KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell-Based High-Throughput Screening (HTS)
2.2. Drugs and Chemicals
2.3. Cell Culture
2.4. Tube Formation Assay
2.5. Cell Adhesion Analysis and Survival Rate (FACS Analysis)
2.6. Total RNA Isolation and Real-Time Reverse Transcription PCR
2.7. Immunocytochemistry
2.8. SwissADME Analysis
2.9. Statistical Analysis
3. Results
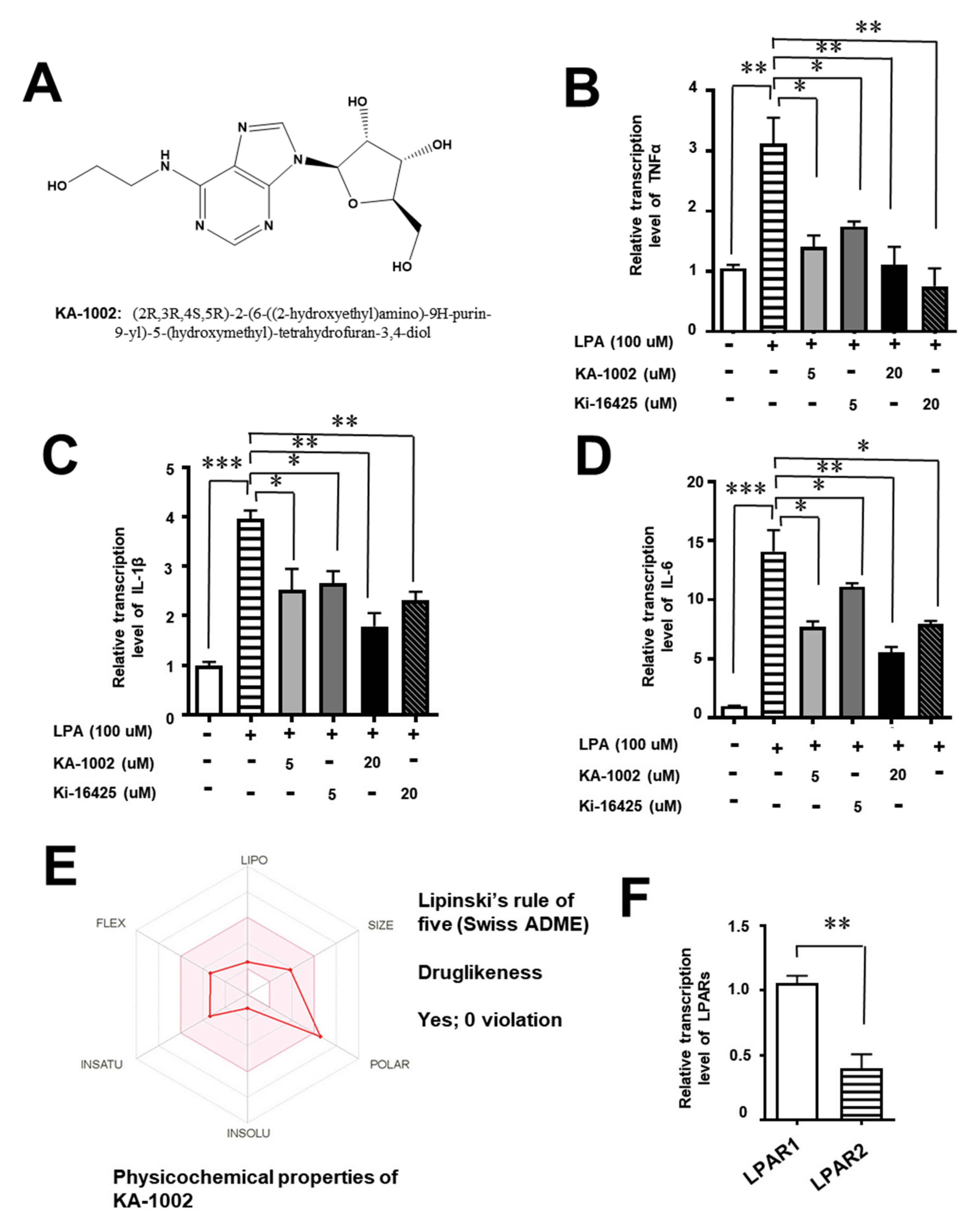
3.1. The LPA-Antagonistic Novel Compound KA-10002 Works against LPA-Induced Inflammatory Cytokine Production from Bovine Pulmonary Blood Endothelial Cells
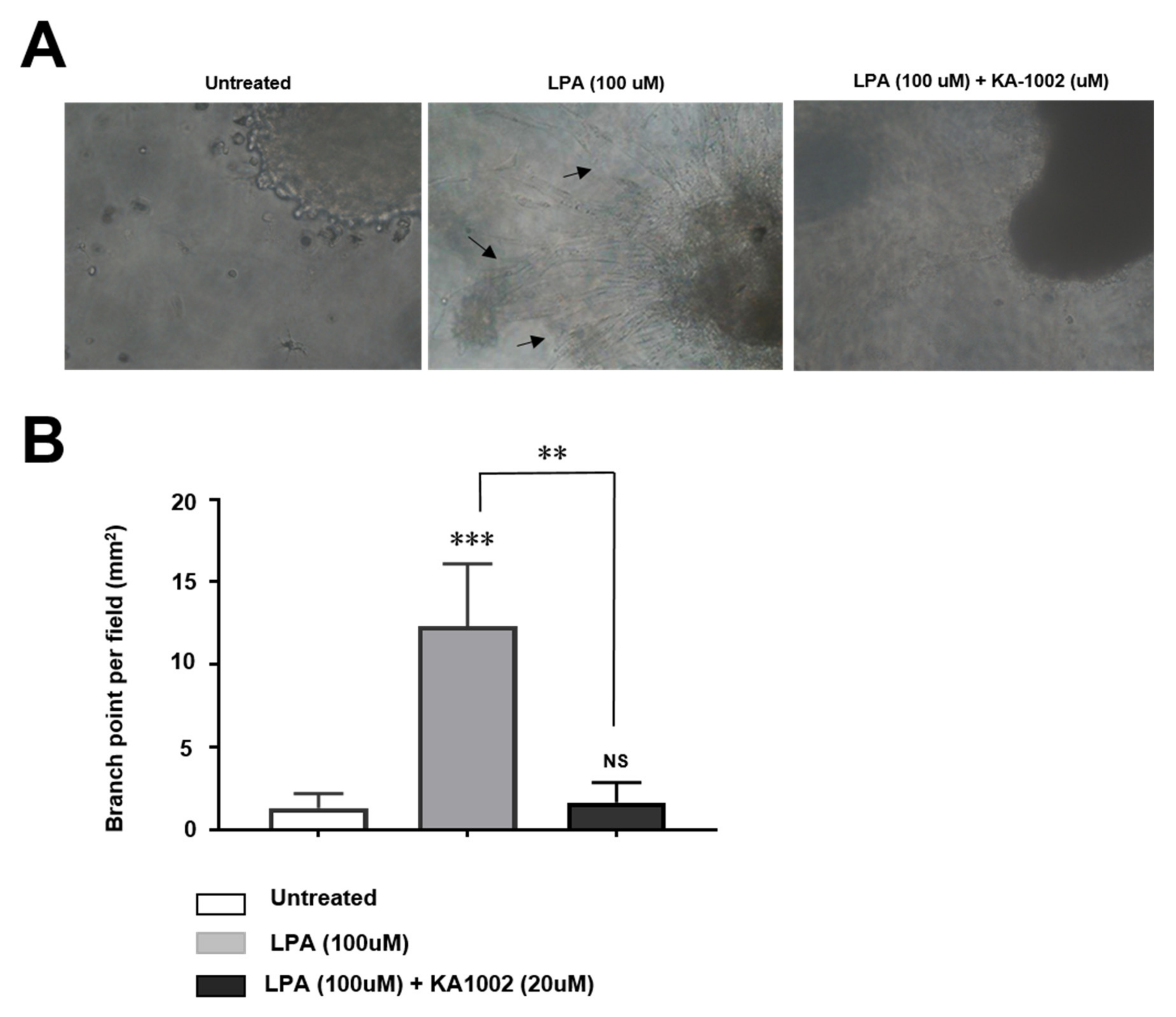
3.2. KA-10002 Alleviates LPA-Induced Inflammatory Angiogenesis
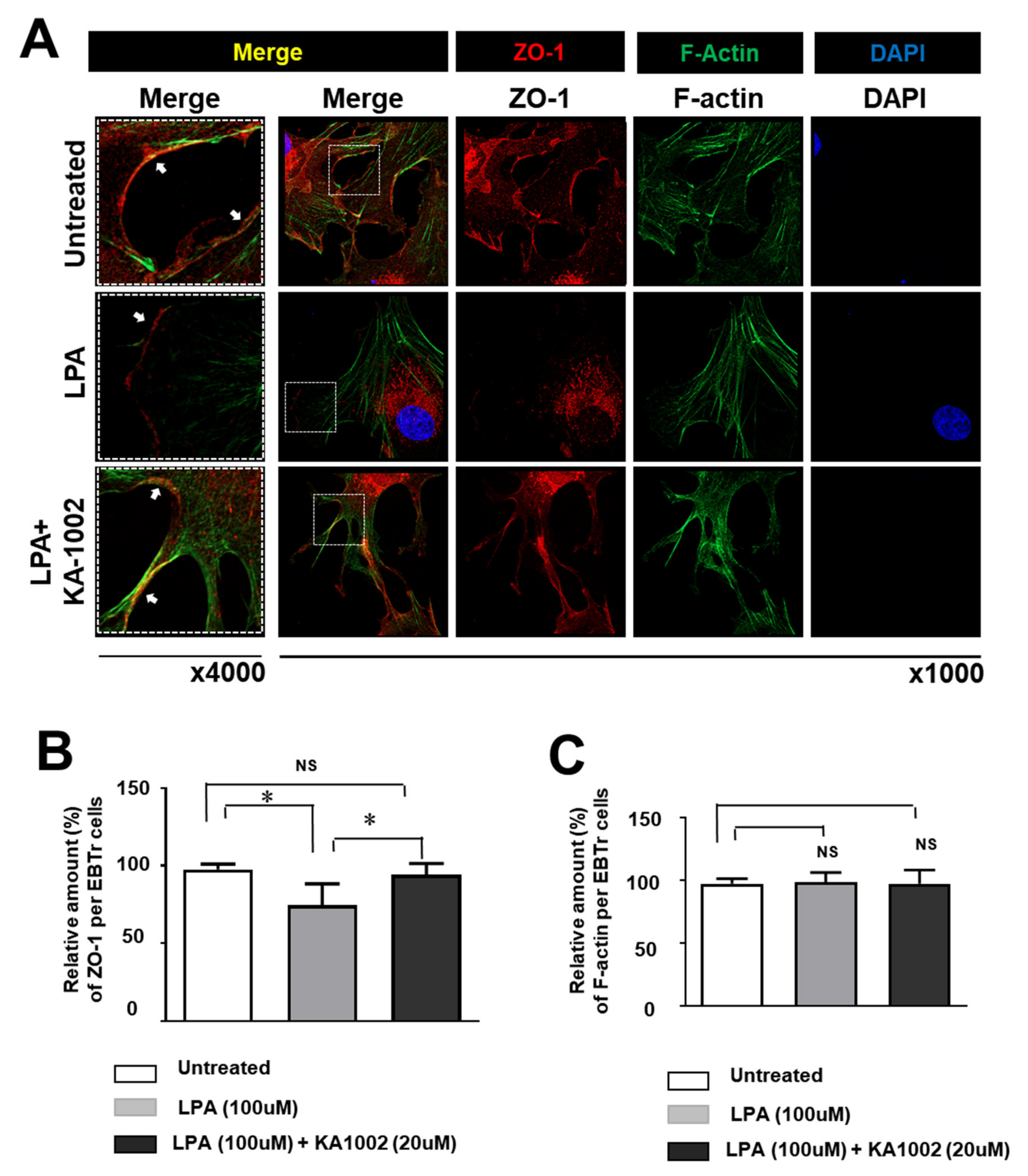
3.3. KA-10002 Alleviated LPA-Induced Downregulated Expression and Disruption of Cellular Arrangement Of ZO-1 in EBTr Cells
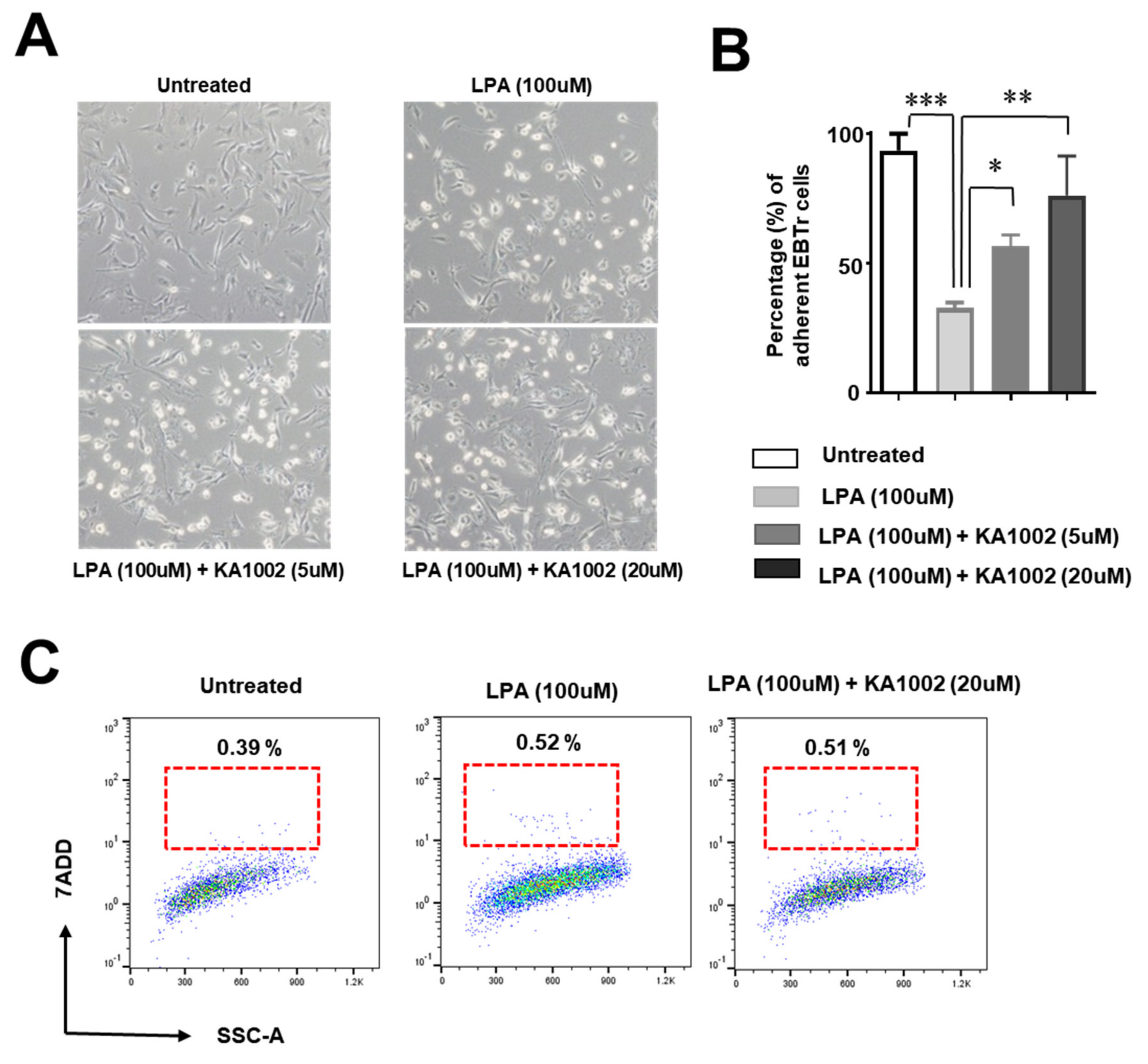
3.4. KA-10002 Alleviated LPA-Induced Loss of Adhesive Capacity in Bovine Tracheal Cells
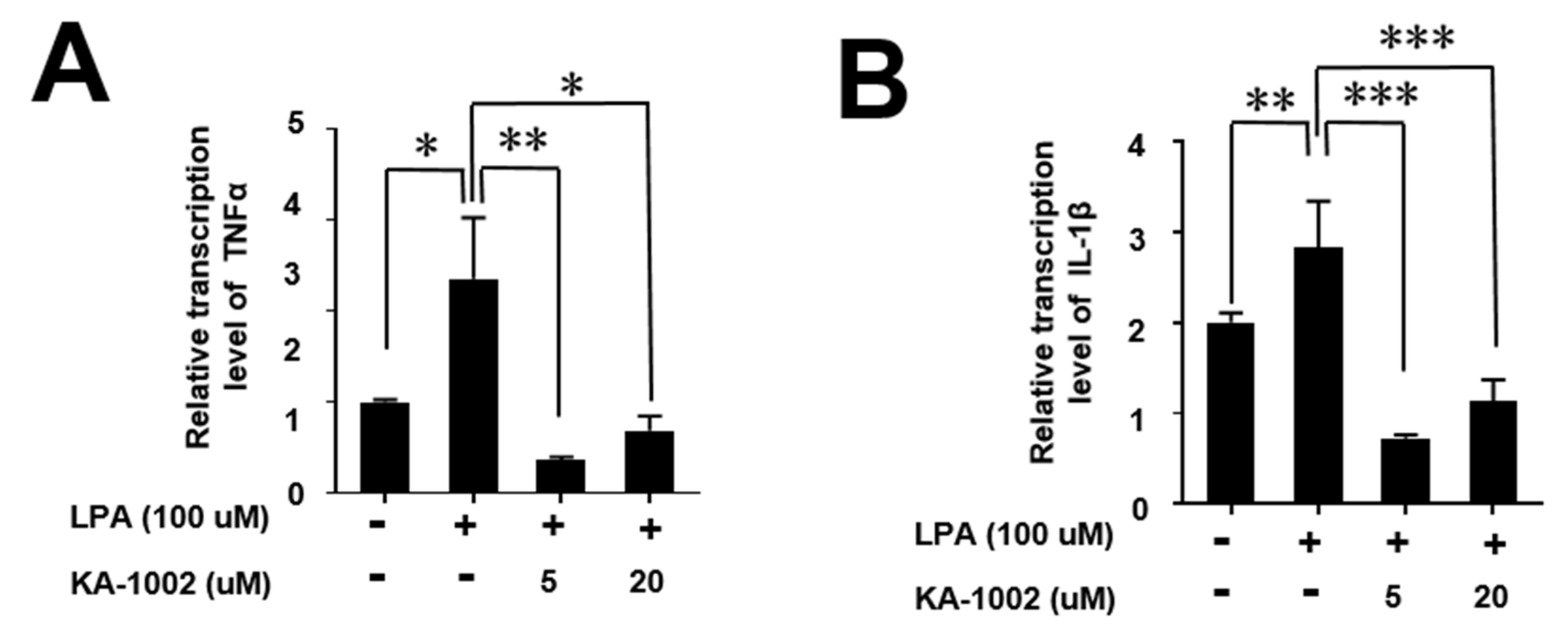
3.5. KA-10002 Alleviated LPA-Induced Inflammatory Cytokine Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubrovsky, S.A.; Van Eenennaam, A.L.; Karle, B.M.; Rossitto, P.V.; Lehenbauer, T.W.; Aly, S.S. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef]
- Rizik, D.G.; Burke, R.F.; Shah, M.G.; Riley, R.D. Recognition and management of an embolized balloon expandable bovine transcatheter heart valve: Treatment of an inverted valve in the aorta. J. Interv. Cardiol. 2014, 27, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A.; Lehmkuhl, H.D.; Cutlip, R.C. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet. Res. 1998, 29, 233–254. [Google Scholar]
- Zhao, Y.; Natarajan, V. Lysophosphatidic acid (LPA) and its receptors: Role in airway inflammation and remodeling. Biochim. Biophys. Acta 2013, 1831, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zhu, W.; Han, Y.; Han, B.; Xu, R.; Deng, L.; Cai, Y.; Cong, X.; Yang, Y.; et al. Specific LPA receptor subtype mediation of LPA-induced hypertrophy of cardiac myocytes and involvement of Akt and NFkappaB signal pathways. J. Cell. Biochem. 2008, 103, 1718–1731. [Google Scholar] [CrossRef]
- Boruszewska, D.; Sinderewicz, E.; Kowalczyk-Zieba, I.; Skarzynski, D.J.; Woclawek-Potocka, I. Influence of lysophosphatidic acid on estradiol production and follicle stimulating hormone action in bovine granulosa cells. Reprod. Biol. 2013, 13, 344–347. [Google Scholar] [CrossRef]
- Panupinthu, N.; Lee, H.Y.; Mills, G.B. Lysophosphatidic acid production and action: Critical new players in breast cancer initiation and progression. Br. J. Cancer 2010, 102, 941–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Alkhatib, A.; Kolls, J.K.; Kondoh, Y.; Lasky, J.A. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J. Thorac. Dis. 2019, 11, S1740–S1754. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Lopez, C.M.; Tucker, A.L.; Lynch, K.R. Lysophosphatidic acid (LPA) and angiogenesis. Angiogenesis 2008, 11, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Gustin, C.; Van Steenbrugge, M.; Raes, M. LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am. J. Physiol. Cell Physiol. 2008, 295, C905–C914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, K.; Arima, N.; Ohgami, M.; Aoki, J. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr. Med. Chem. 2008, 15, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Chen, C.N.; Huang, M.T.; Lee, S.J.; Lin, C.H.; Chang, C.C.; Lee, H. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell Signal. 2008, 20, 1804–1814. [Google Scholar] [CrossRef]
- Hosogaya, S.; Yatomi, Y.; Nakamura, K.; Ohkawa, R.; Okubo, S.; Yokota, H.; Ohta, M.; Yamazaki, H.; Koike, T.; Ozaki, Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: Strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 2008, 45, 364–368. [Google Scholar] [CrossRef]
- Seo, H.; Kim, M.; Choi, Y.; Lee, C.K.; Ka, H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology 2008, 149, 6166–6175. [Google Scholar] [CrossRef]
- Sengupta, S.; Wang, Z.; Tipps, R.; Xu, Y. Biology of LPA in health and disease. Semin. Cell. Dev. Biol. 2004, 15, 503–512. [Google Scholar] [CrossRef]
- Torres, C.; Boruszewska, D.; Batista, M.; Kowalczyk-Zieba, I.; Diniz, P.; Sinderewicz, E.; Saulnier-Blache, J.S.; Woclawek-Potocka, I.; Lopes-da-Costa, L. Lysophosphatidic acid signaling in late cleavage and blastocyst stage bovine embryos. Mediators Inflamm. 2014, 678968. [Google Scholar] [CrossRef] [Green Version]
- Woclawek-Potocka, I. Lysophosphatitic acid action during early pregnancy in the cow: In vivo and in vitro studies. J. Reprod. Dev. 2010, 56, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Jang, J.H.; Oh, S.; Kim, M.; Shin, C.; Jeong, M.; Heo, K.; Park, J.B.; Kim, S.R.; Oh, Y.S. LPA-induced migration of ovarian cancer cells requires activation of ERM proteins via LPA1 and LPA2. Cell Signal. 2018, 44, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Tangkijvanich, P.; Melton, A.C.; Santiskulvong, C.; Yee, H.F., Jr. Rho and p38 MAP kinase signaling pathways mediate LPA-stimulated hepatic myofibroblast migration. J. Biomed. Sci. 2003, 10, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Mellor, H.; Gampel, A.; Scolding, N.J. S1P and LPA trigger Schwann cell actin changes and migration. Eur. J. Neurosci. 2004, 19, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Han, H.J. Autotaxin-LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3-dependent PKC/GSK3beta/beta-catenin and PKC/Rho GTPase pathways. Stem Cells 2015, 33, 819–832. [Google Scholar] [CrossRef]
| Gene Name | 5′-Primer Sequence | 3′-Primer Sequence |
|---|---|---|
| Bovine TNFα | TCTCTCTCACATACCCTGCCA | CCACATCCCGGATCATGCTT |
| Bovine IL-6 | CCAGCCACAAACACTGACCT | CCCCAGCTACTTCATCCGAA |
| Bovine IL-1β | AACGTCCTCCGACGAGTTTC | CCAGCACCAGGGATTTTTGC |
| Bovine LPAR1 | AACACAGGGCCCAATACTCG | CAATTGCAATGGCCAGGAGG |
| Bovine LPAR2 | CCACGAGTCTGTTCGCTACA | GTGGCATTTGCTGTACCCTG |
| Bovine β-actin | TCGGTTGGATCGAGCATTCC | GTGGCTTTTGGGAAGGCAAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.-s.; Kim, M.; Kim, K.S.; Min, Y.K.; Lee, C.H. KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation. Animals 2020, 10, 295. https://doi.org/10.3390/ani10020295
Shin H-s, Kim M, Kim KS, Min YK, Lee CH. KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation. Animals. 2020; 10(2):295. https://doi.org/10.3390/ani10020295
Chicago/Turabian StyleShin, Hee-su, Miok Kim, Kwang Soo Kim, Yong Ki Min, and Chang Hoon Lee. 2020. "KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation" Animals 10, no. 2: 295. https://doi.org/10.3390/ani10020295
APA StyleShin, H.-s., Kim, M., Kim, K. S., Min, Y. K., & Lee, C. H. (2020). KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation. Animals, 10(2), 295. https://doi.org/10.3390/ani10020295





