The Impact of Eggshell Colour on the Quality of Table and Hatching Eggs Derived from Japanese Quail
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- 37.6–38.0 °C temp. and 50%–65% relative humidity in the setting compartment, eggs were turned automatically by 90° every 3 h (8 times a day).
- 37.0–37.5 °C temp. and 75%–80% relative humidity in hatching compartment.
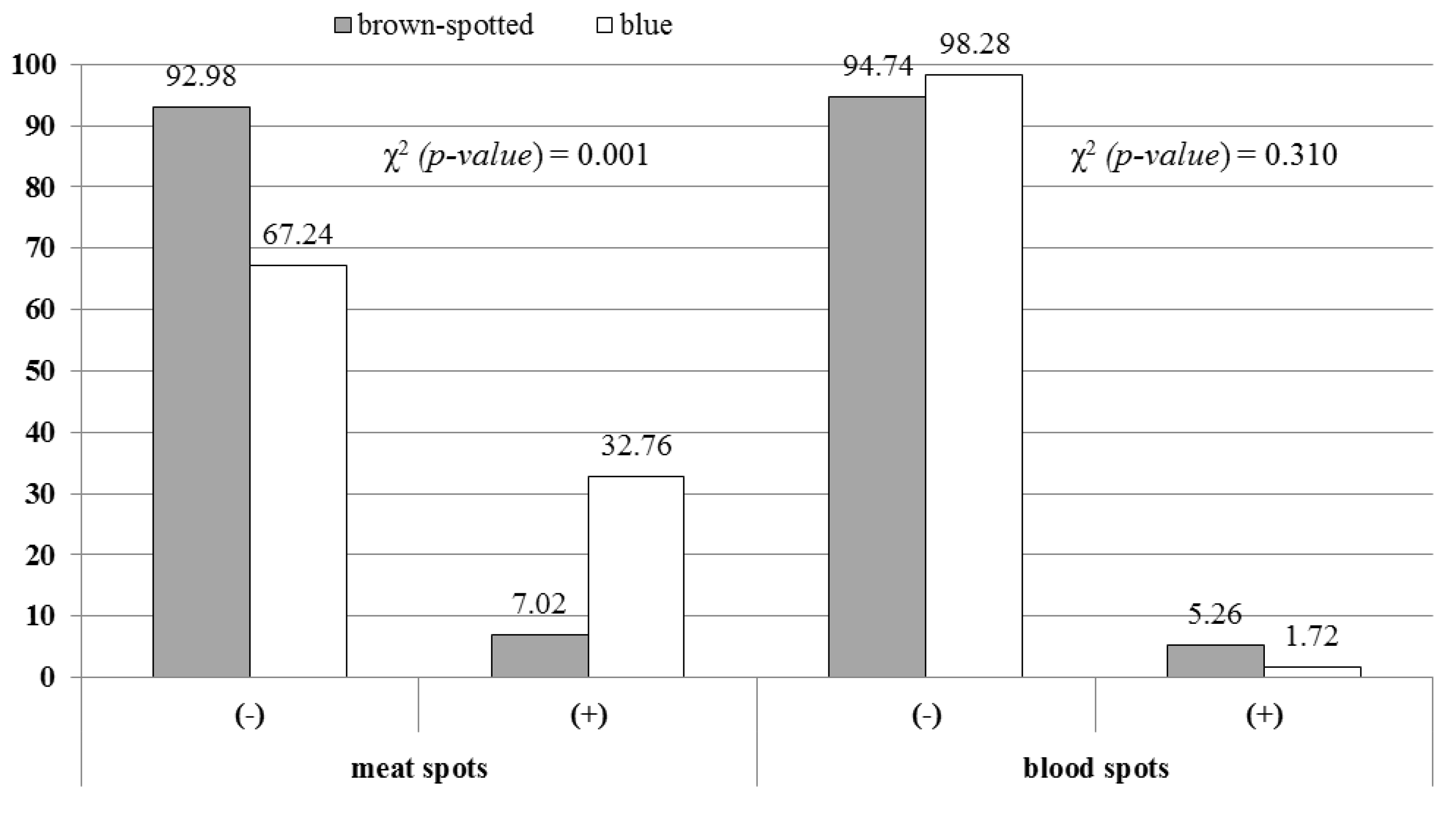
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Solomon, S.E. The eggshell: Strength, structure and function. Br. Poult. Sci. 2010, 51, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Tumova, E.; Zita, L.; Hubeny, M.; Skrivan, M.; Ledvinka, Z. The effect of oviposition time and genotype on egg quality characteristics in egg type hens. Czech J. Anim. Sci. 2007, 52, 26–30. [Google Scholar] [CrossRef]
- Aygun, A. The relationship between eggshell colour and egg quality traits in table eggs. Indian J. Anim. Res. 2014, 48, 290–294. [Google Scholar] [CrossRef]
- Kennedy, G.Y.; Vevers, H.G. A survey of avian eggshell pigments. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1976, 55, 117–123. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, G.Y.; Liu, Z.Z.; Li, J.Y.; Yang, N. A study on eggshell pigmentation: Biliverdin in blue-shelled chickens. Poult. Sci. 2006, 85, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Deng, X.M.; Zhao, C.J.; Li, J.Y.; Xu, G.Y.; Lian, L.S.; Wu, C.X. Study of the deposition process of eggshell pigments using an improved dissolution method. Poult. Sci. 2007, 86, 2236–2238. [Google Scholar] [CrossRef]
- Samiullah, S.; Roberts, J.R. The location of protoporphyrin in the eggshell of brown-shelled eggs. Poult. Sci. 2013, 92, 2783–2788. [Google Scholar] [CrossRef]
- Nowaczewski, S.; Szablewski, T.; Cegielska-Radziejewska, R.; Kontecka, H. Egg morphometry and eggshell quality in ring-necked pheasants kept in cages. Ann. Anim. Sci. 2013, 13, 531–541. [Google Scholar] [CrossRef]
- Taha, A.E. Analyzing of quail eggs hatchability, quality, embryonic mortality and malpositions in relation to their shell colors. J. Anim. Feed Res. 2011, 1, 267–273. [Google Scholar]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. C. R. Palevol. 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Ingram, D.R.; Hatten, L.F.; Homan, K.D. A study on the relationship between eggshell color and eggshell quality in commercial broiler breeders. Int. J. Poult. Sci. 2008, 7, 700–703. [Google Scholar] [CrossRef]
- Bennet, C.D. The influence of shell thickness on hatchability in commercial broiler breeder flocks. J. Poult. Res. 1992, 1, 61–65. [Google Scholar] [CrossRef]
- Sezer, M.; Tekelioglu, O. Quantification of japanese quail eggshell colour by image analysis. Biol. Res. 2009, 42, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tsudzuki, M.; Komori, M.; Mizutani, M. Celadon—An eggshell color mutation in japanese-quail. J. Hered. 1993, 84, 145–147. [Google Scholar] [CrossRef]
- Ogunwole, O.A.; Agboola, A.F.; Mapayi, T.G.; Babayemi, O.J. Consumers’ perception and preference for Japanese quail and the commercial chicken eggs in Akinyele local government area of Oyo State, Nigeria. Trop. Anim. Health Prod. 2015, 18, 108–119. [Google Scholar]
- Williams, K.C. Some factors affecting albumin quality with particular reference to haugh unit score. World Poult. Sci. J. 1992, 48, 5–16. [Google Scholar] [CrossRef]
- Shafey, T.M. Effects of egg size and eggshell conductance on hatchability traits of meat and layer breeder flocks. Asian-Austral. J. Anim. 2002, 15, 1–6. [Google Scholar] [CrossRef]
- Christensen, V.L.; Grimes, J.L.; Wineland, M.J.; Bagley, L.G. Effects of turkey breeder hen age, strain, and length of the incubation period on survival of embryos and hatchlings. J. Appl. Poult. Res. 2001, 10, 5–15. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows Armonk; Version 24.0; IBM Corp.: Armonk, NY, USA, 2016.
- Hassan, H.A.; El-Nesr, S.S.; Osman, A.M.R.; Arram, G.A. Ultrastructure of eggshell, egg weight loss and hatching traits of Japanese Quail varying in eggshell color and pattern using image analysis. Egypt. Poult. Sci. J. 2013, 34, 1–17. [Google Scholar] [CrossRef]
- Zita, L.; Ledvinka, Z.; Klesalova, L. The effect of the age of Japanese quails on certain egg quality traits and their relationships. Veterinarski Arhiv 2013, 83, 223–232. [Google Scholar]
- Sari, M.; Isik, S.; Onk, K.; Tilki, M.; Kirmizibayrak, T. Effects of layer age and different plumage colors on external and internal egg quality characteristics in Japanese quails (Coturnix coturnix japonica). Archiv Fur Geflugelkunde 2012, 76, 254–258. [Google Scholar]
- Johnston, S.A.; Gous, R.M. Modelling the changes in the proportions of the egg components during a laying cycle. Br. Poult. Sci. 2007, 48, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, K.I.A.; Batkowska, J.; Drabik, K.; Gryzinska, M.M. Time of sexual maturity and early egg quality of Japanese quails affected by in ovo injection of medicinal plants. Arch. Anim. Breed. 2019, 62, 423–430. [Google Scholar] [CrossRef]
- Gosler, A.G.; Connor, O.R.; Bonser, R.H.C. Protoporphyrin and eggshell strength: Preliminary findings from a passerine bird. Avian Biol. Res. 2011, 4, 214–223. [Google Scholar] [CrossRef]
- Garcia-Navas, V.; Sanz, J.J.; Merino, S.; Martinez-de la Puente, J.; Lobato, E.; del Cerro, S.; Rivero, J.; de Castaneda, R.R.; Moreno, J. Experimental evidence for the role of calcium in eggshell pigmentation pattern and breeding performance in Blue Tits Cyanistes caeruleus. J. Ornithol. 2011, 152, 71–82. [Google Scholar] [CrossRef]
- Darnell-Middleton, S.L.; Solomon, S.E.; Bain, M.M.; Thorp, B.H. Observations on pigmentation, hatchability and ultrastructure in guinea fowl eggshells. Br. Poult. Sci. 1998, 39, S28–S29. [Google Scholar] [CrossRef]
- Yilmaz, A.; Tepeli, C.; Caglayan, T. External and internal egg quality characteristics in Japanese quails of different plumage color lines. J. Food Agric. Environ. 2011, 9, 375–379. [Google Scholar]
- Alkan, S.; Karabag, K.; Galic, A.; Karsli, T.; Balcioglu, M.S. Effects of Selection for Body Weight and Egg Production on Egg Quality Traits in Japanese Quails (Coturnix coturnix japonica) of Different Lines and Relationships between These Traits. Kafkas Univ. Vet. Fak. Derg. 2010, 16, 239–244. [Google Scholar]
- Godfrey, G.F. The relationship of eggshell color to hatchability in some brown egg laying breeds. Poult. Sci. 1947, 26, 381–388. [Google Scholar] [CrossRef]
- Kozuszek, R.; Kontecka, H.; Nowaczewski, S.; Rosinski, A. Storage time and eggshell colour of pheasant eggs vs. the number of blastodermal cells and hatchability results. Folia Biol.-Krakow 2009, 57, 121–130. [Google Scholar] [CrossRef]
- Soliman, F.N.K.; Elsebai, A.; Abaza, M. Hatchability traits of different colored Japanese quail eggs in relation to egg quality and female blood constituents. Egypt. Poult. Sci. J. 2000, 2, 417–430. [Google Scholar]
- Uddin, M.S.; Paul, D.C.; Huque, Q.M.E. Effect of egg weight and pre-incubation holding periods on hatchability of the Japanese quail eggs in different seasons. Asian-Austral. J. Anim. 1994, 7, 499–503. [Google Scholar] [CrossRef]
- McDaniel, G.R.; Roland, D.A.; Coleman, M.A. Effect of eggshell quality on hatchability and embryonic mortality. Poult. Sci. 1979, 58, 10–13. [Google Scholar] [CrossRef]
- Roque, L.; Soares, M.C. Effects of eggshell quality and broiler breeder age on hatchability. Poult. Sci. 1994, 73, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Krystianiak, S.; Kożuszek, R.; Kontecka, H.; Nowaczewski, S. Quality and ultrastructure of eggshell and hatchability of eggs in relation to eggshell colour in pheasants. Anim. Sci. Pap. Rep. 2005, 23, 5–14. [Google Scholar]
- Baggott, G.K.; Deeming, D.C.; Hémon, S.; Paillat, P. Relationships between eggshell pigmentation, ultrastructure and water vapour conductance in the Houbara bustard (Chlamydotis undulata macqueenii). Avian Poult. Biol. Rev. 2002, 13, 234–235. [Google Scholar]
- Nys, Y.; Hincke, M.T.; Arias, J.L.; Garcia-Ruiz, J.M.; Solomon, S.E. Avian eggshell mineralization. Avian Poult. Biol. Rev. 1999, 10, 143–166. [Google Scholar]
- Goran, G.V.; Crivineanu, V.; Tudoreanu, L.; Udrea, D. Dynamics of some mineral elements in hen eggs. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2010, 67, 88–95. [Google Scholar]
- Adeyeye, E.I. Comparative study on the characteristics of eggshells of some bird species. Bull. Chem. Soc. Ethiopia 2009, 23, 159–166. [Google Scholar] [CrossRef]
| Trait | Eggshell | |
|---|---|---|
| Brown-Spotted | Blue | |
| Shell colour (%) | 29.70 * ± 8.904 | 50.77 * ± 8.147 |
| Whole egg | ||
| Weight (g) | 10.88 * ± 0.725 | 10.27 * ± 0.708 |
| Index | 1.308 ± 0.058 | 1.305 ± 0.052 |
| Specific gravity (g/cm3) | 1.06 ± 0.010 | 1.06 ± 0.006 |
| Proportions (%) | ||
| Shell | 16.40 ± 4.176 | 16.14 ± 3.176 |
| Albumen | 48.42 * ± 8.906 | 52.29 * ± 6.893 |
| Yolk | 35.18 * ± 6.948 | 31.57 * ± 5.891 |
| Shell | ||
| Strength (N) | 12.04 * ± 4.111 | 10.01 * ± 4.452 |
| Weight (g) | 1.81 ± 0.466 | 1.70 ± 0.365 |
| Thickness (mm) | 0.201 ± 0.049 | 0.197 ± 0.086 |
| Surface area (cm2) | 23.47 * ± 1.035 | 22.59 * ± 1.044 |
| Volume (cm3) | 0.472 ± 0.117 | 0.444 ± 0.185 |
| Density (g/cm3) | 4.01 ± 1.331 | 4.19 ± 1.403 |
| Yolk | ||
| Colour (pts) | 7.44 ± 2.383 | 7.56 ± 1.191 |
| Index (%) | 38.01 * ± 4.196 | 41.52 * ± 4.659 |
| Weight (g) | 3.88 * ± 0.763 | 3.33 * ± 0.655 |
| Albumen | ||
| Height (mm) | 9.47 * ± 1.473 | 9.40 * ± 1.622 |
| HU | 82.97 * ± 12.498 | 89.15 * ± 4.606 |
| Weight (g) | 5.38 ± 1.126 | 5.52 ± 0.816 |
| Trait | SC | EW | SG | SS | SW | ST | SDD | YC | YW | AH |
|---|---|---|---|---|---|---|---|---|---|---|
| EW | −0.253 ** | |||||||||
| SG | 0.044 | 0.115 | ||||||||
| SS | −0.027 | 0.210 * | 0.502 ** | |||||||
| SW | −0.051 | 0.070 | 0.068 | 0.033 | ||||||
| ST | −0.169 | 0.217 * | 0.226 * | 0.418 ** | −0.128 | |||||
| SDD | 0.079 | −0.264 ** | −0.071 | −0.232 * | 0.690 ** | 0.715 ** | ||||
| YC | −0.120 | 0.036 | 0.409 ** | 0.076 | 0.292 ** | −0.065 | 0.257 ** | |||
| YW | −0.316 ** | 0.510 ** | 0.207 * | 0.156 | 0.253 ** | 0.086 | 0.053 | 0.246 ** | ||
| AH | 0.423 ** | 0.035 | −0.146 | −0.080 | −0.106 | −0.126 | −0.073 | −0.208 * | −0.224 * | |
| AW | 0.032 | 0.528 ** | 0.055 | 0.102 | −0.365 ** | 0.106 | −0.416 ** | −0.173 | −0.205 * | 0.245 ** |
| Trait | Eggshell | |
|---|---|---|
| Brown-Spotted | Blue | |
| Shell colour (%) | 30.11 * ± 7.399 | 52.71 * ± 5.740 |
| Fertility (%) | 96.67 ± 6.172 | 90.00 ± 12.536 |
| Embryos died during the 1st phase of incubation (%) | 7.33 ± 8.837 | 7.33 ± 7.037 |
| Embryos died during the 2nd phase of incubation (%) | 4.00 ± 5.071 | 6.00 ± 12.421 |
| Crippled chicks (%) | 0.00 * ± 0.000 | 6.67 * ± 8.165 |
| Hatchability of fertile eggs (%) | 86.43 ± 11.698 | 77.61 ± 14.019 |
| Hatchability of set eggs (%) | 84.00 * ± 11.832 | 68.67 * ± 12.459 |
| Trait | Eggshell | ||
|---|---|---|---|
| Brown-Spotted | Blue | ||
| Initial Egg Weight (g) | 10.68 * ± 0.194 | 10.06 * ± 0.046 | |
| 1st phase of incubation (up to 14th day) | Weight of infertile eggs (g) | 7.69 ± 2.587 | 7.65 ± 1.601 |
| Weight loss of infertile eggs (%) | 30.10 ± 23.553 | 30.40 ± 14.580 | |
| Shell conductance of infertile eggs (mg H2O/d/mmHg) | 4.89 ± 3.824 | 4.94 ± 2.367 | |
| Weight of eggs with dead embryo (g) | 8.29 ± 2.269 | 7.49 ± 1.105 | |
| Weight loss of eggs with dead embryo (%) | 26.62 ± 24.488 | 33.61 ± 10.097 | |
| Shell conductance of eggs with dead embryo (mg H2O/d/mmHg) | 6.45 ± 5.950 | 9.13 ± 5.652 | |
| Weight of eggs with alive embryo (g) | 9.34 * ± 0.245 | 8.70 * ± 0.499 | |
| Weight loss of eggs with alive embryo (%) | 15.00 * ± 2.229 | 20.89 * ± 4.537 | |
| Shell conductance of eggs with alive embryo (mg H2O/d/mmHg) | 2.44 * ± 0.362 | 3.40 * ± 0.737 | |
| 2nd phase of incubation (up to 17.5th day) | Weight of unhatched eggs (g) | 8.72 ± 0.811 | 8.73 ± 0.815 |
| Weight loss of unhatched eggs (%) | 20.67 ± 7.392 | 20.65 ± 7.398 | |
| Shell conductance of unhatched eggs (mg H2O/d/mmHg) | 2.69 ± 0.958 | 2.68 ± 0.963 | |
| Chick weight (g) | 6.47 * ± 0.394 | 6.05 * ± 0.169 | |
| Proportion of chick in initial egg weight (%) | 60.08 ± 2.464 | 60.16 ± 1.644 | |
| Trait | SC | EW | F | HFE | HSE | EWA | WLA | ESCA | BW |
|---|---|---|---|---|---|---|---|---|---|
| EW | −0.753 ** | ||||||||
| F | −0.266 | 0.098 | |||||||
| HFE | −0.384 * | 0.365 * | −0.075 | ||||||
| HSE | −0.514 ** | 0.454 ** | 0.429 ** | 0.799 ** | |||||
| EWA | −0.589 ** | 0.753 ** | 0.080 | 0.433 ** | 0.463 ** | ||||
| WLA | 0.593 ** | −0.757 ** | −0.080 | −0.431 ** | −0.462 ** | ||||
| ESCA | 0.589 ** | −0.753 ** | −0.080 | −0.433 ** | −0.463 ** | ||||
| BW | −0.457 ** | 0.650 ** | −0.156 | 0.161 | 0.105 | 0.582 ** | −0.587 ** | −0.582 ** | |
| BW/EW | 0.192 | 0.005 | −0.450 ** | −0.167 | −0.379 * | 0.113 | −0.115 | −0.113 | 0.673 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabik, K.; Batkowska, J.; Vasiukov, K.; Pluta, A. The Impact of Eggshell Colour on the Quality of Table and Hatching Eggs Derived from Japanese Quail. Animals 2020, 10, 264. https://doi.org/10.3390/ani10020264
Drabik K, Batkowska J, Vasiukov K, Pluta A. The Impact of Eggshell Colour on the Quality of Table and Hatching Eggs Derived from Japanese Quail. Animals. 2020; 10(2):264. https://doi.org/10.3390/ani10020264
Chicago/Turabian StyleDrabik, Kamil, Justyna Batkowska, Kostiantyn Vasiukov, and Adrian Pluta. 2020. "The Impact of Eggshell Colour on the Quality of Table and Hatching Eggs Derived from Japanese Quail" Animals 10, no. 2: 264. https://doi.org/10.3390/ani10020264
APA StyleDrabik, K., Batkowska, J., Vasiukov, K., & Pluta, A. (2020). The Impact of Eggshell Colour on the Quality of Table and Hatching Eggs Derived from Japanese Quail. Animals, 10(2), 264. https://doi.org/10.3390/ani10020264







