. Cortisol responses of calves to two methods of disbudding used with or without local anaesthetic. N. Zeal. Vet. J. 1996, 44, 9–14. [Google Scholar] [CrossRef]
- Moberg, G.P.; Mench, J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Pub.: Wallingford, New Zealand, 2000. [Google Scholar]
- McCarthy, D.; Lomax, S.; Windsor, P.A.; White, P.J. Effect of a topical anaesthetic formulation on the cortisol response to surgical castration of unweaned beef calves. Animal 2016, 10, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F.; Arthington, J.D.; Austin, B.R.; Yelich, J.V. Effects of acclimation to handling on performance, reproductive, and physiological responses of Brahman-crossbred heifers. J. Anim. Sci. 2009, 87, 3403–3412. [Google Scholar] [CrossRef] [Green Version]
- Andrade, O.; Orihuela, A.; Solano, J.; Galina, C.S. Some effects of repeated handling and the use of a mask on stress responses in Zebu cattle during restraint. Appl. Anim. Behav. Sci. 2001, 71, 175–181. [Google Scholar] [CrossRef]
- Crookshank, H.R.; Elissalde, M.H.; White, R.G.; Clanton, D.C.; Smalley, H.E. Effect of transportation and handling of calves upon blood-serum composition. J. Anim. Sci. 1979, 48, 430–435. [Google Scholar] [CrossRef]
- Paull, D.R.; Lee, C.; Colditz, I.G.; Fisher, A.D. Effects of a topical anaesthetic formulation and systemic carprofen, given singly or in combination, on the cortisol and behavioural responses of Merino lambs to castration. Aust. Vet. J. 2009, 87, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Paull, D.R.; Lee, C.; Colditz, I.G.; Atkinson, S.J.; Fisher, A.D. The effect of a topical anaesthetic formulation, systemic flunixin and carprofen, singly or in combination, on cortisol and behavioural responses of merino lambs to mulesing. Aust. Vet. J. 2007, 85, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifying some responses to pain as a stressor. Biol. Anim. Stress 2000, 171–198. [Google Scholar] [CrossRef]
| Treatment Group | Block Total (n) | ||||
|---|---|---|---|---|---|
| CON (n) | D (n) | DTA (n) | |||
| Block | 1 | 4 | 3 | 3 | 10 |
| 2 | 3 | 4 | 3 | 10 | |
| 3 | 3 | 3 | 4 | 10 | |
| Treatment group total (n) | 10 | 10 | 10 | ||
| AUC Time Period | CON | D | DTA | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean | s.e.m. | Mean | s.e.m. | Mean | s.e.m. | ||
| AUC0–24 h | 514.7 a | 160.5 | 1307.9 b | 331.8 | 1317.5 b | 299 | 0.02 |
| AUC0–40 min | 23.7 | 6.2 | 37.6 | 3.9 | 42.5 | 10.7 | 0.07 |
| AUC40 min–1.5 h | 39.0 a | 7.8 | 70.7 b | 9.7 | 66.1 b | 10.9 | 0.02 |
| AUC1.5–4 h | 82.0 a | 20.2 | 181.5 b | 37.2 | 169.6 b | 35.8 | 0.01 |
| AUC4–24 h | 330.6 a | 143.8 | 988.9 b | 284.3 | 1030.1 b | 249.2 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, C.; Lomax, S.; Windsor, P. The Effect of Topical Anaesthesia on the Cortisol Responses of Calves Undergoing Dehorning. Animals 2020, 10, 312. https://doi.org/10.3390/ani10020312
Espinoza C, Lomax S, Windsor P. The Effect of Topical Anaesthesia on the Cortisol Responses of Calves Undergoing Dehorning. Animals. 2020; 10(2):312. https://doi.org/10.3390/ani10020312
Chicago/Turabian StyleEspinoza, Crystal, Sabrina Lomax, and Peter Windsor. 2020. "The Effect of Topical Anaesthesia on the Cortisol Responses of Calves Undergoing Dehorning" Animals 10, no. 2: 312. https://doi.org/10.3390/ani10020312
APA StyleEspinoza, C., Lomax, S., & Windsor, P. (2020). The Effect of Topical Anaesthesia on the Cortisol Responses of Calves Undergoing Dehorning. Animals, 10(2), 312. https://doi.org/10.3390/ani10020312







