Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Turtle Models
2.2. Study Sites
2.3. Field Trials
2.4. Data Analyses
3. Results
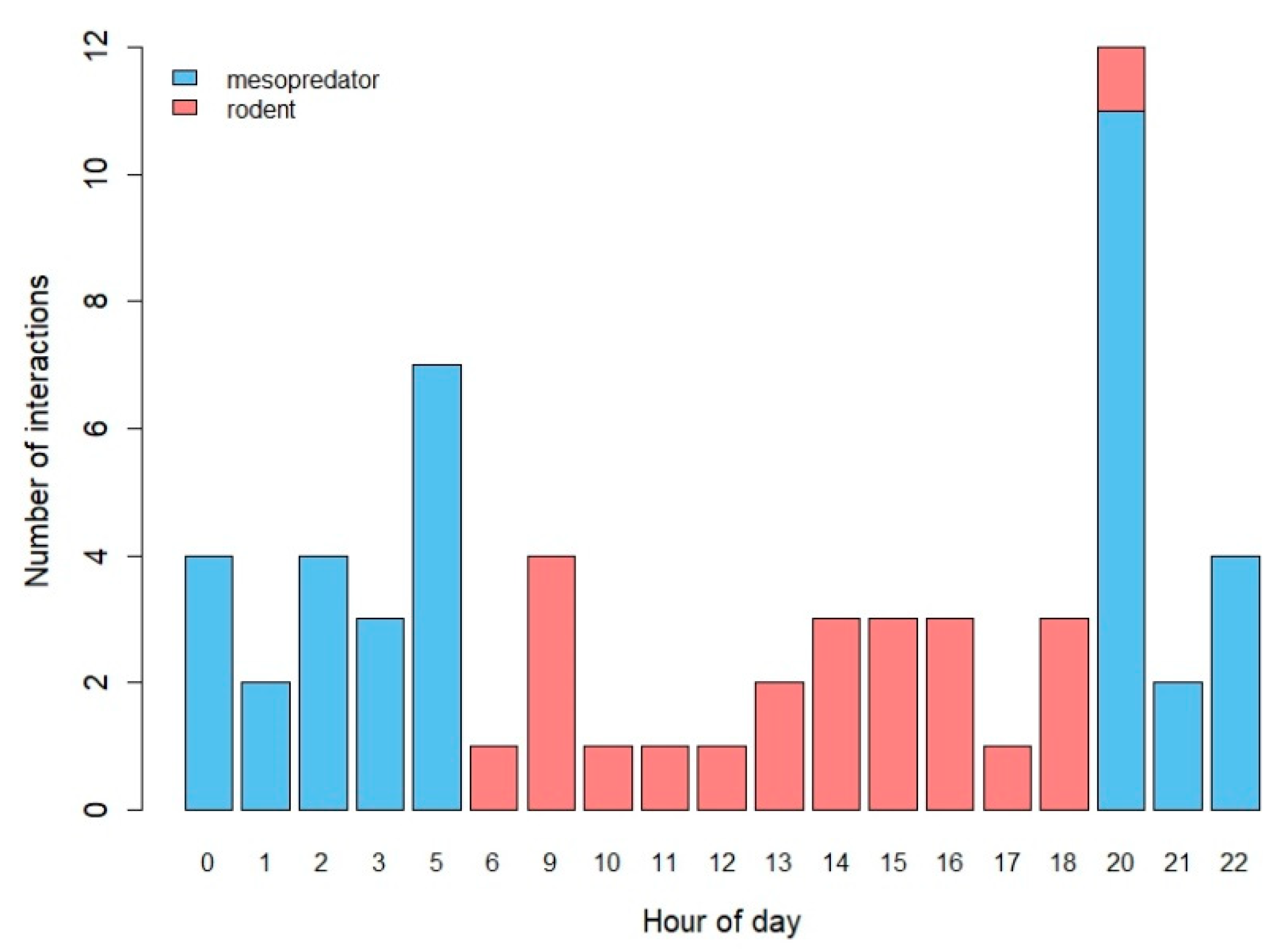
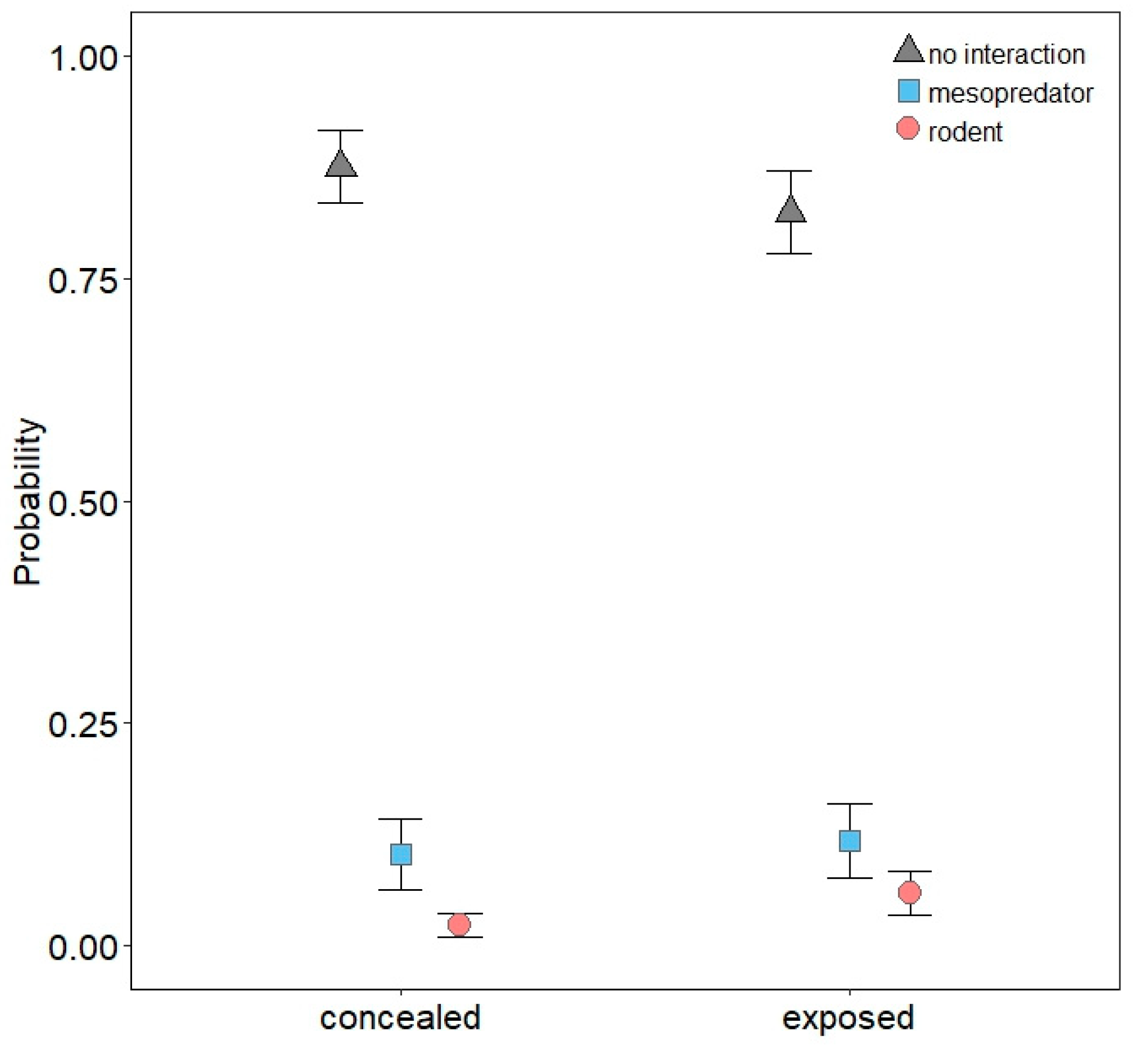
3.1. Descriptive Results
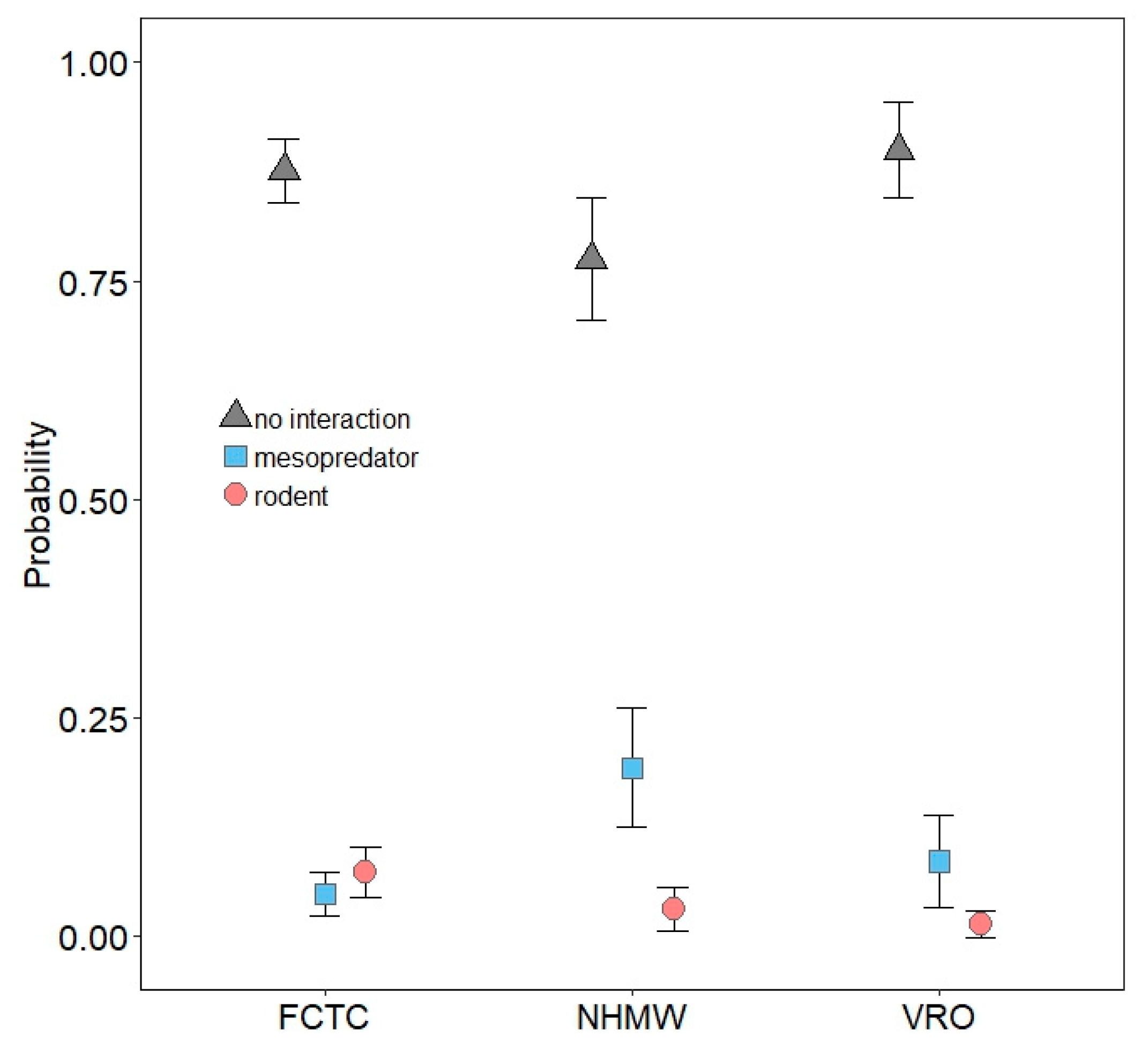
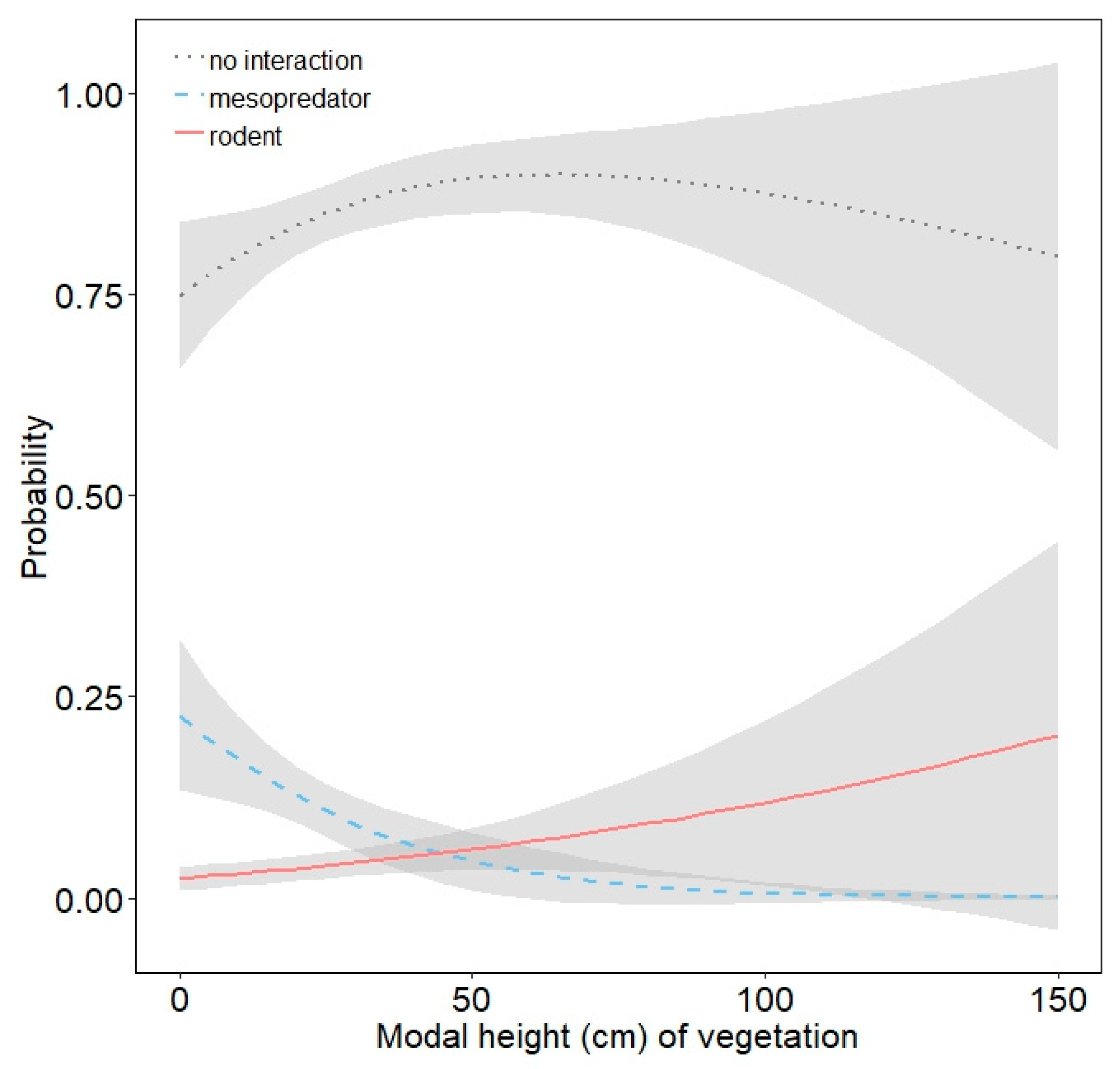
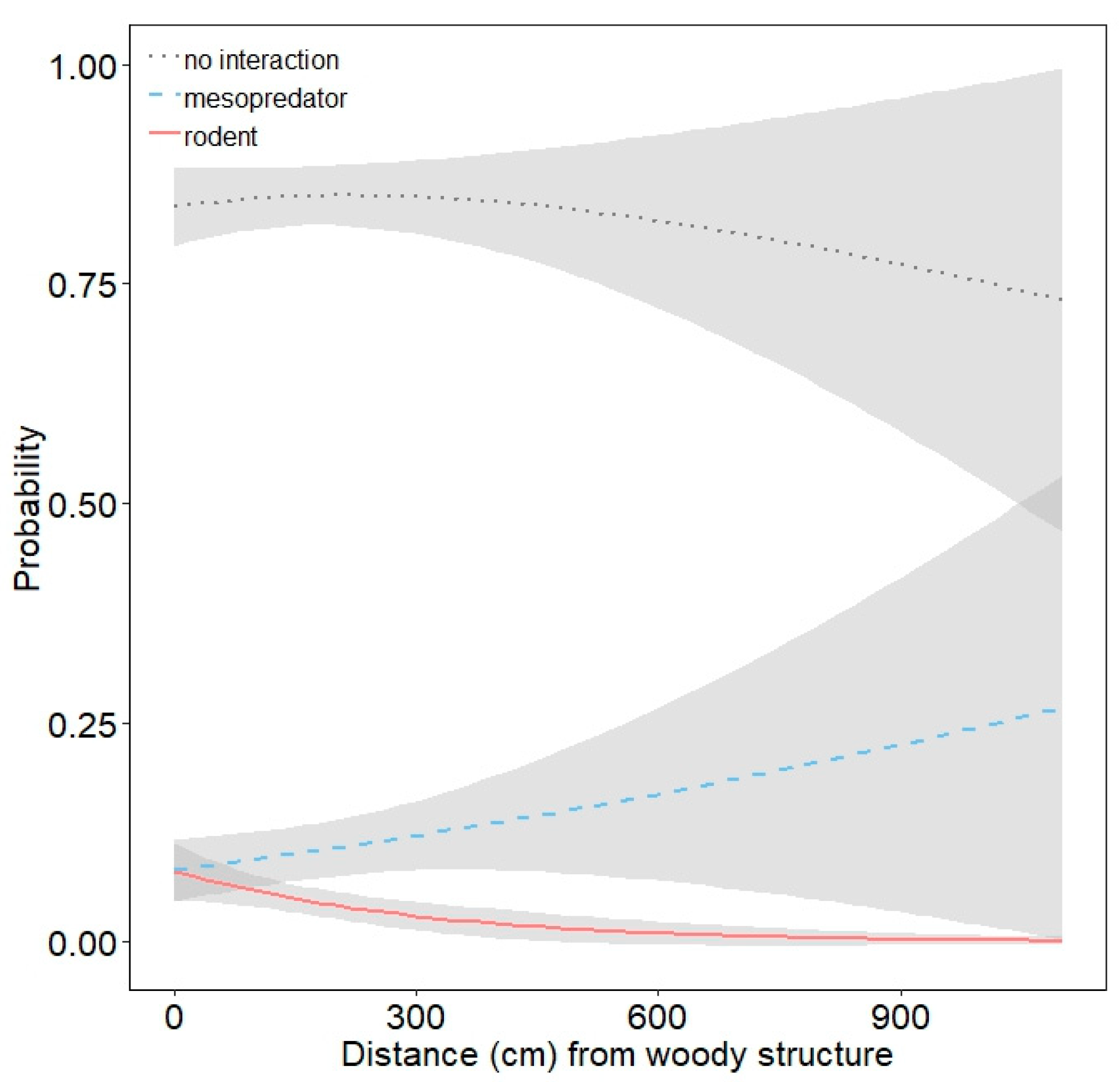
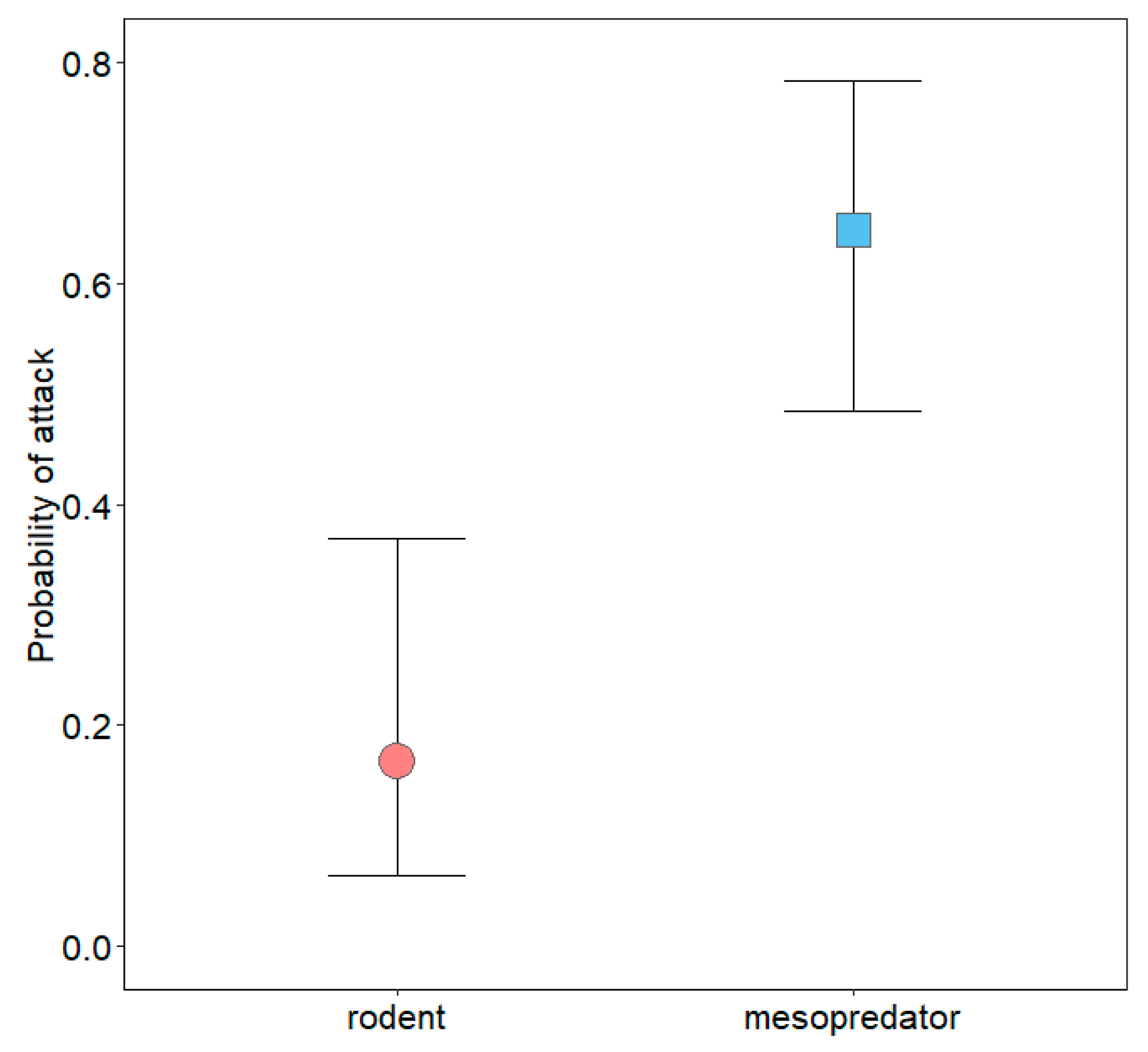
3.2. Mesopredator Interactions
3.3. Rodent Interactions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Lovich, J.E.; Ennen, J.R.; Agha, M.; Gibbons, J.W. Where have all the turtles gone, and why does it matter? BioScience 2018, 68, 771–781. [Google Scholar] [CrossRef]
- Dodd, C.K., Jr. North American Box Turtles: A Natural History; University of Oklahoma Press: Norman, OK, USA, 2001. [Google Scholar]
- Browne, C.L.; Hecnar, S.J. Species loss and shifting population structure of freshwater turtles despite habitat protection. Biol. Conserv. 2007, 138, 421–429. [Google Scholar] [CrossRef]
- Oddie, M.A.Y.; Coombes, S.M.; Davy, C.M. Investigation of cues used by predators to detect snapping turtle (Chelydra serpentina) nests. Can. J. Zool. 2015, 93, 299–305. [Google Scholar] [CrossRef]
- Heithaus, M.R.; Wirsing, A.J.; Thomson, J.A.; Burkholder, D.A. A review of lethal and non-lethal effects of predators on adult marine turtles. J. Exp. Mar. Biol. Ecol. 2008, 356, 43–51. [Google Scholar] [CrossRef]
- Sinclair, A.R.E.; Pech, R.P.; Dickman, C.R.; Hik, D.; Mahon, P.; Newsome, A.E. Predicting effects of predation on conservation of endangered prey. Conserv. Biol. 1998, 12, 564–575. [Google Scholar] [CrossRef]
- Ernst, C.H.; Lovich, J.E.; Barbour, R.W. Turtles of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1994. [Google Scholar]
- Fincham, J.E.; Lambrechts, N. How many tortoises do a pair of pied crows (Corvus albus) need to kill to feed their chicks? Ornithol. Obs. 2014, 5, 135–138. [Google Scholar]
- Nagy, K.A.; Hillard, L.S.; Tuma, M.W.; Morafka, D.J. Head-started desert tortoises (Gopherus agassizii): Movements, survivorship and mortality causes following their release. Herpetol. Conserv. Biol. 2015, 10, 203–215. [Google Scholar]
- Tetzlaff, S.J.; Sperry, J.H.; DeGregorio, B.A. Attempted depredation of a juvenile Eastern Box Turtle, Terrapene carolina (Linnaeus, 1758), by Sandhill Cranes, Antigone canadensis (Linnaeus, 1758). Herpetol. Notes 2018, 11, 589–591. [Google Scholar]
- Callahan, J.R. Squirrels as predators. Great Basin Nat. 1993, 53, 137–144. [Google Scholar]
- Belzer, W.R.; Seibert, S.; Atkinson, B. Putative chipmunk predation of juvenile eastern box turtles. Turt. Tortoise Newsl. 2000, 5, 8–9. [Google Scholar]
- Jones, M.T.; Sievert, P.R. Elevated mortality of hatchling Blanding’s turtles (Emydoidea blandingii) in residential landscapes. Herpetol. Conserv. Biol. 2012, 7, 89–94. [Google Scholar]
- Pike, D.A.; Pizzatto, L.; Pike, B.A.; Shine, R. Estimating survival rates of uncatchable animals: The myth of high juvenile mortality in reptiles. Ecology 2008, 89, 607–611. [Google Scholar] [CrossRef]
- Arsovski, D.; Tomović, L.; Golubović, A.; Nikolić, S.; Sterijovski, B.; Ajtić, R.; Ballouard, J.; Bonnet, X. When carapace governs size: Variation among age classes and individuals in a free-ranging ectotherm with delayed maturity. Oecologia 2018, 186, 953–963. [Google Scholar] [CrossRef]
- Paterson, J.E.; Steinberg, B.D.; Litzgus, J.D. Revealing a cryptic life-history stage: Differences in habitat selection and survivorship between hatchlings of two turtle species at risk (Glyptemys insculpta and Emydoidea blandingii). Wildl. Res. 2012, 39, 408–418. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Atwood, T.C.; Gese, E.M.; Kunkel, K.E. Spatial partitioning of predation risk in a multiple predator-multiple prey system. J. Wildl. Manag. 2009, 73, 876–884. [Google Scholar] [CrossRef]
- Thaker, M.; Vanak, A.T.; Owen, C.R.; Ogden, M.B.; Niemann, S.M.; Slotow, R. Minimizing predation risk in a landscape of multiple predators: Effects on the spatial distribution of African ungulates. Ecology 2011, 92, 398–407. [Google Scholar] [CrossRef]
- Remsen, J.V.; Robinson, S.K. A classification scheme for foraging behavior of birds in terrestrial habitats. Stud. Avian Biol. 1990, 13, 144–160. [Google Scholar]
- Santisteban, L.; Sieving, K.E.; Avery, M.L. Use of sensory cues by fish crows Corvus ossifragus preying on artificial bird nests. J. Avian Biol. 2002, 33, 245–252. [Google Scholar] [CrossRef]
- McQuade, D.B.; Williams, E.H.; Eichenbaum, H.B. Cues used for localizing food by the gray squirrel (Sciurus carolinensis). Ethology 1986, 72, 22–30. [Google Scholar] [CrossRef]
- Duncan, R.D.; Jenkins, S.H. Use of visual cues in foraging by a diurnal herbivore, Belding’s ground squirrel. Can. J. Zool. 1998, 76, 1766–1770. [Google Scholar] [CrossRef]
- Conover, M.R. Predator–Prey Dynamics, the Role of Olfaction; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Fogarty, D.T.; Elmore, R.D.; Fuhlendorf, S.D.; Loss, S.R. Variation and drivers of airflow patterns associated with olfactory concealment and habitat selection. Ecology 2018, 99, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.; Shine, R. Reptiles. In Practical Census Techniques for Animal Populations; Sutherland, W., Ed.; Cambridge University Press: Cambridge, UK, 2004; pp. 218–226. [Google Scholar]
- Bateman, P.W.; Fleming, P.A.; Wolfe, A.K. A different kind of ecological modelling: The use of clay model organisms to explore predator–prey interactions in vertebrates. J. Zool. 2017, 301, 251–262. [Google Scholar] [CrossRef]
- Behm, J.E.; Waite, B.R.; Hsieh, S.T.; Helmus, M.R. Benefits and limitations of three-dimensional printing technology for ecological research. BMC Ecol. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Humphries, S. 3D Printing: Applications in evolution and ecology. Ecol. Evol. 2019, 9, 4289–4301. [Google Scholar] [CrossRef]
- Akcali, C.K.; Pérez-Mendoza, H.A.; Salazar-Valenzuela, D.; Kikuchi, D.W.; Guayasamin, J.M.; Pfennig, D.W. Evaluating the utility of camera traps in field studies of predation. PeerJ 2019, 7, e6487. [Google Scholar] [CrossRef]
- Hansen, N.A.; Sato, C.F.; Michael, D.R.; Lindenmayer, D.B.; Driscoll, D.A. Predation risk for reptiles is highest at remnant edges in agricultural landscapes. J. Appl. Ecol. 2019, 56, 31–43. [Google Scholar] [CrossRef]
- Lawson, R.R.; Fogarty, D.T.; Loss, S.R. Use of visual and olfactory sensory cues by an apex predator in deciduous forests. Can. J. Zool. 2019, 97, 488–494. [Google Scholar] [CrossRef]
- Van Dijk, P.P. Terrapene Carolina (Errata Version Published in 2016). The IUCN Red List of Threatened Species 2011. Available online: https://www.iucn.org/sites/dev/files/import/downloads/arabian_oryx_factsheet.pdf (accessed on 30 July 2019).
- Budischak, S.A.; Hester, J.M.; Price, S.J.; Dorcas, M.E. Natural history of Terrapene carolina (Box Turtles) in an urbanized landscape. Southeast. Nat. 2006, 5, 191–204. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Sperry, J.H.; Kingsbury, B.A.; DeGregorio, B.A. Captive-rearing duration may be more important than environmental enrichment for enhancing turtle head-starting success. Glob. Ecol. Conserv. 2019, 20, e00797. [Google Scholar] [CrossRef]
- Blouin-Demers, G.; Weatherhead, P.J. Habitat use by black rat snakes (Elaphe obsoleta obsoleta) in fragmented forests. Ecology 2001, 82, 2882–2896. [Google Scholar] [CrossRef]
- Ryan, D.A.; Larson, J.S. Chipmunks in residential environments. Urban Ecol. 1976, 2, 173–178. [Google Scholar] [CrossRef]
- Sato, C.F.; Wood, J.T.; Schroder, M.; Green, K.; Osborne, W.S.; Michael, D.R.; Lindenmayer, D.B. An experiment to test key hypotheses of the drivers of reptile distribution in subalpine ski resorts. J. Appl. Ecol. 2014, 51, 13–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 3rd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.42.1. 2018. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 30 July 2019).
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Second International Symposium on Information Theory; Petrov, B.N., Csaki, B.F., Eds.; Academiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Arnold, T.W. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010, 74, 1175–1178. [Google Scholar] [CrossRef]
- Tucker, C.R.; Strickland, J.T.; Edmond, B.S.; Delaney, D.K.; Ligon, D.B. Activity patterns of ornate box turtles (Terrapene ornata) in northwestern Illinois. Copeia 2015, 103, 502–511. [Google Scholar] [CrossRef]
- Fritzell, E.K. Habitat use by prairie raccoons during the waterfowl breeding season. J. Wildl. Manag. 1978, 42, 118–127. [Google Scholar] [CrossRef]
- Pedlar, J.H.; Fahrig, L.; Merriam, H.G. Raccoon habitat use at 2 spatial scales. J. Wildl. Manag. 1997, 61, 102–112. [Google Scholar] [CrossRef]
- Kamler, J.F.; Gipson, P.S. Space and habitat use by male and female raccoon, Procyon lotor, in Kansas. Can. Field Nat. 2003, 117, 218–223. [Google Scholar] [CrossRef]
- Barding, E.E.; Nelson, T.A. Raccoons use habitat edges in northern Illinois. Am. Midl. Nat. 2008, 159, 394–402. [Google Scholar] [CrossRef]
- Frey, S.N.; Conover, M.R. Habitat use by meso-predators in a corridor environment. J. Wildl. Manag. 2006, 70, 1111–1118. [Google Scholar] [CrossRef]
- DeGregorio, B.A.; Weatherhead, P.J.; Sperry, J.H. Power lines, roads, and avian nest survival: Effects on predator identity and predation intensity. Ecol. Evol. 2014, 4, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Burgin, S. Patterns of bird predation on reptiles in small woodland remnant edges in peri-urban north-western Sydney, Australia. Landsc. Ecol. 2008, 23, 1039–1047. [Google Scholar] [CrossRef]
- Bowman, G.B.; Harris, L.D. Effect of spatial heterogeneity on ground-nest depredation. J. Wildl. Manag. 1980, 44, 806–813. [Google Scholar] [CrossRef]
- Andruskiw, M.; Fryxell, J.M.; Thompson, I.D.; Baker, J.A. Habitat-mediated variation in predation risk by the American marten. Ecology 2008, 89, 2273–2280. [Google Scholar] [CrossRef]
- Sperry, J.H.; Weatherhead, P.J. Ratsnakes and brush piles: Intended and unintended consequences of improving habitat for wildlife? Am. Midl. Nat. 2010, 163, 311–317. [Google Scholar] [CrossRef]
- Paluh, D.J.; Hantak, M.M.; Saporito, R.A. A test of aposematism in the dendrobatid poison frog Oophaga pumilio: The importance of movement in clay model experiments. J. Herpetol. 2014, 48, 249–254. [Google Scholar] [CrossRef]
- Webb, J.K.; Whiting, M.J. Why don’t small snakes bask? Juvenile broad-headed snakes trade thermal benefits for safety. Oikos 2005, 110, 515–522. [Google Scholar] [CrossRef]
- Garrott, R.A.; White, P.J.; Vanderbilt White, C.A. Overabundance: An issue for conservation biologists? Conserv. Biol. 1993, 7, 946–949. [Google Scholar] [CrossRef]
- Crooks, K.R.; Soule, M.E. Mesopredator release and avifaunal extinctions in a fragmented system. Nature 1999, 400, 563–566. [Google Scholar] [CrossRef]
- Boarman, W.I. Managing a subsidized predator population: Reducing common raven predation on desert tortoises. Environ. Manag. 2003, 32, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.J.; Van Dyke, J.U.; Thompson, M.B. Critically evaluating best management practices for preventing freshwater turtle extinctions. Conserv. Biol. 2017, 31, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.L. Head-starting turtles: Learning from experience. Herpetol. Conserv. Biol. 2015, 10, 299–308. [Google Scholar]
- Treves, A.; Krofel, M.; McManus, J. Predator control should not be a shot in the dark. Front. Ecol. Environ. 2016, 14, 380–388. [Google Scholar] [CrossRef]
- Taylor, G.; Canessa, S.; Clarke, R.H.; Ingwersen, D.; Armstrong, D.P.; Seddon, P.J.; Ewen, J.G. Is reintroduction biology an effective applied science? TREE 2017, 32, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Khannoon, E.R.; Lunt, D.H.; Schulz, S.; Hardege, J.D. Divergence of scent pheromones in allopatric populations of Acanthodactylus boskianus (Squamata: Lacertidae). Zool. Sci. 2013, 30, 380–385. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Fuller, N.W.; Moore, P.R.; Gilmore, D.F.; Savary, B.J.; Risch, T.S. Fatty acid methyl ester profiles of bat wing surface lipids. Lipids 2014, 49, 1143–1150. [Google Scholar] [CrossRef]




| Species | Detections | Attacks |
|---|---|---|
| Raccoon (Procyon lotor) | 11 | 22 |
| Virginia opossum (Didelphis virginiana) | 2 | 2 |
| Eastern chipmunk (Tamias striatus) | 15 | 3 |
| Eastern grey squirrel (Sciurus carolinensis) | 1 | 0 |
| Eastern fox squirrel (Sciurus niger) | 4 | 1 |
| Wild turkey (Meleagris gallopavo) | 1 | 1 |
| Unidentified small mammal | 2 | 0 |
| Mesopredator | Odds Ratio Estimate | 85% CI | |
| NHMW | 4.759 | 2.432 | 9.313 |
| VRO | 1.785 | 0.816 | 3.907 |
| forest | 1.315 | 0.691 | 2.501 |
| grassland | 0.156 | 0.032 | 0.755 |
| wetland | 2.351 | 1.064 | 5.193 |
| modal vegetation height | 0.962 | 0.938 | 0.986 |
| maximum vegetation height | 0.996 | 0.985 | 1.006 |
| distance from woody structure | 1.001 | 0.999 | 1.003 |
| exposure treatment | 1.259 | 0.734 | 2.161 |
| scent treatment | 1.101 | 0.629 | 1.929 |
| exposure and scent treatment interaction | 1.664 | 0.568 | 4.878 |
| Rodent | Odds Ratio Estimate | 85% CI | |
| NHMW | 0.655 | 0.285 | 1.508 |
| VRO | 0.218 | 0.069 | 0.686 |
| forest | 0.866 | 0.420 | 1.784 |
| grassland | 0.000 | 0.000 | 171.546 |
| wetland | 0.459 | 0.171 | 1.236 |
| modal vegetation height | 1.007 | 0.993 | 1.021 |
| maximum vegetation height | 0.992 | 0.981 | 1.003 |
| distance from woody structure | 0.997 | 0.994 | 0.999 |
| exposure treatment | 2.871 | 1.440 | 5.727 |
| scent treatment | 1.182 | 0.591 | 2.367 |
| exposure and scent treatment interaction | 2.199 | 0.550 | 8.795 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetzlaff, S.J.; Estrada, A.; DeGregorio, B.A.; Sperry, J.H. Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models. Animals 2020, 10, 275. https://doi.org/10.3390/ani10020275
Tetzlaff SJ, Estrada A, DeGregorio BA, Sperry JH. Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models. Animals. 2020; 10(2):275. https://doi.org/10.3390/ani10020275
Chicago/Turabian StyleTetzlaff, Sasha J., Alondra Estrada, Brett A. DeGregorio, and Jinelle H. Sperry. 2020. "Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models" Animals 10, no. 2: 275. https://doi.org/10.3390/ani10020275
APA StyleTetzlaff, S. J., Estrada, A., DeGregorio, B. A., & Sperry, J. H. (2020). Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models. Animals, 10(2), 275. https://doi.org/10.3390/ani10020275





