The Effect of Kinesiotape on Flexion-Extension of the Thoracolumbar Back in Horses at Trot
Simple Summary
Abstract
1. Introduction
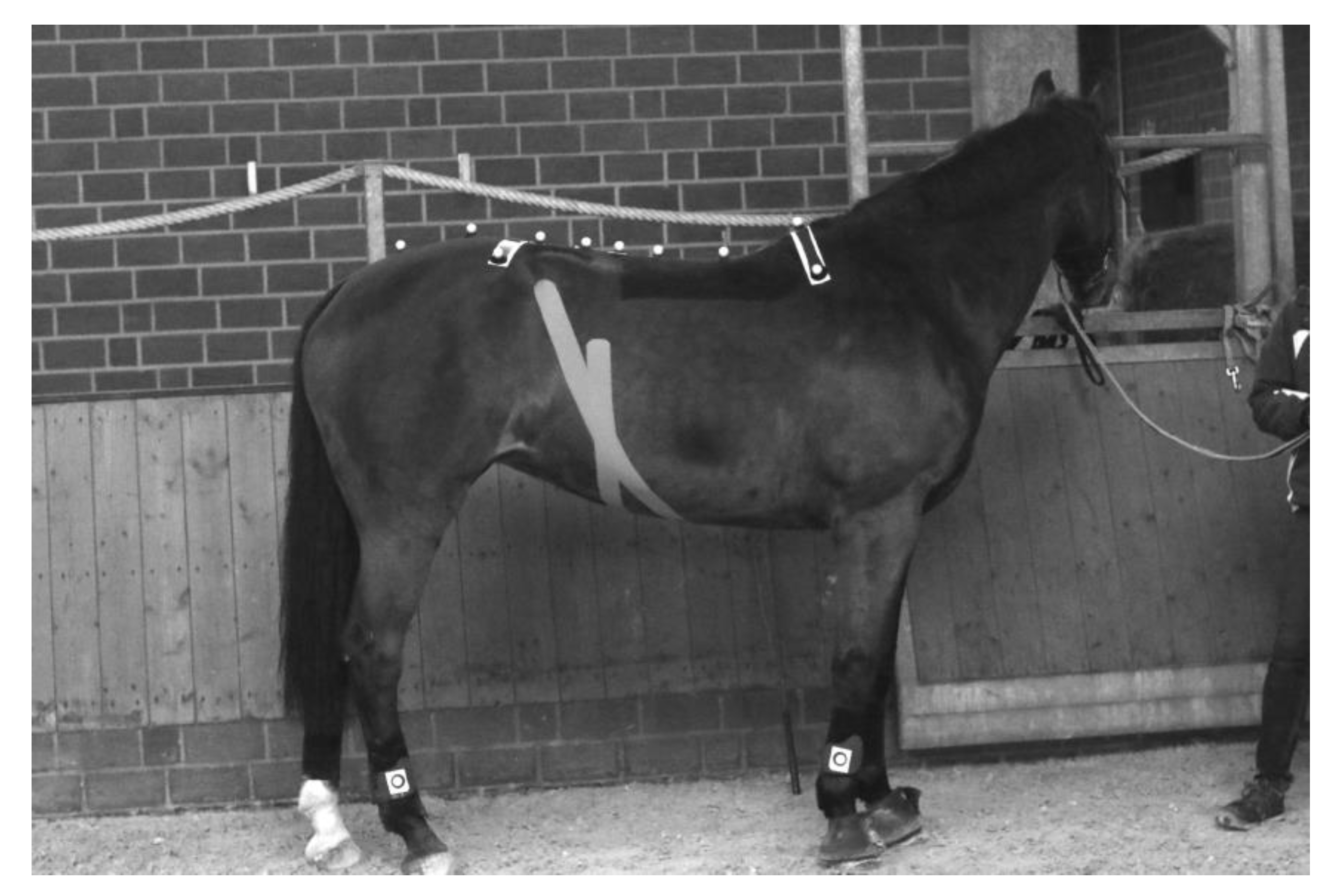
2. Material and Methods
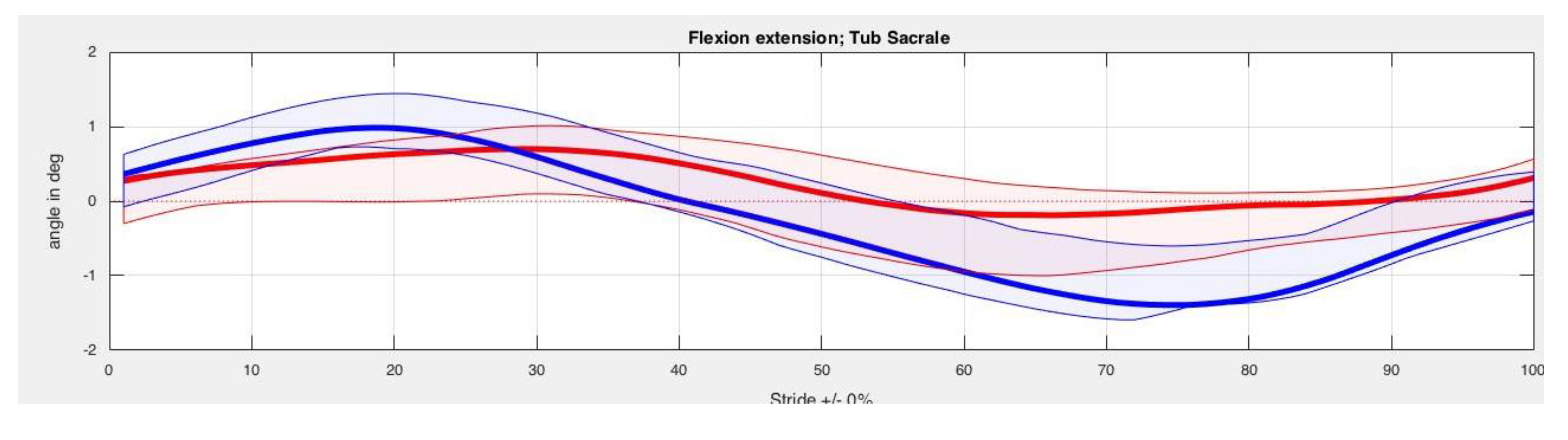
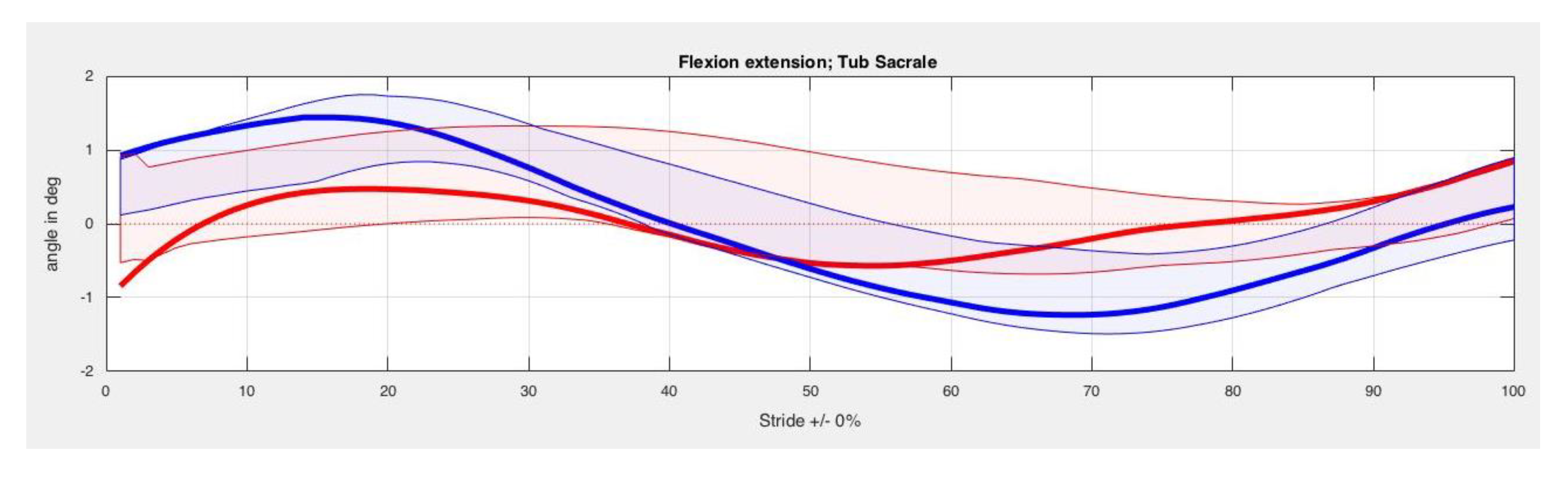
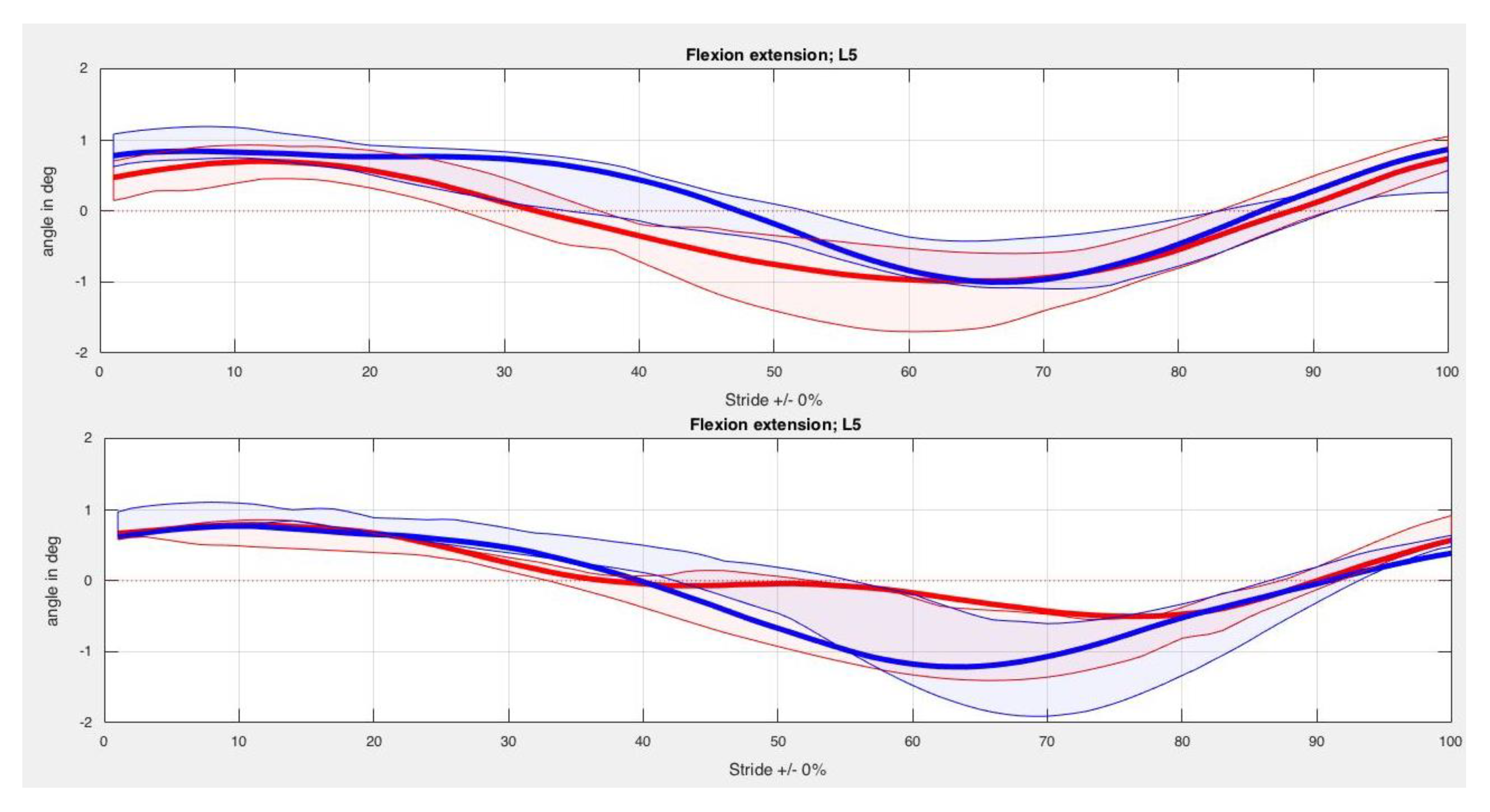
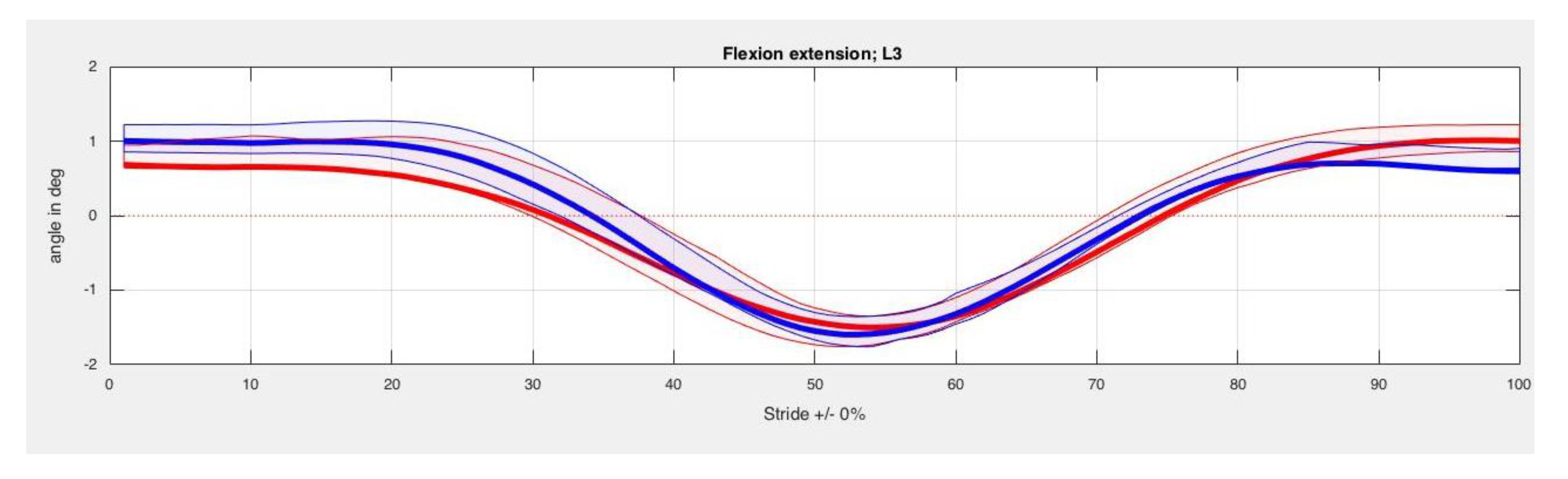
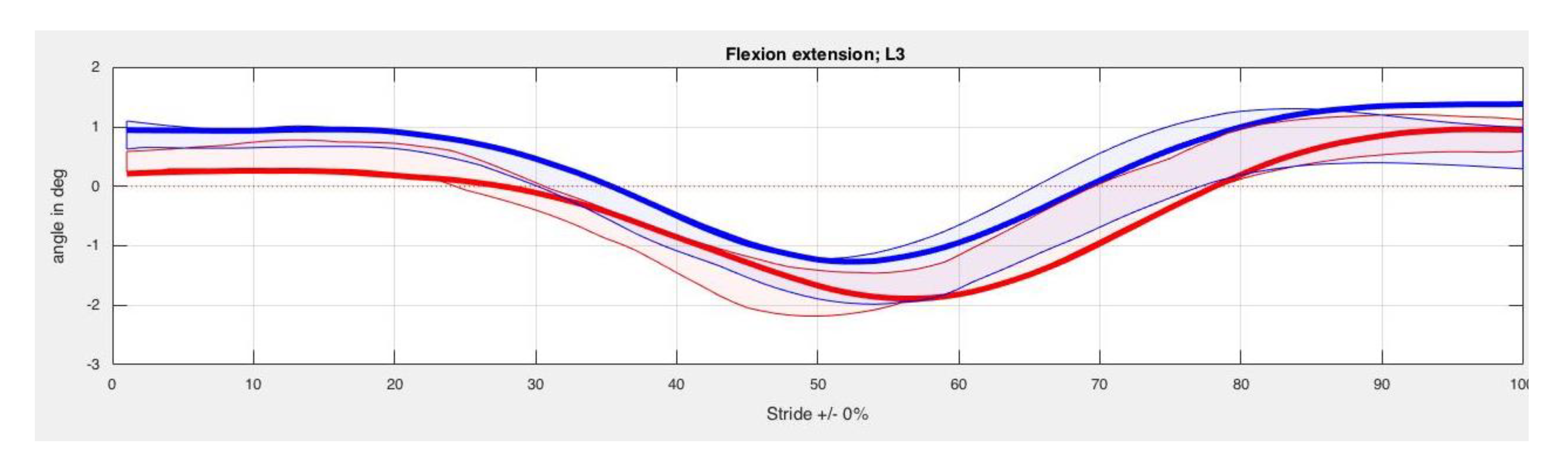
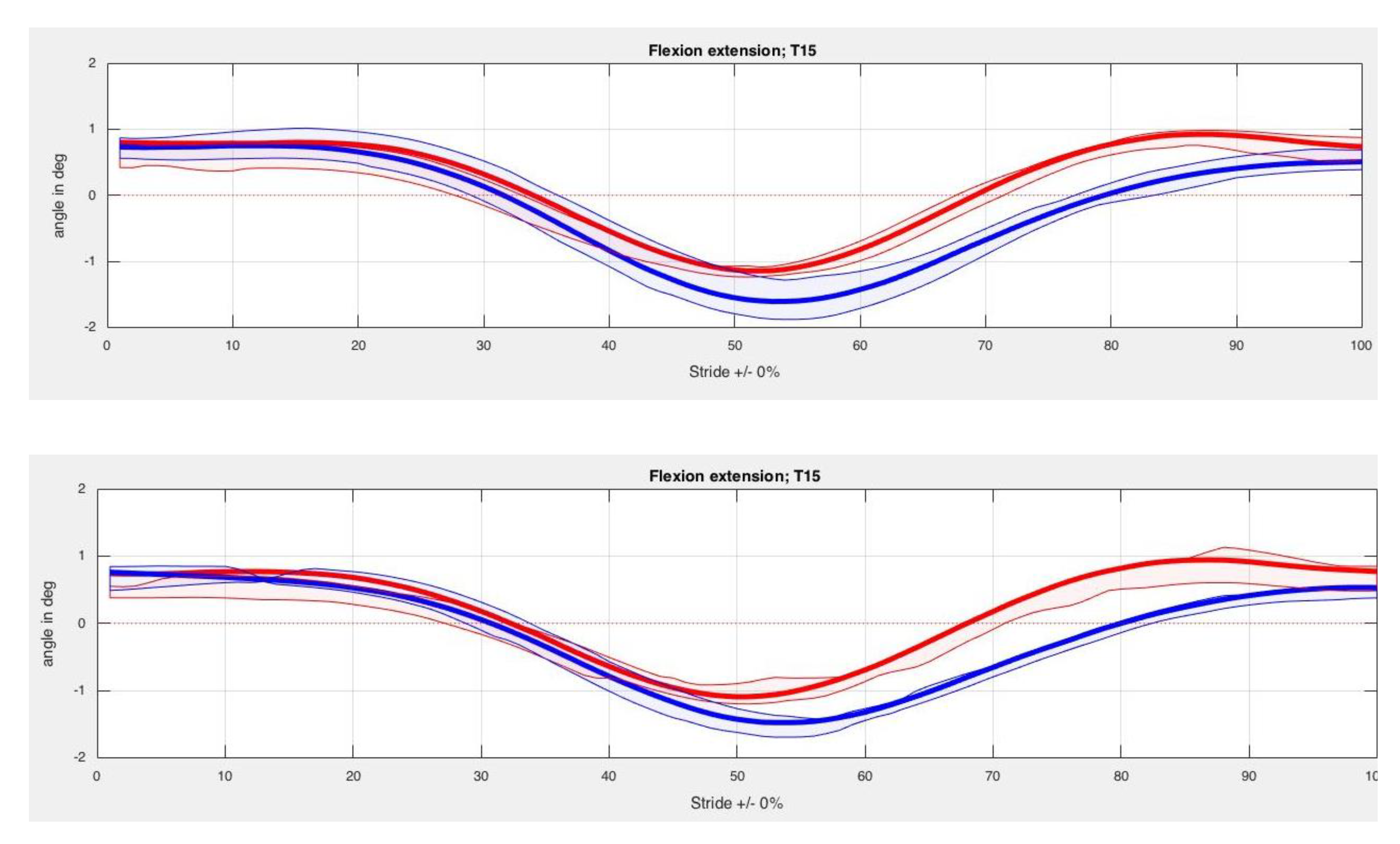
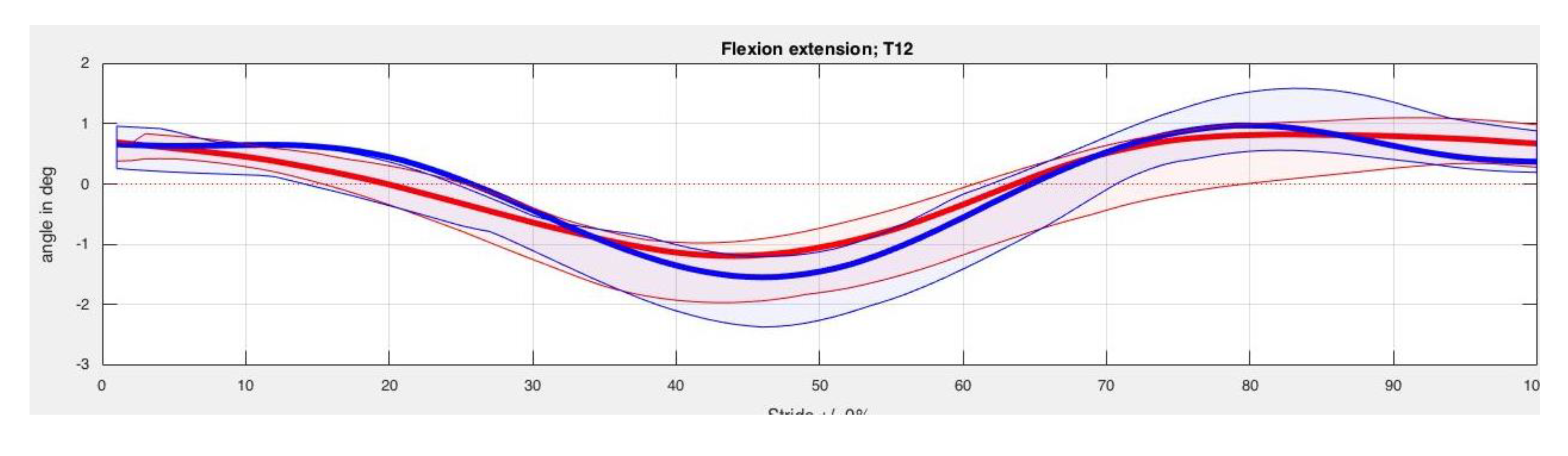
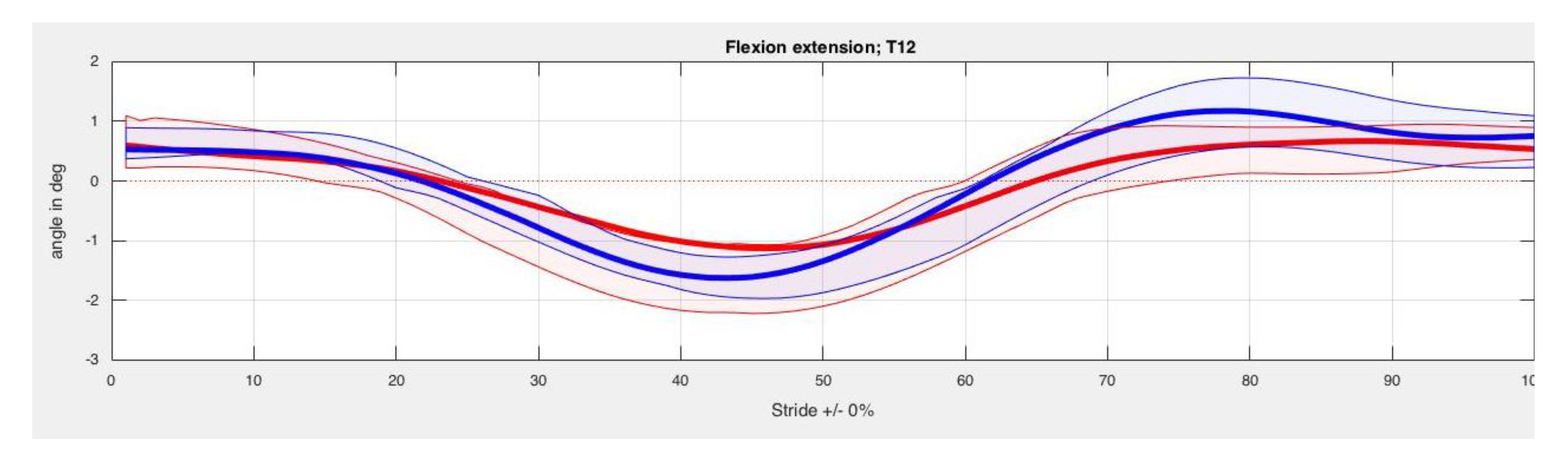
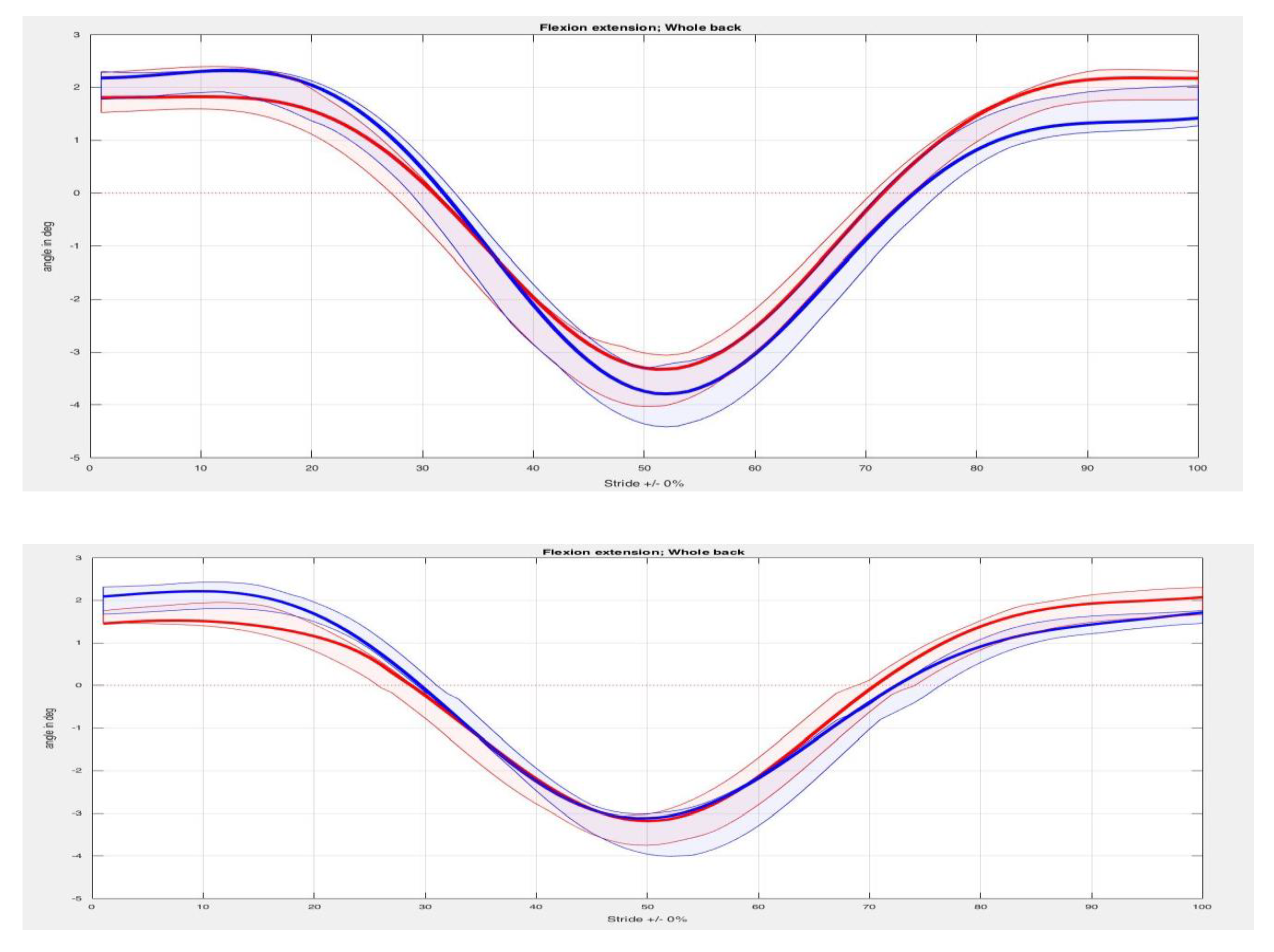
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Muscle Tone Score | Classification | Description |
|---|---|---|
| 0 | Hypotonicity | Loss of muscle tone |
| 1 | Normal | Normal, expected muscle tone for a horse standing square |
| 2 | Mild | Mildly to moderately increased muscle tone |
| 3 | Moderate | Moderately to severely increased muscle tone |
| 4 | Severe | Severely increased muscle tone |
| Pain Score | Classification | Description |
|---|---|---|
| 0 | Pain free | No reaction |
| 1 | Mild | Nose wrinkling, ear flattening, slight spasm on palpation without associated movements |
| 2 | Moderate | Head jerk, teeth bearing, tail lashing, stamping foreleg, aggressive tail flattening, rising hind leg, spasm on palpation with associated local movement (i.e., Pelvic tilt) |
| 3 | Severe | Kicking, biting, rearing, sour attitude, restless, sinking away from the hand |
References
- Stubbs, N.C.; Hodges, P.W.; McGowan, C.M.; Jefcott, L.B.; Hodgson, D.R.; Cowin, G. Functional anatomy of the caudal thoracolumbar and lumbosacral spine in the horse. EVJ 2006, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hyytiäinen, H.K.; Mykkänen, A.K.; Hielm-Björkman, A.K.; Stubbs, N.C.; McGowan, C.M. Muscle fibre type distribution of the thoracolumbar and hindlimb regions of horses: Relating fibre type and functional role. Acta Vet. Scand. 2014, 56, 1–8. [Google Scholar]
- Jeffcott, L.B. Disorders of the thoracolumbar spine of the horse—A survey of 443 cases. EVJ 1980, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Wennerstrand, J. Clinical perspectives on Equine back Kinematics. Ph.D. Thesis, Doctor in Veterinary Medicine, Swedish University of Agricultural Sciences, Uppsala, Sweden.
- Stubbs, N.C.; Riggs, C.M.; Hodges, P.W.; Jefcott, L.B.; Hodgson, D.R.; Clayton, H.M.; McGowan, C.M. Osseous spinal pathology and epaxial muscle ultrasonography in Thoroughbred race horses. EVJ 2010, 38, 654–661. [Google Scholar]
- McGowan, C.M.; Stubbs, N.; Jull, G.A. Equine physiotherapy: A comparative view of the science underlying the profession. EVJ 2007, 39, 90–94. [Google Scholar] [CrossRef]
- Clayton, H.M. Core Training and Rehabilitation in Horses. Vet. Clin. North Am. Equine Pract. 2016, 32, 49–71. [Google Scholar] [CrossRef]
- Walker, V.A.; Dyson, S.J.; Murray, R.C. Effect of Pessoa training aid on temporal, linear and angular variables of the working trot. Vet. J. 2013, 198, 404–411. [Google Scholar] [CrossRef]
- Wennerstrand, J.; Johnston, C.; Rhodin, M.; Roethlisberger-Holm, K.; Drevemo, S. The effect of weighted boots on movement of the back in the asymptomatic riding horse. ECEP 2006, 3, 13–18. [Google Scholar] [CrossRef]
- Cottriall, S.; Ritruechai, P.; Wakeling, J.M. The effects of training aids on the longissimus dorsi of the equine back. Comp. Exerc. Physiol. 2009, 5, 111–114. [Google Scholar] [CrossRef]
- Simons, V.; Weller, R.; Stubbs, N.C.; Rombach, N.; Pfau, T. Objective assessment of back kinematics and movement asymmetry in horses: Effect of elastic resistance band training. EVJ 2015, 47, 11. [Google Scholar] [CrossRef]
- Yoshida, A.; Kahanov, L. The Effect of Kinesio Taping on Lower Trunk Range of Motions. Res. Sports Med. 2007, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mahapatra, D. Taping in sports: A brief update. JHSE 2012, 7, 544–552. [Google Scholar] [CrossRef]
- Bicici, S.; Karatas, N.; Baltaci, G. Effect of athletic taping and Kinesiotaping on measurement of functional performance in basketball players with chronic inversion ankle sprains. Int. J. Sports Phys. Ther. 2012, 9, 665–667. [Google Scholar]
- Hyeyoung, K.; Byonghee, L. The effect of Kinesio Tape on Isokinetic Muscular Function of Horse Racing Jockeys. J. Phys. Ther. Sci. 2013, 25, 1273–1277. [Google Scholar]
- Miralles, I.; Monterde, S.; del Rio, O.; Valero, S.; Montull, S.; Salvat, I. Has Kinesio Tape Effects on Ankle Proprioception? A Randomized Clinical Trial. CK 2014, 68, 9–18. [Google Scholar]
- Gusella, A.; Bettulo, M.; Contiero, F. Kinesiology taping and muscular activity: A myofascial hypothesis and a randomised, blinded trial on healthy individuals. J. Bodyw. Mov. Ther. 2014, 18, 405–411. [Google Scholar] [CrossRef]
- Maughan, R.J.; Lindinger, M.I. Preparing for and competing in the heat: The human perspective. EVJ 1995, 20, 8–15. [Google Scholar] [CrossRef]
- Goel, A.; Shah, A.; Kothari, M.; Gaikwad, S.; Dhande, P.L. Comparative qualitative analysis of osseous anatomy of the craniovertebral junction of tiger, horse, deer and humans. JCVJS 2011, 2, 32–37. [Google Scholar]
- Spaas, J.H.; Guest, D.J.; Van de Valle, G.R. Tendon regeneration in Human and Equine Athletes. J. Sports Med. 2012, 42, 871–890. [Google Scholar] [CrossRef]
- Theoret, C.L.; Wilmink, J.M. Aberrant wound healing in the horse: Naturally occurring conditions reminiscent of those observed in man. Wound Repair Regen. 2013, 21, 365–371. [Google Scholar] [CrossRef]
- Theoret, C.L.; Olutoye, O.O.; Parnell, L.K.; Hicks, J. Equine Exberant Granulation Tissue and Human Keloids: A Comparative Histopathologic Study. VS 2013, 42, 783–789. [Google Scholar] [PubMed]
- Smith, R.K.; Garvician, E.R.; Fortier, L.A. The current ‘state of play’ of regenerative medicine in horses: What the horse can tell the human. JRGM 2014, 9, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Miller, W.H. Structure and function of the skin. In Equine Dermatology, 2nd ed.; Scott, D.W., Miller, W.H., Eds.; Elsevier-Saunders: St. Louis, MO, USA, 2011; pp. 1–34. [Google Scholar]
- Tong, L. Using science to answer the question: Does Whipping Hurt Horses? Available online: http://www.abc.net.au/catalyst/download/Horse_Whipping_report_Dr_Lydia_Tong.pdf (accessed on 18 February 2016).
- Paulekas, R.; Haussler, K.K. Principles and practice of therapeutic exercises for horses. JVES 2009, 29, 870–893. [Google Scholar] [CrossRef]
- Ross, M.W. Movement. In Lameness in the Horse, 2nd ed.; Ross, M., Dyson, S., Eds.; Elsevier-Saunders: St. Louis, MO, USA, 2011; pp. 64–80. [Google Scholar]
- De Heus, P.; Van Oossanen, G.; Van Dierendonck, M.C.; Back, W. A Pressure Algometer Is a Useful Tool to Objectively Monitor the Effect of Diagnostic Palpation by a Physiotherapist in Warmblood Horses. JVES 2010, 30, 310–321. [Google Scholar] [CrossRef]
- Varcoe-Cocks, K.; Sagar, K.N.; Jeffcott, L.B.; McGowan, C.M. Pressure algometry to quantify muscle pain in racehorses with suspected sacroiliac dysfunction. EVJ 2006, 38, 558–562. [Google Scholar] [CrossRef]
- Warner, S.M.; Koch, T.O.; Pfau, T. Inertial sensors for assessment of back movement in horses during locomotion over ground. EVJ 2010, 42, 417–424. [Google Scholar] [CrossRef]
- van den Bogert, A.J.; van Weeren, R.; Schamhardt, H.C. Correction for skin displacement errors in movement analysis of the horse. J. Biomech. 1990, 23, 97–101. [Google Scholar] [CrossRef]
- Poore, L.-A.; Licka, T.L. A Quantitative review of the Equianalysis System for the Equine Locomotion Analysis. JVES 2011, 31, 717–721. [Google Scholar]
- Faber, M.; Schamhardt, H.; van Weeren, R. Methodology and validity of assessing kinematics of the thoracolumbar vertebral column in horses on the basis of skin-fixated markers. Am. J. Vet. Res. 2001, 62, 301–306. [Google Scholar] [CrossRef]
- Greve, L.; Dyson, S.; Pfau, T. Thoracolumbar movement in sound horses trotting in hand and on the lunge. Vet. J. 2017, 220, 95–104. [Google Scholar] [CrossRef]
- Zsoldos, R.R.; Kotschwar, A.; Kotschwar, A.B.; Rodriguez, C.P.; Peham, C.; Licka, T. Activity of the equine rectus abdominis and oblique external abdominal muscles measured by surface EMG during walk and trot on the treadmill. EVJ 2010, 42, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Audigié, F.; Valette, J.P.; Pourcelot, P.; Denoix, J.-M. Effects of treadmill speed on the mechanics of the back in the trotting saddle horse. EVJ 2001, 33, 154–159. [Google Scholar]
- Robert, C.; Valette, J.P.; Denoix, J.-M. The effects of treadmill inclination and speed on the activity of three trunk muscles in the trotting horse. EVJ 2001, 33, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, N.C.; Kaiser, L.J.; Hauptman, J.; Clayton, H.M. Dynamic mobilisation exercises increase cross sectional area of musculus multifidus. EVJ 2011, 43, 522–529. [Google Scholar]
- Van Weeren, P.R. Functional kinematics of the back. Pferdeheilkunde 2006, 22, 602–608. [Google Scholar] [CrossRef]
- Rhodin, M.; Wennerstrand, J.; Drevemo, S.; Roethlisberger-Holm, K.; Johnston, C. The influence of head and neck position on kinematics of the back in riding horses at the walk and trot. EVJ 2005, 37, 7–11. [Google Scholar] [CrossRef]
- Gómez Alvarez, C.B.; Rhodin, M.; Bobbert, M.F.; Meyer, H.; Weishaupt, M.A.; Johnston, C.; Van Weeren, P.R. The effect of head and neck position on the thoracolumbar kinematics in the ridden horse. EVJ 2006, 36, 445–451. [Google Scholar]
- Rhodin, M.; Gómez Alvarez, C.B.; Byström, A.; Johnston, C.; van Weeren, R.; Roepstorff, L.; Weishaupt, M.A. The effect of different head and neck positions on the caudal back and hindlimb kinematics in the elite dressage horse at trot. EVJ 2009, 44, 274–279. [Google Scholar] [CrossRef]
- Grönberg, P. Muscles. In ABC of the horse—Anatomy, Biomechanics, Conditioning; Grönberg, P., Ed.; Otava Book Printing Ltd.: Helsinki, Finland, 2002; pp. 81–199. [Google Scholar]
- Budras, K.-D.; Jahrmärker, G.; Starke, D.; Richter, R. bdominal wall and cavity. In Anatomy of the Horse, 5th ed.; Blackwell, Hannover,: Schlütersee, Germany, 2009; pp. 64–71. [Google Scholar]
- Denoix, J.M. Spinal biomechanics and functional anatomy. Vet. Clin. North Am. Equine Pract. 1999, 15, 27–60. [Google Scholar] [CrossRef]
- Ptak, A.; Konieczny, G.; Stefanska, M. The influence of short-term Kinesiology Taping on force-velocity parameters of the rectus abdominis muscle. JBMR 2013, 26, 91–297. [Google Scholar] [CrossRef]
- Södring Elbrönd, V.; Mark Schultz, R. Myofascia—The unexplored tissue: Myofascial kinetic lines in horses, a model for describing locomotion using comparative dissection studies derived from human lines. Arch. Med. Res. 2015, 3, 1–22. [Google Scholar]
- Molle, S. Kinesio Taping Fundamentals for the Equine Athlete. Vet. Clin. North Am. Equine Pract. 2016, 32, 103–113. [Google Scholar] [CrossRef] [PubMed]
- van Iwaarden, A.; Stubbs, N.C.; Clayton, H.M. Topographical Anatomy of the Equine M. Cutaneus trunci in Relation to the position of the Saddle and Girth. JVES 2012, 32, 519–524. [Google Scholar] [CrossRef]
- Essig, C.M.; Merrit, J.S.; Stubbs, N.C.; Clayton, H.M. Localization of the cutaneous trunci muscle reflex in horses. Am. J. Vet. Res. 2013, 74, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Brouwer, B.; Miles, T.S.; Pitcher, J.B.; Thompson, P.D. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp. Brain Res. 2000, 131, 135–143. [Google Scholar] [CrossRef]
- Wennerstrand, J.; Johnston, C.; Roethlisberger_Holm, K.; Erichsen, C.; Eksell, P.; Drevemo, S. Kinematic evaluation of the back in the sport horse with back pain. EVJ 2004, 36, 707–711. [Google Scholar] [CrossRef]
- Nelson, C.F.; Lawrence, D.J.; Triano, J.J.; Bronfort, G.; Perle, S.M.; Metz, R.D.; Hegetschweiler, K.; LaBrot, T. Chiropractic as spine care: A model for the profession. Chiropr. Man. Therap. 2005, 13, 1–17. [Google Scholar] [CrossRef]
- Zaneb, H.; Kaufmann, V.; Stanek, C.; Peham, C.; Licka, T. Quantitative differences in activities of back and pelvic limb muscles during walking and trotting between chronically lame and nonlame horses. Am. J. Vet. Res. 2009, 70, 1129–1134. [Google Scholar] [CrossRef]
- Lesimple, C.; Fureix, C.; De Margerie, E.; Seneque, E.; Menguy, H.; Hausberger, M. Towards a Postural Indicator of Back Pain in Horses (Equus caballus). PLoS ONE 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Walker, V.A.; Tranquille, C.A.; Dyson, S.J.; Spear, J.; Murray, R.C. Association of a Subjective Muscle Score With Increased Angles of Flexion During Sitting Trot in Dressage Horses. JVES 2016, 40, 6–15. [Google Scholar] [CrossRef]
- Landman, M.A.; de Blaauw, J.A.; van Weeren, R. Field study of the prevalence of lameness in horses with back pain. Vet. Rec. 2004, 155, 165–168. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.; Soutello, R.V.G.; da Fonseca, R.; Costa, C.; Paulo, R.D.L.; Fachiolli, D.F.; Clayton, H.M. Gymnastic Training and Dynamic Mobilization Exercises Improve Stride Quality and Increase Epaxial Muscle Size in Therapy Horses’. JVES 2015, 35, 888–893. [Google Scholar] [CrossRef]
- Haussler, K.K.; Bertram, J.E.; Gellman, K.; Hermanson, J.W. Segmental in vivo vertebral kinematics at the walk, trot and canter: A preliminary study. EVJ 2001, 33, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Tabor, G. The effect of dynamic mobilisation exercises on the equine multifidus muscle and thoracic profile. Master’s Thesis, Master of Equitation Science, University of Plymouth, Plymouth, UK, 2015. [Google Scholar]
| Horse | Age | Breed | Gender | Discipline | Lameness Scale 0–5 | Muscle Tone 0–4 | Pain Score 0–3 |
|---|---|---|---|---|---|---|---|
| 1 | 9 | Wbl | Mare | J | RF 1 | 2 | 2 |
| 2 | 12 | Wbl | Mare | J | RF 1 | 1 | 2 |
| 3 | 9 | Wbl | Mare | J | 0 | 2 | 2 |
| 4 | 9 | Wbl | Gelding | D | 0 | 1 | 1 |
| 5 | 6 | Pony | Mare | J/D | 0 | 1 | 1 |
| 6 | 5 | Wbl | Gelding | J/D | 0 | 2 | 2 |
| 7 | 15 | Wbl | Gelding | D | RF 1 | 3 | 3 |
| 8 | 5 | Friesian | Gelding | D | 0 | 1 | 0 |
| Back Segment | Kinesiotape Mean (SD) in Degrees | Control Mean (SD) in Degrees | p-Value |
|---|---|---|---|
| TS Max left | 20.20 (3.91) | 20.34 (3.74) | 0.33 |
| TS Min left | 17.78 (3.38) | 18.05 (3.43) | 0.40 |
| TS ROM left | 2.42 (1.07) | 2.29 (1.17) | 0.40 |
| TS Max right | 20.50 (3.30) | 20.50 (3.54) | 0.89 |
| TS Min right | 18.29 (3.13) | 18.47 (3.49) | 0.78 |
| TS ROM right | 2.21 (0.71) | 2.03 (0.85) | 0.89 |
| L5 Max left | 0.18 (6.31) | 1.52 (2.74) | 0.67 |
| L5 Min left | −1.99 (7.48) | −0.35 (2.90) | 0.33 |
| L5 ROM left | 2.16 (1.31) | 1.86 (0.66) | 0.67 |
| L5 Max right | 0.20 (5.84) | 1.45 (2.58) | 0.89 |
| L5 Min right | −2.35 (7.39) | −0.36 (2.71) | 0.78 |
| L5 ROM right | 2.56 (1.72) | 1.80 (0.40) | 0.16 |
| L3 Max left | 1.23 (5.74) | −0.75 (2.47) | 0.48 |
| L3 Min left | −1.35 (5.44) | −2.92 (2.38) | 0.58 |
| L3 ROM left | 2.57 (1.01) | 2.17 (1.16) | 0.58 |
| L3 Max right | 1.42 (6.01) | −0.78 (2.54) | 0.58 |
| L3 Min right | −1.49 (4.84) | −3.15 (2.49) | 0.89 |
| L3 ROM right | 2.91 (1.47) | 2.37 (1.18) | 0.29 |
| T15 Max left | −5.65 (2.19) | −5.81 (2.07) | 0.33 |
| T15 Min left | −8.06 (2.50) | −7.91 (2.23) | 0.89 |
| T15 ROM left | 2.41 (0.70) | 2.11 (0.90) | 0.26 |
| T15 Max right | −5.60 (2.15) | −5.71 (2.18) | 0.89 |
| T15 Min right | −7.96 (2.21) | −7.65 (1.97) | 0.29 |
| T15 ROM right | 2.37 (0.69) | 1.94 (0.85) | 0.40 |
| T12 Max left | −13.84 (3.25) | −13.73 (2.48) | 0.78 |
| T12 Min left | −16.21 (3.55) | −15.91 (2.78) | 0.78 |
| T12 ROM left | 2.37 (0.72) | 2.17 (0.92) | 0.61 |
| T12 Max right | −13.37 (3.26) | −13.55 (2.59) | 1.00 |
| T12 Min right | −16.04 (3.60) | −15.76 (2.81) | 0.58 |
| T12 ROM right | 2.66 (0.57) | 2.21 (0.89) | 0.26 |
| WB Max left | 0.37 (0.33) | 0.31 (0.34) | 0.18 |
| WB Min left | −0.05 (0.13) | 0.02 (0.35) | 0.74 |
| WB ROM left | 0.43 (0.27) | 0.29 (0.26) | 0.21 |
| WB Max right | 0.35 (0.31) | 0.32 (0.30) | 0.55 |
| WB Min right | −0.07 (0.14) | 0.00 (0.37) | 0.89 |
| WB ROM right | 0.42 (0.26) | 0.32 (0.23) | 0.67 |
| Horse and Segment | Flex-Ext, ROM, Left Max in Degrees | Flex-Ext, ROM, Left Min in Degrees | Flex-Ext, Left ROM in Degrees | Flex-Ext, ROM, Right Max in Degrees | Flex-Ext, ROM, Right Min in Degrees | Flex-Ext, Right ROM in Degrees |
|---|---|---|---|---|---|---|
| 1. TS | −0.21 | 0.32 | −0.53 | 0.19 | 1.00 | −0.81 |
| 1. L5 | 0.42 | 0.54 | −0.13 | −0.09 | 0.34 | −0.43 |
| 1. L3 | −0.09 | 0.06 | −0.16 | 0.03 | 0.07 | −0.04 |
| 1. T15 | 0.11 | 0.03 | 0.08 | −0.31 | −0.32 | 0.01 |
| 1. T12 | −1.41 | −2.03 | 0.62 | −1.45 | −0.95 | −0.50 |
| 1. WB | 0.05 | 0.01 | 0.04 | −0.02 | 0.00 | −0.01 |
| 2. TS | 0.38 | −0.06 | 0.44 | −0.32 | −0.04 | −0.29 |
| 2. L5 | −0.66 | −0.49 | −0.17 | −0.37 | −0.76 | 0.39 |
| 2. L3 | 0.27 | 0.15 | 0.12 | 0.17 | −0.10 | 0.27 |
| 2. T15 | 0.45 | 0.32 | 0.13 | 0.27 | 0.32 | −0.05 |
| 2. T12 | 0.91 | 2.07 | −1.16 | 0.73 | 0.98 | −0.25 |
| 2. WB | 0.00 | 0.05 | −0.05 | −0.03 | 0.08 | −0.11 |
| 3. TS | −0.23 | 0.13 | −0.36 | 0.31 | 0.46 | −0.16 |
| 3. L5 | −0.05 | −0.18 | 0.13 | −0.22 | −0.04 | −0.18 |
| 3. L3 | 0.72 | −0.19 | 0.91 | 0.31 | 0.06 | 0.25 |
| 3. T15 | −0.10 | −0.46 | 0.36 | −0.31 | −0.71 | 0.40 |
| 3. T12 | −1.34 | −1.54 | 0.21 | −1.23 | −2.00 | 0.76 |
| 3. WB | 0.10 | 0.00 | 0.10 | −0.04 | −0.02 | −0.02 |
| 4. TS | −0.73 | −0.79 | 0.06 | 0.21 | 0.51 | −0.30 |
| 4. L5 | 0.13 | 0.56 | −0.43 | −0.02 | −0.14 | 0.11 |
| 4. L3 | 0.01 | 0.09 | −0.07 | 0.49 | 0.74 | −0.25 |
| 4. T15 | −0.05 | 0.20 | −0.24 | 0.03 | 0.31 | −0.29 |
| 4. T12 | 0.02 | −1.36 | 1.38 | 0.66 | −0.27 | 0.94 |
| 4. WB | 0.03 | 0.02 | 0.01 | 0.00 | 0.03 | −0.02 |
| 5. TS | −0.63 | −1.58 | 0.95 | −1.24 | −2.24 | 1.00 |
| 5. L5 | 1.41 | 1.30 | 0.11 | 1.76 | 1.39 | 0.37 |
| 5. L3 | − 2.90 | −2.20 | −0.70 | −3.06 | −2.96 | −0.10 |
| 5. T15 | 0.89 | 1.36 | −0.47 | 0.76 | 1.20 | −0.44 |
| 5. T12 | 0.66 | −1.48 | −0.22 | −1.41 | −1.08 | −0.33 |
| 5. WB | −0.16 | 0.11 | 0.21 | 0.22 | 0.14 | 0.08 |
| 6. TS | 0.11 | −0.33 | 0.22 | −0.62 | −0.74 | 0.21 |
| 6. L5 | −13.09 | −16.37 | 3.28 | −12.50 | −17.37 | 4.88 |
| 6. L3 | 17.26 | 16.73 | 0.52 | 18.88 | 16.32 | 2.56 |
| 6. T15 | −0.89 | −1.81 | 0.92 | −0.29 | −1.87 | 1.58 |
| 6. T12 | 0.66 | 1.90 | −1.25 | 0.84 | 1.34 | −0.50 |
| 6. WB | −0.16 | −0.10 | −0.06 | 0.05 | −0.10 | 0.15 |
| 7. TS | 0.57 | 0.55 | 0.02 | 0.69 | 0.48 | 0.21 |
| 7. L5 | 0.63 | 0.55 | 0.08 | 1.16 | 0.68 | 0.49 |
| 7. L3 | −0.37 | −0.21 | −0.16 | −0.32 | −0.24 | −0.08 |
| 7. T15 | 0.21 | 0.11 | 0.10 | −0.16 | −0.34 | 0.18 |
| 7. T12 | −0.47 | −0.48 | 0.01 | 0.24 | −0.87 | 1.11 |
| 7. WB | 0.05 | 0.04 | 0.02 | 0.04 | 0.04 | 0.01 |
| 8. TS | −0.13 | −0.33 | 0.20 | 0.83 | −0.81 | 1.64 |
| 8. L5 | 0.48 | 0.98 | −0.50 | 0.32 | −0.07 | 0.39 |
| 8. L3 | 0.92 | −1.85 | 2.77 | 1.07 | −0.62 | 1.69 |
| 8. T15 | 0.65 | −0.92 | 1.58 | 0.92 | −1.07 | 1.99 |
| 8. T12 | 2.52 | 0.53 | 1.99 | 3.02 | 0.63 | 2.39 |
| 8. WB | 0.12 | −0.73 | 0.85 | −0.01 | −0.77 | 0.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ericson, C.; Stenfeldt, P.; Hardeman, A.; Jacobson, I. The Effect of Kinesiotape on Flexion-Extension of the Thoracolumbar Back in Horses at Trot. Animals 2020, 10, 301. https://doi.org/10.3390/ani10020301
Ericson C, Stenfeldt P, Hardeman A, Jacobson I. The Effect of Kinesiotape on Flexion-Extension of the Thoracolumbar Back in Horses at Trot. Animals. 2020; 10(2):301. https://doi.org/10.3390/ani10020301
Chicago/Turabian StyleEricson, Cajsa, Pernilla Stenfeldt, Aagje Hardeman, and Inger Jacobson. 2020. "The Effect of Kinesiotape on Flexion-Extension of the Thoracolumbar Back in Horses at Trot" Animals 10, no. 2: 301. https://doi.org/10.3390/ani10020301
APA StyleEricson, C., Stenfeldt, P., Hardeman, A., & Jacobson, I. (2020). The Effect of Kinesiotape on Flexion-Extension of the Thoracolumbar Back in Horses at Trot. Animals, 10(2), 301. https://doi.org/10.3390/ani10020301





