Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Farm and Animals
2.2. Treatment Protocols
2.3. Behavioural Recording and Monitoring
2.4. Statistical Analysis
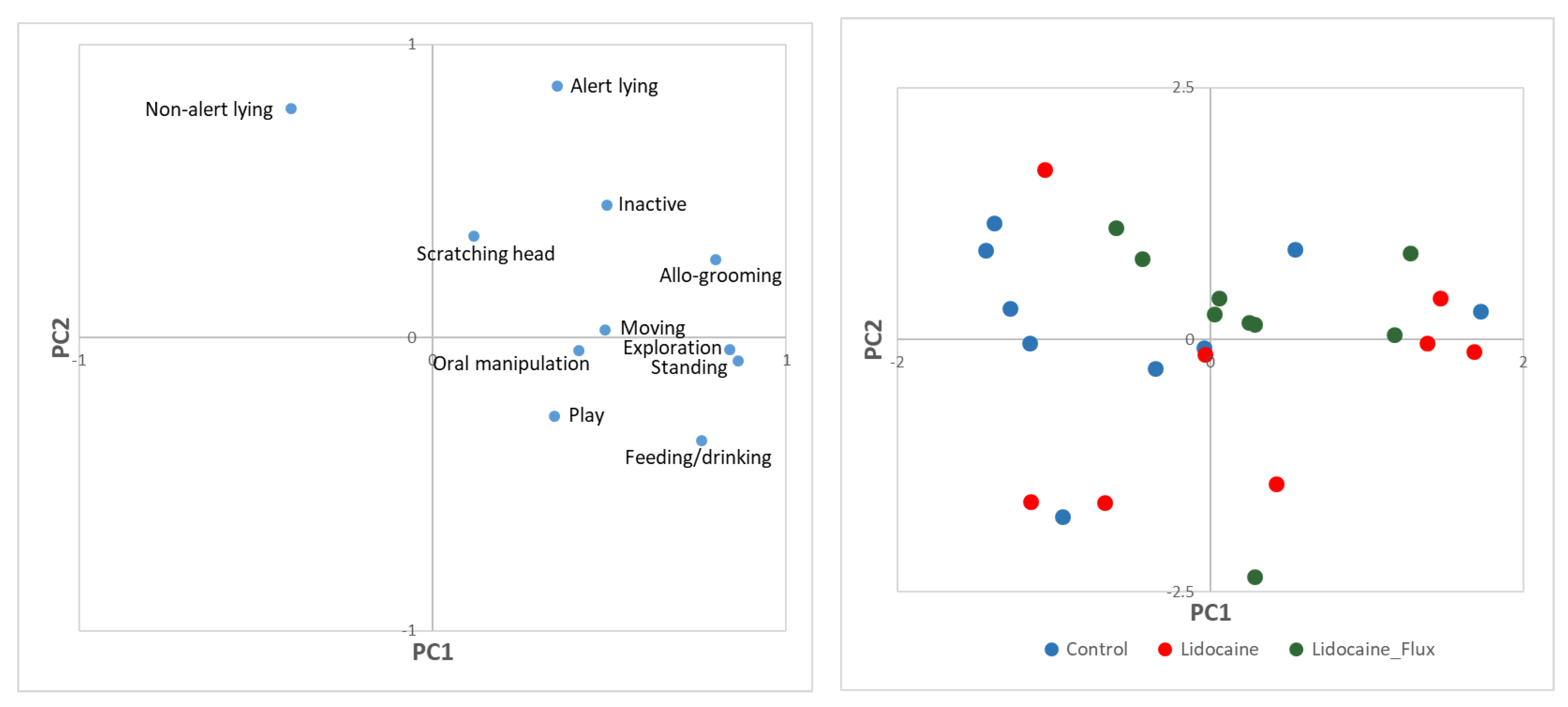
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alvarez, L.; Gutiérrez, J. A first description of the physiological and behavioural responses to disbudding in goat kids. Anim. Welf. 2010, 19, 55–59. [Google Scholar]
- Wagmann, N.; Spadavecchia, C.; Morath-Huss, U.; Schüpbach-Regula, G.; Zanolari, P. Evaluation of anaesthesia and analgesia quality during disbudding of goat kids by certified Swiss farmers. BMC Vet. Res. 2018, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Sherman, D. Goat Medicine, 2nd ed.; Wiley-Blackwell Press: Hoboken, NJ, USA, 2009. [Google Scholar]
- Loretz, C.; Wechsler, B.; Hauser, R.; Rüsch, P. A comparison of space requirements of horned and hornless goats at the feed barrier and in the lying area. Appl. Anim. Behav. Sci. 2004, 87, 275–283. [Google Scholar] [CrossRef]
- Stafford, K.J.; Mellor, D.J. Addressing the pain associated with disbudding and dehorning in cattle. Appl. Anim. Behav. Sci. 2011, 135, 226–231. [Google Scholar] [CrossRef]
- Wathes, C. Guarding the welfare of farm animals. Vet. Rec. 2010, 167, 583–584. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Behavioural response of dairy goat kids to cautery disbudding. Appl. Anim. Behav. Sci. 2017, 194, 42–47. [Google Scholar] [CrossRef]
- Merskey, H.; Bogduk, N. (Eds.) IASP Part III Pain Terms: A Current List with Definitions and Notes on Usage. In Classification of Chronic Pain; IASP Press: Seattle, WA, USA, 1994; pp. 209–214. [Google Scholar]
- McLennan, K.M.; Rebelo, C.J.B.; Corke, M.J.; Holmes, M.A.; Leach, M.C.; Constantino-Casas, F. Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Appl. Anim. Behav. Sci. 2016, 176, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Schibler, L. Fine Mapping Suggests that the Goat Polled Intersex Syndrome and the Human Blepharophimosis Ptosis Epicanthus Syndrome Map to a 100-kb Homologous Region. Genome Res. 2000, 10, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Evaluation of alternatives to cautery disbudding of dairy goat kids using physiological measures of immediate and longer-term pain. J. Dairy Sci. 2018, 101, 5374–5387. [Google Scholar] [CrossRef] [Green Version]
- Hartnack, A.K.; Jordan, M.E.; Roussel, A.J. Complications associated with surgical dehorning in goats: A retrospective study of 239 cases. Vet. Surg. 2018, 47, 188–192. [Google Scholar] [CrossRef]
- Matthews, J.; Dustan, B. Disbudding of goat kids. In Pract. 2019, 41, 433–444. [Google Scholar] [CrossRef] [Green Version]
- BVA; GVS. BVA and Goat Veterinary Society Policy Position on Goat Kid Disbudding and Analgesia. 2018. Available online: https://www.bva.co.uk/media/2749/goat-disbudding.pdf (accessed on 10 September 2019).
- Council of Europe. Recommendations Concerning Goats. 1992. p. Art. 28. Available online: https://www.coe.int/t/e/legal_affairs/legal_co-operation/biological_safety_and_use_of_animals/farming/Rec%20goats%20E.asp (accessed on 10 September 2019).
- Graf, B.; Senn, M. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl. Anim. Behav. Sci. 1999, 62, 153–171. [Google Scholar] [CrossRef]
- Stilwell, G.; Schubert, H.; Broom, D.M. Short communication: Effects of analgesic use postcalving on cow welfare and production. J. Dairy Sci. 2014, 97, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, P.M.; Weary, D.M. Reducing Pain After Dehorning in Dairy Calves. J. Dairy Sci. 2000, 83, 2037–2041. [Google Scholar] [CrossRef]
- Alvarez, L.; De Luna, J.B.; Gamboa, D.; Reyes, M.; Sánchez, A.; Terrazas, A.; Rojas, S.; Galindo, F. Cortisol and pain-related behavior in disbudded goat kids with and without cornual nerve block. Physiol. Behav. 2015, 138, 58–61. [Google Scholar] [CrossRef]
- Ingvast-Larsson, C.; Högberg, M.; Mengistu, U.; Olsén, L.; Bondesson, U.; Olsson, K. Pharmacokinetics of meloxicam in adult goats and its analgesic effect in disbudded kids. J. Vet. Pharmacol. Ther. 2011, 34, 64–69. [Google Scholar] [CrossRef]
- Chandrahas, C.; Saini, A.; Saijpaul, S. Performance assessment of Beetal kids due to disbudding pre-medication under stall-fed conditions. J. Appl. Anim. Res. 2015, 43, 69–76. [Google Scholar]
- Molaei, M.M.; Mostafavi, A.; Kheirandish, R.; Azari, O.; Shaddel, M. Study of disbudding goat kids following injection of clove oil essence in horn bud region. Vet. Res. forum an Int. Q. J. 2015, 6, 17–22. [Google Scholar]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Dowling, S.K.; Cave, V.M.; Lowe, G.L.; Sutherland, M.A. Effect of isoflurane alone or in combination with meloxicam on the behavior and physiology of goat kids following cautery disbudding. J. Dairy Sci. 2018, 101, 3193–3204. [Google Scholar] [CrossRef]
- Molony, V.; Kent, J.E. Assessment of Acute Pain in Farm Animals Using Behavioral and Physiological Measurements. J. Anim. Sci. 1997, 75, 266–272. [Google Scholar] [CrossRef]
- Endo, N.; Yamane, H.; Rahayu, L.P.; Tanaka, T. Effect of repeated adrenocorticotropic hormone administration on reproductive function and hair cortisol concentration during the estrous cycle in goats. Gen. Comp. Endocrinol. 2018, 259, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kavaliers, M. Evolutionary and comparative aspects of nociception. Brain Res. Bull. 1988, 21, 923–931. [Google Scholar] [CrossRef]
- Mogil, J.S.; Crager, S.E. What should we be measuring in behavioral studies of chronic pain in animals? Pain 2004, 112, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Sobayil, F.A. A new simple device for dehorning in small ruminants. Small Rumin. Res. 2007, 67, 232–234. [Google Scholar] [CrossRef]
- Friard, O.P.; Gamba, M. Behavioral Observation Research Interactive Software (BORIS) 2016. Available online: https://iris.unito.it/handle/2318/1589424#.XkHAdWj7TIU (accessed on 20 September 2019).
- Flecknell, P.A. Recognition and Alleviation of Pain in Animals. In Advances in Animal Welfare Science 1985; Springer: Dordrecht, The Netherlands, 1986; pp. 61–77. [Google Scholar]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifying some responses to pain as a stressor. In The Biology of Animal Stress: Basic Principles and Implications for Welfare; CABI Press: Wallingford, UK, 2000; pp. 171–198. [Google Scholar]
- Mattiello; Battini; De Rosa; Napolitano; Dwyer How Can We Assess Positive Welfare in Ruminants? Animals 2019, 9, 758. [CrossRef] [PubMed] [Green Version]
- Morisse, J.P.; Cotte, J.P.; Huonnic, D. Effect of dehorning on behaviour and plasma cortisol responses in young calves. Appl. Anim. Behav. Sci. 1995, 43, 239–247. [Google Scholar] [CrossRef]
- Chandrahas, C.; Saini, A.L.; Malik, D.S.; Mohindroo, J. Effect of local anaesthetics and non-steroidal analgesics on maintenace behaviour of hot- iron disbudded beetal kids under stall-fed conditions. Indian J. Anim. Res. 2013, 47, 407–411. [Google Scholar]
- McMeekan, C.M.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N.; Gregory, N.G. Effects of regional analgesia and/or a non-steroidal anti-inflammatory analgesic on the acute cortisol response to dehorning in calves. Res. Vet. Sci. 1998, 64, 147–150. [Google Scholar] [CrossRef]
- Stilwell, G.; de Carvalho, R.C.; Lima, M.S.; Broom, D.M. Effect of caustic paste disbudding, using local anaesthesia with and without analgesia, on behaviour and cortisol of calves. Appl. Anim. Behav. Sci. 2009, 116, 35–44. [Google Scholar] [CrossRef]
- McGlone, J.J.; Nicholson, R.I.; Hellman, J.M.; Herzog, D.N. The development of pain in young pigs associated with castration and attempts to prevent castration-induced behavioral changes. J. Anim. Sci. 1993, 71, 1441–1446. [Google Scholar] [CrossRef] [Green Version]
- Mintline, E.M.; Stewart, M.; Rogers, A.R.; Cox, N.R.; Verkerk, G.A.; Stookey, J.M.; Webster, J.R.; Tucker, C.B. Play behavior as an indicator of animal welfare: Disbudding in dairy calves. Appl. Anim. Behav. Sci. 2013, 144, 22–30. [Google Scholar] [CrossRef]
- Mooring, M.S.; Gavazzi, A.J.; Hart, B.L. Effects of castration on grooming in goats. Physiol. Behav. 1998, 64, 707–713. [Google Scholar] [CrossRef]
- Kakuma, Y. Hormonal control of grooming behavior in domestic goats. Physiol. Behav. 2003, 78, 61–66. [Google Scholar] [CrossRef]
- Anzuino, K.; Bell, N.J.; Bazeley, K.J.; Nicol, C.J. Assessment of welfare on 24 commercial UK dairy goat farms based on direct observations. Vet. Rec. 2010, 167, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Behaviour Code | Behaviour Type | Description | Key | Excluded Behaviours |
|---|---|---|---|---|
| Alert lying | State event | The kid is lying down, eyes open, head up, reactive to external stimuli | V | A, D, G, P, M, 0, S |
| Allo-grooming | State event | The kid is licking or sniffing other kids | L | A, D, E, G, P, T, I, O, 0, F |
| Evacuation | Point event | The kid urinates or defecates | U | |
| Exploration | State event | The kid is licking or sniffing the housing structures or is climbing up | E | A, L, D, G, P, T, I, O, 0, F |
| Feeding/drinking | State event | The kid’s head is at the feed rack or at the drinker | A | L, D, V, E, G, P, T, I, O, M, 0, F |
| Inactive | State event | The kid is idling inactive and does not interact with the environment nor with other kids | I | A, L, D, V, E, G, P, T, O, M, 0, F |
| Moving | State event | The kid is walking or running to move around | M | A, L, D, V, G, P, T, I, O, 0, F |
| Moving the tail | Point event | The kid is moving the tail | C | |
| Non-alert lying | State event | The kid is lying down, eyes closed, nonreactive to any external stimulus. 1 = head up; 2 = head down (on the soil or on its own body) | D | A, L, V, E, G, P T, I O, M, 0, S, F |
| Nonvisible | State event | The kid is not visible (zero), e.g., hidden in a blind corner | 0 | A, L, D, V, E, G, P, T, I, O, M, F, S |
| Oral manipulation | State event | The kid is biting or chewing an object or the litter, or is moving its mouth with no apparent purpose | O | A, L, D, V, E, G, P, T, I, M, 0, F |
| Play | State event | The kid is playing alone (runs, jumps on the walls or in the air) | P | A, L, D, V, E, G, T, I, O, M, 0, F |
| Scratching head | State event | The kid is scratching its head with the legs or against an object | T | A, L, D, V, E, G, P, I, O, M, 0, F |
| Self-grooming | State event | The kid is licking itself (any part of the body) | F | A, L, D, V, E, G, P, T, I, O, M |
| Shaking | Point event | The kid shakes the head or ears | Q | |
| Social play | State event | The kid is playing with other kids or is encouraging them to play (runs, chases, jumps, play flights) | G | F |
| Standing | State event | The kid is standing on four legs | S | D, V, 0 |
| Stargazing | Point event | The kid brings the head back, looks up, with no visual stimulus present | X | |
| Stretching | Point event | The kid is stretching | Z | |
| Vocalisation | Point event | The kid emits any type of sound | B | |
| Yawning | Point event | The kid yawns | Y |
| Behaviour | Control-GC (n = 9) | Lidocaine-GL (n = 8) | Lidocaine_Flux GL+F (n = 9) | p Value |
|---|---|---|---|---|
| Feeding/drinking | 4.39 ± 3.41 | 5.85 ± 3.75 | 5.63 ± 2.32 | 0.594 |
| Allo-grooming | 1.12 ± 1.25 | 1.51 ± 1.23 | 1.29 ± 0.72 | 0.764 |
| Non-alert lying | 43.49 ± 29.48 | 29.05 ± 28.15 | 34.03 ± 17.36 | 0.501 |
| Alert lying | 24.46 ± 14.98 | 21.29 ± 16.55 | 29.32 ± 11.69 | 0.521 |
| Exploration | 2.66 ± 1.95a | 6.25 ± 4.57b | 4.92 ± 3.04ab | 0.096 |
| Playing | 0.52 ± 0.81a | 1.50 ± 1.81ab | 1.94 ± 1.46b | 0.113 |
| Scratching head | 0.68 ± 0.71a | 1.74 ± 1.82ab | 3.04 ± 3.18b | 0.089 |
| Inactive | 17.32 ± 13.04 | 13.01 ± 11.84 | 20.72 ± 13.59 | 0.480 |
| Oral manipulation | 0.77 ± 1.03 | 0.44 ± 1.04 | 0.60 ± 0.81 | 0.783 |
| Moving | 2.52 ± 2.78 | 2.85 ± 1.40 | 2.78 ± 1.33 | 0.936 |
| Standing | 10.58 ± 5.48a | 19.05 ± 7.47b | 18.21 ± 5.25b | 0.014* |
| Behaviour | Control-GC (n = 9) | Lidocaine-GL (n = 8) | Lidocaine_Flux GL+F (n = 9) | p Value |
|---|---|---|---|---|
| Feeding/drinking | 8.22 ± 5.61a | 15.38 ± 8.67b | 13.67 ± 5.59ab | 0.090 |
| Allo-grooming | 10.11 ± 8.04 | 16.25 ±11.55 | 14.11 ± 6.62 | 0.361 |
| Non-alert lying | 8.33 ± 4.50 | 8.13 ± 8.24 | 8.56 ± 4.45 | 0.989 |
| Alert lying | 19.11 ± 12.49 | 21.38 ± 17.84 | 18.44 ± 7.60 | 0.890 |
| Exploration | 18.44 ± 10.63a | 39.88 ± 21.25b | 31.11 ± 13.02ab | 0.030* |
| Playing | 4.44 ± 4.28a | 12.00 ± 11.20ab | 13.56 ± 8.13b | 0.062 |
| Scratching head | 11.89 ± 11.43 | 30.50 ± 33.37 | 33.22 ± 28.87 | 0.192 |
| Inactive | 25.11 ± 15.69 | 27.00 ± 18.68 | 28.33 ± 17.39 | 0.924 |
| Oral manipulation | 1.78 ± 2.77 | 1.75 ± 2.49 | 2.78 ± 3.73 | 0.729 |
| Moving | 31.11 ± 18.72a | 65.50 ± 38.95b | 56.44 ± 33.81ab | 0.081 |
| Standing | 39.44 ± 19.91a | 76.38 ± 38.50b | 63.89 ± 28.23b | 0.047* |
| Behaviour | Control-GC (n = 9) | Lidocaine-GL (n = 8) | Lidocaine_Flux-GL+F (n = 9) | p Value |
|---|---|---|---|---|
| Feeding/drinking | 49.45 ± 33.23 | 34.14 ± 19.95 | 37.99 ± 14.25 | 0.400 |
| Allo-grooming | 8.18 ± 4.62 | 8.34 ± 3.03 | 8.07 ± 2.18 | 0.987 |
| Non-alert lying | 411.89 ± 242.49 | 346.28 ± 348.39 | 360.04 ± 181.40 | 0.860 |
| Alert lying | 134.29 ± 120.13 | 77.35 ± 52.86 | 142.53 ± 62.82 | 0 .256 |
| Exploration | 12.69 ± 4.92 | 13.18 ± 6.07 | 14.02 ± 7.65 | 0.904 |
| Playing | 0.08 ± 0.07 | 0.11 ± 0.07 | 0.16 ± 0.13 | 0.238 |
| Scratching head | 5.43 ± 3.13 | 5.03 ± 1.09 | 7.09 ± 3.36 | 0.283 |
| Inactive | 59.36 ± 52.29 | 34.31 ± 20.38 | 61.08 ± 46.17 | 0.374 |
| Oral manipulation | 27.62 ± 51.56 | 8.96 ± 12.96 | 10.50 ± 11.81 | 0.413 |
| Moving | 6.86 ± 3.25a | 4.12 ± 1.40b | 4.66 ± 1.01b | 0.033* |
| Standing | 26.40 ± 10.19 | 23.34 ± 6.99 | 27.91 ± 8.78 | 0.565 |
| Behaviour. | Control-GC (n = 9) | Lidocaine-GL (n = 8) | Lidocaine_Flux (GL+F) (n = 9) | p Value |
|---|---|---|---|---|
| Eliminatory Behaviour | 1.33 ± 1.22 | 1.75 ± 0.71 | 1.78 ± 1.48 | 0.705 |
| Moving tail | 39.00 ± 44.59 | 36.63 ± 31.00 | 47.78 ± 33.93 | 0.720 |
| Yawning | 0.00 ± 0.00 | 0.25 ± 0.71 | 0.22 ± 0.67 | 0.573 |
| Shaking | 14.33 ± 12.92 | 25.00 ± 24.91 | 29.22 ± 11.78 | 0.133 |
| Stargazing | 0.78 ± 1.30 | 0.63 ± 0.916 | 1.67 ± 2.83 | 0.980 |
| Stretching | 1.44 ± 1.42 | 1.38 ± 1.19 | 1.44 ± 1.88 | 0.936 |
| Vocalization | 0.33 ± 0.50 | 19.25 ± 51.22 | 0.67 ± 1.00 | 0.105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajuda, I.; Battini, M.; Mattiello, S.; Arcuri, C.; Stilwell, G. Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding. Animals 2020, 10, 277. https://doi.org/10.3390/ani10020277
Ajuda I, Battini M, Mattiello S, Arcuri C, Stilwell G. Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding. Animals. 2020; 10(2):277. https://doi.org/10.3390/ani10020277
Chicago/Turabian StyleAjuda, Inês, Monica Battini, Silvana Mattiello, Cecilia Arcuri, and George Stilwell. 2020. "Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding" Animals 10, no. 2: 277. https://doi.org/10.3390/ani10020277
APA StyleAjuda, I., Battini, M., Mattiello, S., Arcuri, C., & Stilwell, G. (2020). Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding. Animals, 10(2), 277. https://doi.org/10.3390/ani10020277






