Glutamate Supply Reactivates Ovarian Function while Increases Serum Insulin and Triiodothyronine Concentrations in Criollo x Saanen-Alpine Yearlings’ Goats during the Anestrous Season
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. General
2.2. Location, Environmental Conditions, Animals, and their Management
2.3. Blood Sampling, Progesterone Quantification, and Confirmation of Anestrus Status
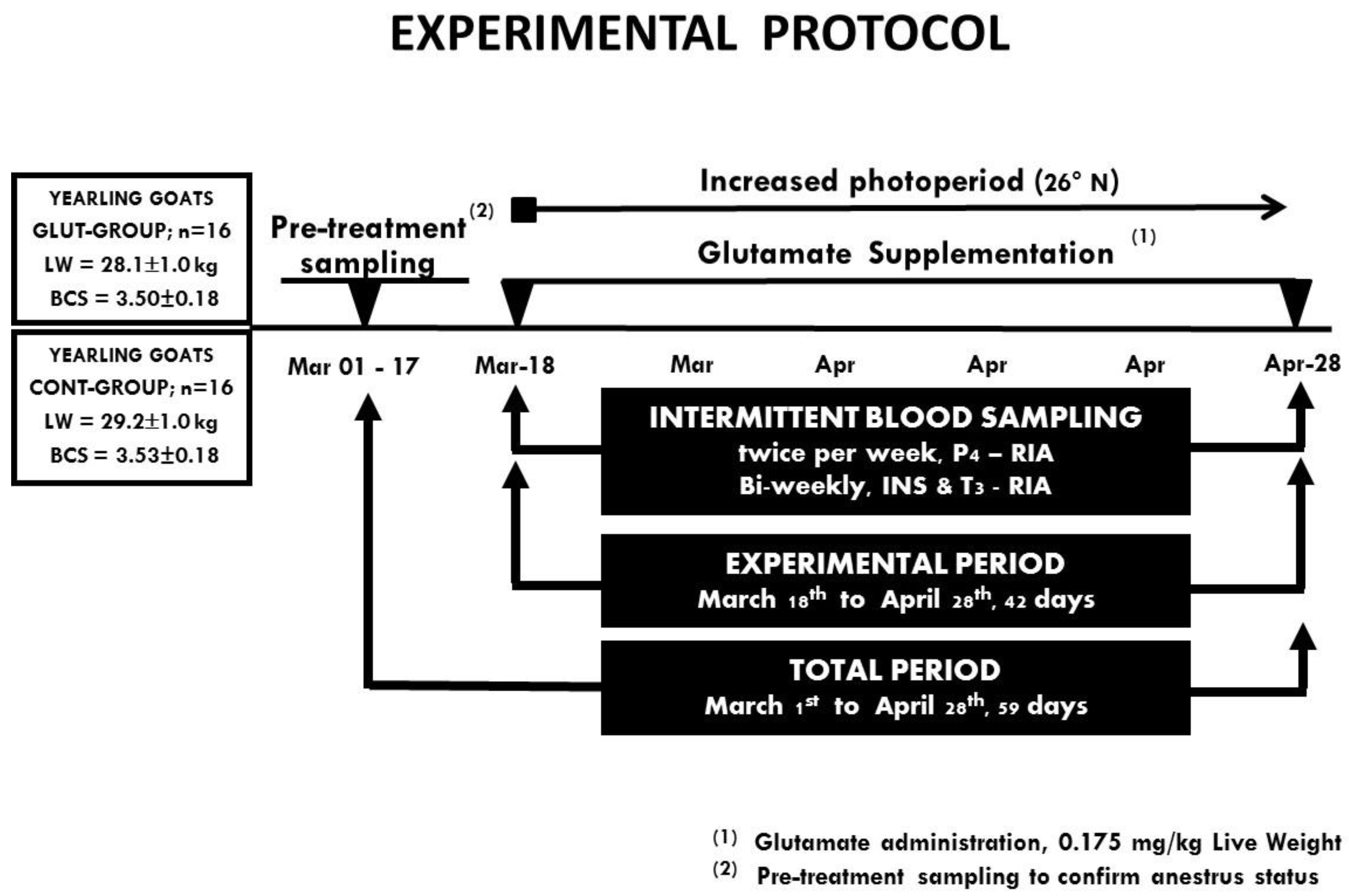
2.4. Experimental Design and Treatments
2.5. Intermittent Blood Sampling for Serum Progesterone, Insulin, and Triiodothyronine Levels and Ovarian Function
2.6. Statistical Analyses
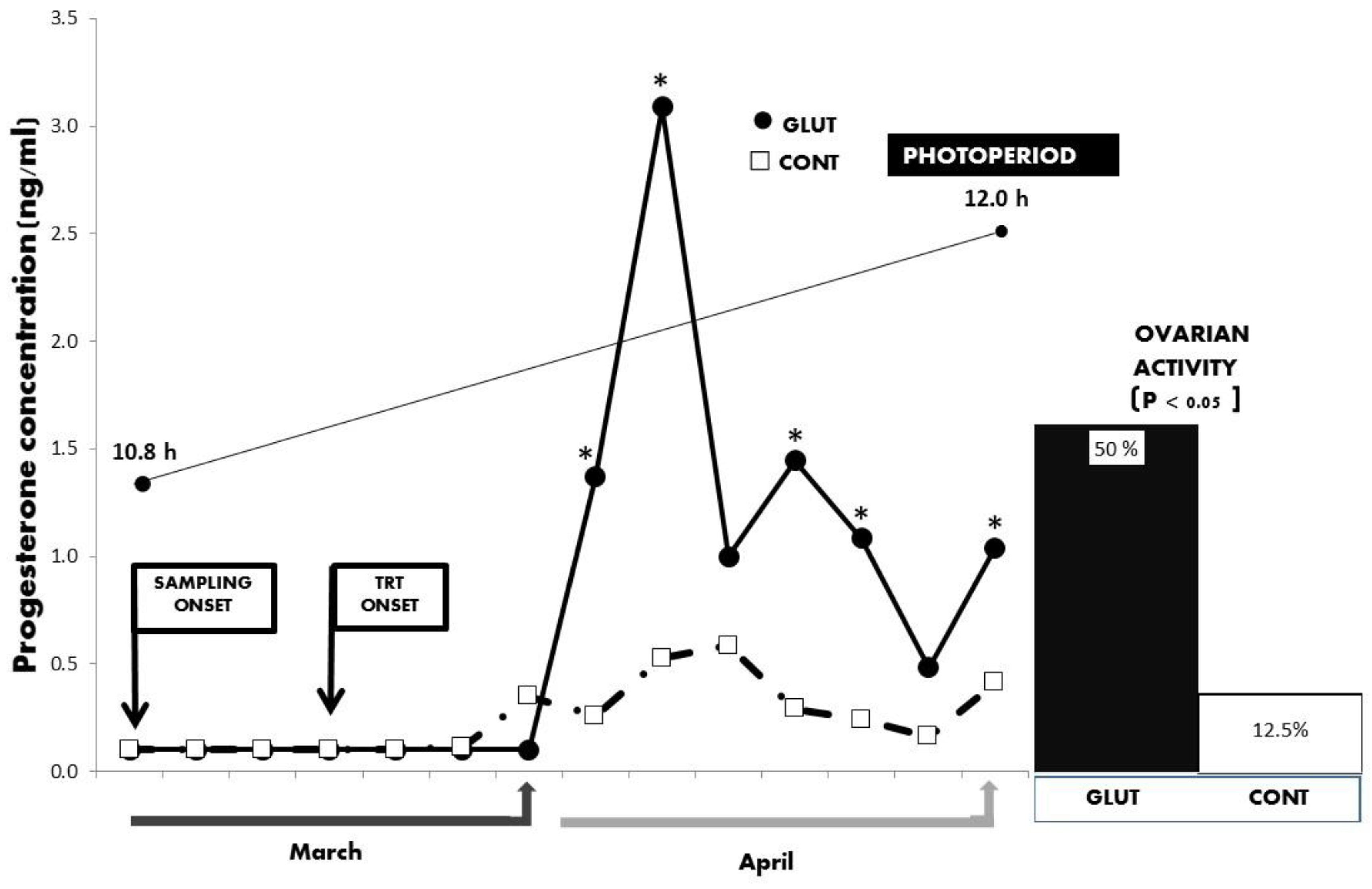
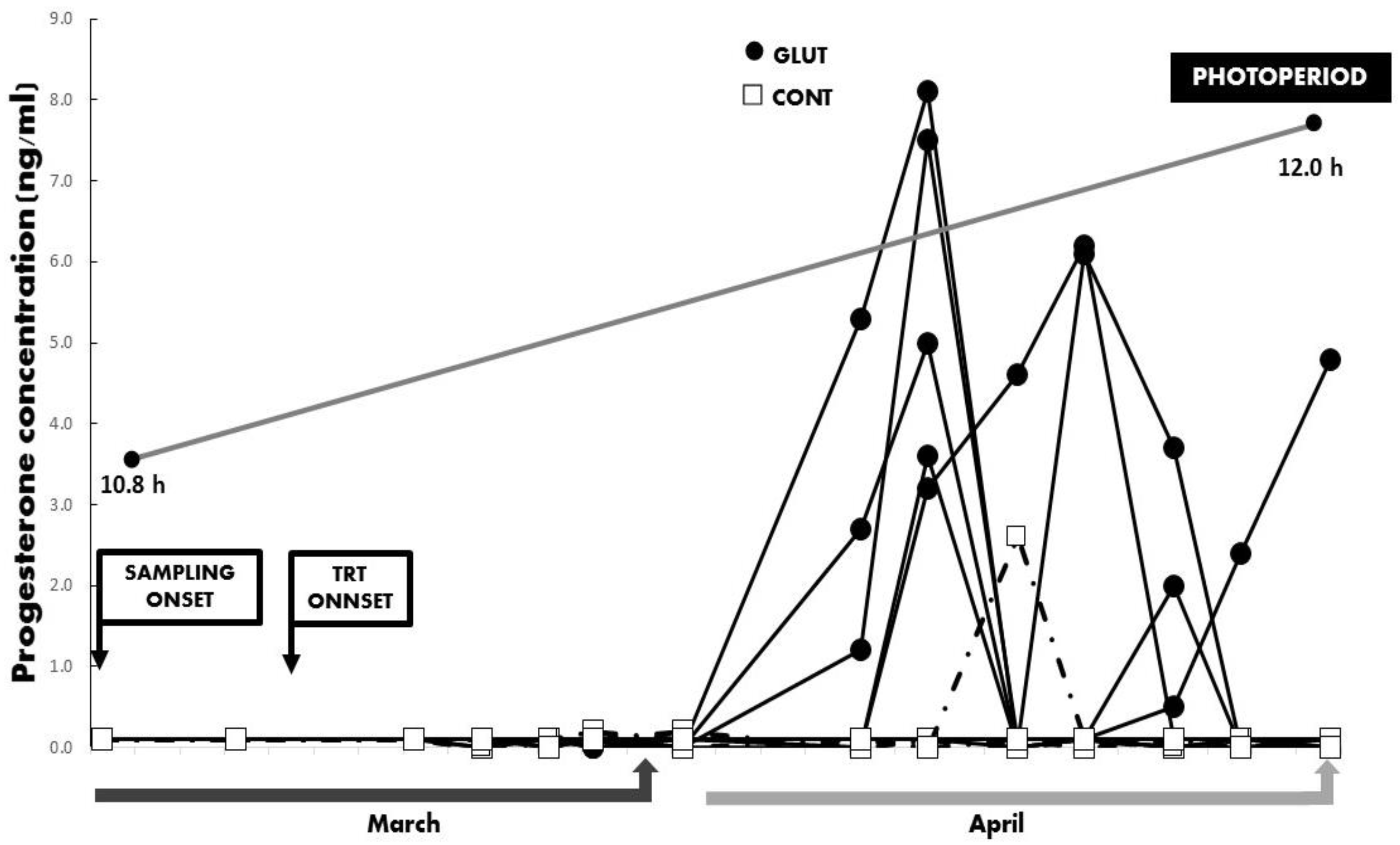
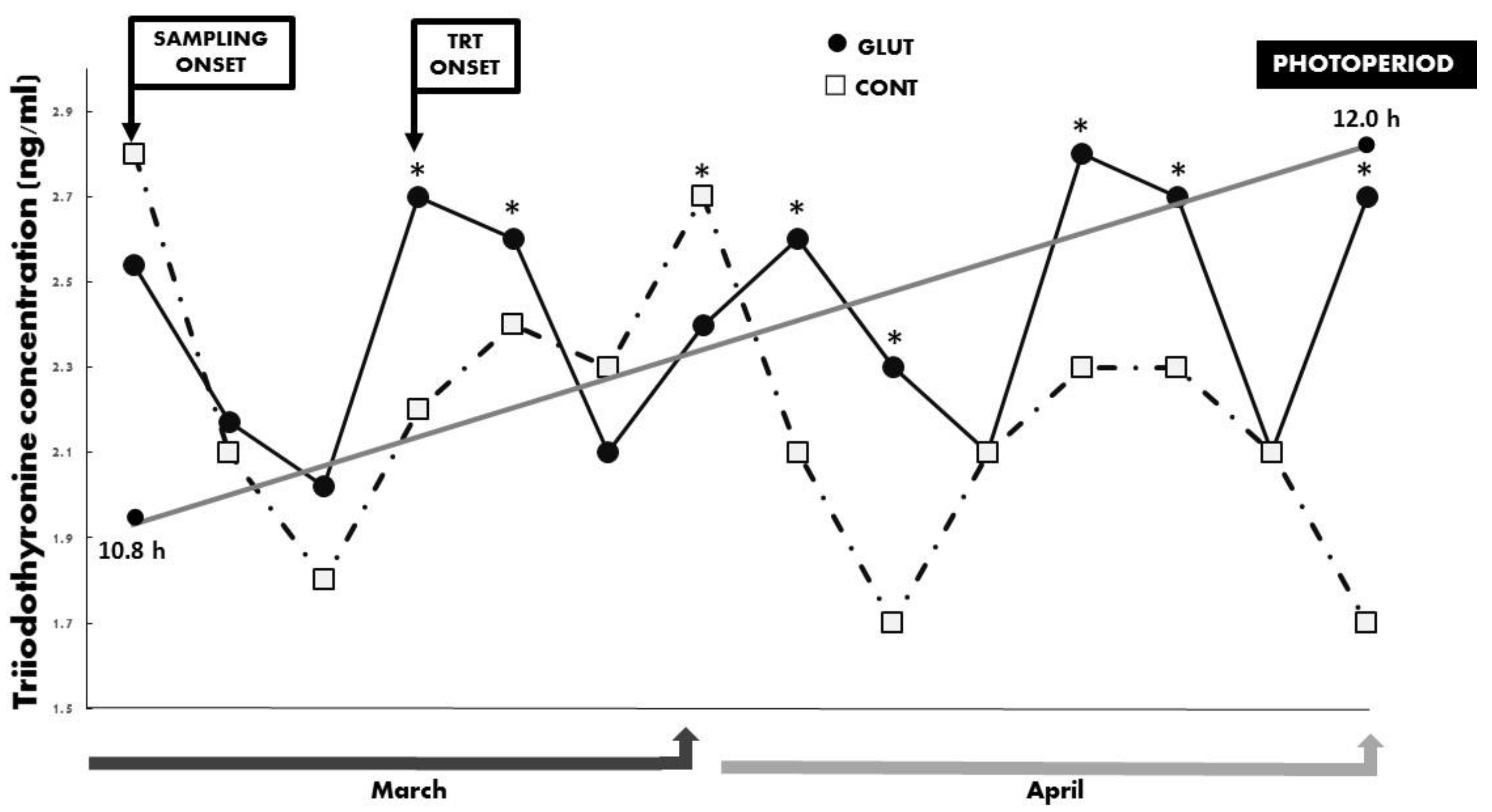
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Repository Resources
References
- Gonzalez-Bulnes, A.; Meza-Herrera, C.A.; Rekik, M.; Ben Salem, H.; Kridli, R.T. Limiting factors and strategies for improving reproductive outputs of small ruminants reared in semi-arid environments. In Semi-Arid Environments: Agriculture, Water Supply and Vegetation; Degenovine, K.M., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; Chapter 2; pp. 41–60. ISBN 978-1-61761-541-2. [Google Scholar]
- Escareño, L.; Wurzinger, M.; Iñiguez, L.; Soelkner, J.; Salinas, H.; Meza-Herrera, C.A. Dairy goat production systems in dry areas: Status-quo, perspectives and challenges. Trop. Anim. Health Prod. 2013, 45, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Morales, J.; Meza-Herrera, C.A.; Escobar-Medina, F.J.; Gamez- Vazquez, H.G.; Ramirez-Andrade, B.M.; Diaz-Gomez, M.O.; Gonzalez-Bulnes, A. Relative roles of photoperiodic and nutritional cues in modulating ovarian activity in goats. Reprod. Biol. 2009, 9, 283–294. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Tena-Sempere, M. Interface between nutrition and reproduction. In Animal Reproduction in Livestock—Encyclopedia of Life Support Systems; Astiz, S., Gonzalez, A., Eds.; Eolss Publishers: Oxford, UK, 2012. [Google Scholar]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebling, F.J. The neuroendocrine timing of puberty. Reproduction 2009, 129, 675–683. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Veliz-Deras, F.G.; Wurzinger, M.; Lopez-Ariza, B.; Arellano-Rodriguez, G. The kiss1, kisspeptin, gpr-54 complex: A critical modulator of GnRH neurons during pubertal activation. J. Appl. Biomed. 2010, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Meza-Herrera, C.A.; Gonzalez-Bulnes, A.; Kridli, R.; Mellado, M.; Arechiga-Flores, C.F.; Salinas, H.; Luginbhul, J.M. Neuroendocrine, metabolic and genomic cues signaling the onset of puberty in females. Reprod. Dom. Anim. 2011, 45, e495–e502. [Google Scholar] [CrossRef]
- Maffuci, J.A.; Gore, A.C. Hypothalamic neural systems controlling the female reproductive life cycle: Gonadotropin-releasing hormone, glutamate and GABA. Internat. Rev. Cell Mol. Biol. 2009, 274, 127. [Google Scholar]
- Meza-Herrera, C.A. Puberty, kisspeptin and glutamate: A ceaseless golden braid. Adv. Exp. Med. Biol. 2012, 52, 97–124. [Google Scholar]
- Perfito, N.; Bentley, G.E. Opportunism, photoperiodism and puberty: Different mechanisms or variations on a Theme? Integ. Compar. Biol. 2009, 49, 538–549. [Google Scholar] [CrossRef]
- Urbanski, H.F.; Steven, G.K.; Vasilios, T.G. Mechanisms mediating the response of GnRH neurones to excitatory amino acids. Rev. Reprod. 1996, 1, 173–181. [Google Scholar] [CrossRef]
- Parent, A.S.; Matagne, V.; Bourguignon, J.P. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine 2005, 28, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Van den Pol, A.N.; Wuarin, J.P.; Dudek, F.E. Glutamate the dominant excitatory transmitter in neuroendocrine regulation. Science 1990, 250, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.W.; Mahesh, V.B. Excitatory amino acids: Evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocrine Rev. 1997, 18, 678–700. [Google Scholar] [CrossRef]
- Zamorano, L.; Mahesh, P.; De Sevilla, V.; Brann, D. Excitatory amino acid receptors and puberty. Steroids 1998, 63, 268–270. [Google Scholar] [CrossRef]
- Roth, C.; Schricker, M.; Lakomek, M.; Strege, A.; Heiden, I.; Luft, H.; Munzel, U.; Wuttke, W.; Jarry, H. Autoregulation of the gonadotropin-releasing hormone (GnRH) system during puberty: Effects of antagonistic versus agonistic GnRH analogs in a female rat model. J. Endocrinol. 2001, 169, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, J.; Elias, C.F. The ventral premammillary nucleus links metabolic cues and reproduction. Front. Endocrinol. 2011, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Leshan, R.L.; Ptaff, D.W. The hypothalamic ventral premammillary neucelus: A key site in leptin´s regulation of reproduction. J. Chem. Neuroanat. 2014, 61-62, 239–247. [Google Scholar] [CrossRef]
- d’Anglemont de Tassigny, X.; Ackroyd, K.J.; Chatzidaki, E.E.; Colledge, W.H. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J. Neurosci. 2010, 30, 8581–8590. [Google Scholar] [CrossRef] [Green Version]
- Kallo, I.; Molnar, C.S.; Szpke, S.; Fekete, C.; Hrabouvszky, E.; Lipostis, Z. Area-specific analysis of the distribution of hypothalamic neurons projecting to the rat ventral tegmental area with special reference to GABAergic and glutamatergic efferents. Front. Neuroanat. 2015, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Meza-Herrera, C.A.; Torres-Moreno, M.; Lopez-Medrano, J.I.; Gonzalez-Bulnes, A.; Veliz, F.G.; Mellado, M.; Wurzinger, M.; Soto-Sanchez, M.J.; Calderon-Leyva, M.G. Glutamate supply positively affects serum release of triiodothyronine and insulin across time without increases of glucose during the onset of puberty in the female goat. Anim. Reprod. Sci. 2011, 125, 74–80. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Calderon-Leyva, G.; Soto-Sanchez, M.J.; Serradilla, J.M.; Garcia-Martinez, A.; Mellado, M.; Veliz-Deras, F.G. Glutamate supply positively affects cholesterol concentrations without increases in total protein and urea around the onset of puberty in goats. Anim. Reprod. Sci. 2014, 147, 106–111. [Google Scholar] [CrossRef] [PubMed]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Aumont, G.; Poisot, F.; Saminadin, G.; Borel, H.; Alexandre, G. Body condition score and adipose cell size determination for in vivo assessment of body composition and post-mortem predictors of carcass components in Creole goats. Small Rumin. Res. 1994, 15, 77–85. [Google Scholar] [CrossRef]
- Schneider, F.A.; Hallford, D.M. Use of rapid progesterone radioimmunoassay to predict pregnancy and fetal numbers in ewes. Sheep Goat Res. J. 1996, 12, 33–38. [Google Scholar]
- Cushwa, W.T.; Bradford, G.H.; Stabenfeldt, Y.M.; Berger, M.; Rally, M.R. Ram influence on ovarian and sexual activity in anestrous ewes: Effects of isolation of ewes from rams before joining and date of ramintroduction. J. Anim. Sci. 1992, 70, 1195–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, W.A., Jr.; Hatfield, P.G.; Hallford, D.M.; Fitzgerald, J.A.; Petersen, M.K.; Stellflug, J.N. Effect of selection for life time production of lamb weaned on hormonal factors that affect growth in Targhee ewes and lambs. J. Anim. Sci. 1996, 74, 2152–2157. [Google Scholar] [CrossRef]
- Sanson, D.W.; Hallford, D.M. Growth response and carcass characteristics and serum glucose and insulin in lambs fed tolazamide. Nutr. Reprod. Intern. 1984, 29, 461–471. [Google Scholar]
- Littell, C.R.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1988, 76, 1216–1231. [Google Scholar] [CrossRef] [Green Version]
- Dardente, H.; Lomet, D.; Robert, V.; Decourt, C.; Beltramo, M.; Pellicer-Rubio, M.T. Seasonal breeding in mammals: From basic science to applications and back. Theriogenology 2016, 86, 324–332. [Google Scholar] [CrossRef]
- Mayer, M.L. The structure and function of glutamate receptors: Mg2+ block to X-ray diffraction. Neuropharmacology 2016, 112, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, N.; Pandolfi, M.; Ponzo, O.; Carbone, S.; Szwarcfarb, B.; Scacchi, P.; Reynoso, R. Evidence to suggest glutamic acid involvement in bisphenol A effect at the hypothalamic level in prepubertal male rats. Neuro Endocrinol. Lett. 2010, 31, 512–516. [Google Scholar]
- Meza-Herrera, C.A.; Gonzalez-Velazquez, A.; Veliz-Deras, F.G.; Rodriguez-Martinez, R.; Arellano-Rodriguez, G.; Serradilla, J.M.; Garcia-Martinez, A.; Avendaño-Reyes, L.; Macias-Cruz, U. Short-term glutamate administration positively affects the number of antral follicles and the ovulation rate in cycling adult goats. Reprod. Biol. 2014, 13, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Kaiser, U.B. Metabolic influences on neuroendocrine regulation of reproduction. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 335–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sliwowska, J.H.; Fergani, C.; Gawałek, M.; Skowronska, B.; Fichna, P.; Lehman, M.N. Insulin: Its Role in the Central Control of Reproduction. Physiol. Behav. 2014, 113, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tata, J.R. A hormone for all seasons. Perspect. Biol. Med. Winter 2007, 50, 89–103. [Google Scholar] [CrossRef]
- Blache, D.; Zhang, S.; Martin, G.B. Dynamic and integrative aspects of the regulation of reproduction by metabolic status in male sheep. Reprod. Nutr. Dev. 2006, 46, 379–390. [Google Scholar] [CrossRef]
- Slebodzinsk, A.B. Ovarian iodide uptake and triiodothyronine generation in follicular fluid: The enigma of the thyroid ovary interaction. Domest. Anim. Endocrinol. 2005, 29, 97–103. [Google Scholar] [CrossRef]
- Anderson, G.M.; Connors, J.M.; Hardy, S.L.; Valent, M.; Robert, L. Thyroid hormones mediate steroid-independent seasonal changes in luteinizing hormone pulsatility in the ewe. Biol. Reprod. 2002, 66, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Arufe, M.C.; Duran, R.; Perez-Vences, A.; Alfonso, M. Endogenous excitarory aminoacid neurotransmission regulates thyroid stimulating hormone and thyroid secretion in conscious freely moving male rats. Endocrine 2002, 17, 193–197. [Google Scholar] [CrossRef]
- Gunnarsson, D.; Selstam, G.; Ridderstråle, Y.; Holm, L.; Ekstedt, E.; Madej, A. Effects of dietary phytoestrogens on plasma testosterone and triiodothyronine (T3) levels in male goat kids. Acta Vet. Scand. 2009, 10, 51. [Google Scholar] [CrossRef] [Green Version]
- Sechman, A.; Pawlowska, K.; Rzasa, J. Influence of triiodothyronine (T3) on secretion of steroids and thyroid hormone receptor expression in chicken ovarian follicles. Domest. Anim. Endocrinol. 2009, 37, 61–73. [Google Scholar] [CrossRef]
- Vissenberg, R.; Manders, V.D.; Mastenbroek, S.; Fliers, E.; Afink, G.B.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P.H. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezawa, T.; Mogi, K.; Li You, J.; Sako, R.; Yamanouchi, K.; Nishihara, M. Modulation of growth hormone pulsatility by sex steroids in female goats. Endocrinology 2005, 146, 2736–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Herrera, C.A.; Vergara-Hernández, H.P.; Paleta-Ochoa, A.; Álvarez-Ruíz, A.R.; Veliz-Deras, F.G.; Arellano-Rodriguez, G.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Rodriguez-Martinez, R.; Carrillo, E. Glutamate Supply Reactivates Ovarian Function while Increases Serum Insulin and Triiodothyronine Concentrations in Criollo x Saanen-Alpine Yearlings’ Goats during the Anestrous Season. Animals 2020, 10, 234. https://doi.org/10.3390/ani10020234
Meza-Herrera CA, Vergara-Hernández HP, Paleta-Ochoa A, Álvarez-Ruíz AR, Veliz-Deras FG, Arellano-Rodriguez G, Rosales-Nieto CA, Macias-Cruz U, Rodriguez-Martinez R, Carrillo E. Glutamate Supply Reactivates Ovarian Function while Increases Serum Insulin and Triiodothyronine Concentrations in Criollo x Saanen-Alpine Yearlings’ Goats during the Anestrous Season. Animals. 2020; 10(2):234. https://doi.org/10.3390/ani10020234
Chicago/Turabian StyleMeza-Herrera, César A., Hector P. Vergara-Hernández, Alicia Paleta-Ochoa, Alma R. Álvarez-Ruíz, Francisco G. Veliz-Deras, Gerardo Arellano-Rodriguez, Cesar A. Rosales-Nieto, Ulises Macias-Cruz, Rafael Rodriguez-Martinez, and Evaristo Carrillo. 2020. "Glutamate Supply Reactivates Ovarian Function while Increases Serum Insulin and Triiodothyronine Concentrations in Criollo x Saanen-Alpine Yearlings’ Goats during the Anestrous Season" Animals 10, no. 2: 234. https://doi.org/10.3390/ani10020234
APA StyleMeza-Herrera, C. A., Vergara-Hernández, H. P., Paleta-Ochoa, A., Álvarez-Ruíz, A. R., Veliz-Deras, F. G., Arellano-Rodriguez, G., Rosales-Nieto, C. A., Macias-Cruz, U., Rodriguez-Martinez, R., & Carrillo, E. (2020). Glutamate Supply Reactivates Ovarian Function while Increases Serum Insulin and Triiodothyronine Concentrations in Criollo x Saanen-Alpine Yearlings’ Goats during the Anestrous Season. Animals, 10(2), 234. https://doi.org/10.3390/ani10020234








