Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Administration of BPA
2.2. Tissue Collection and Storage
2.3. Immunofluorescence Labelling
2.4. Evaluation of the Number of Enteric Neurons and Intramural Nerve Fibers
2.5. Statistical Analysis
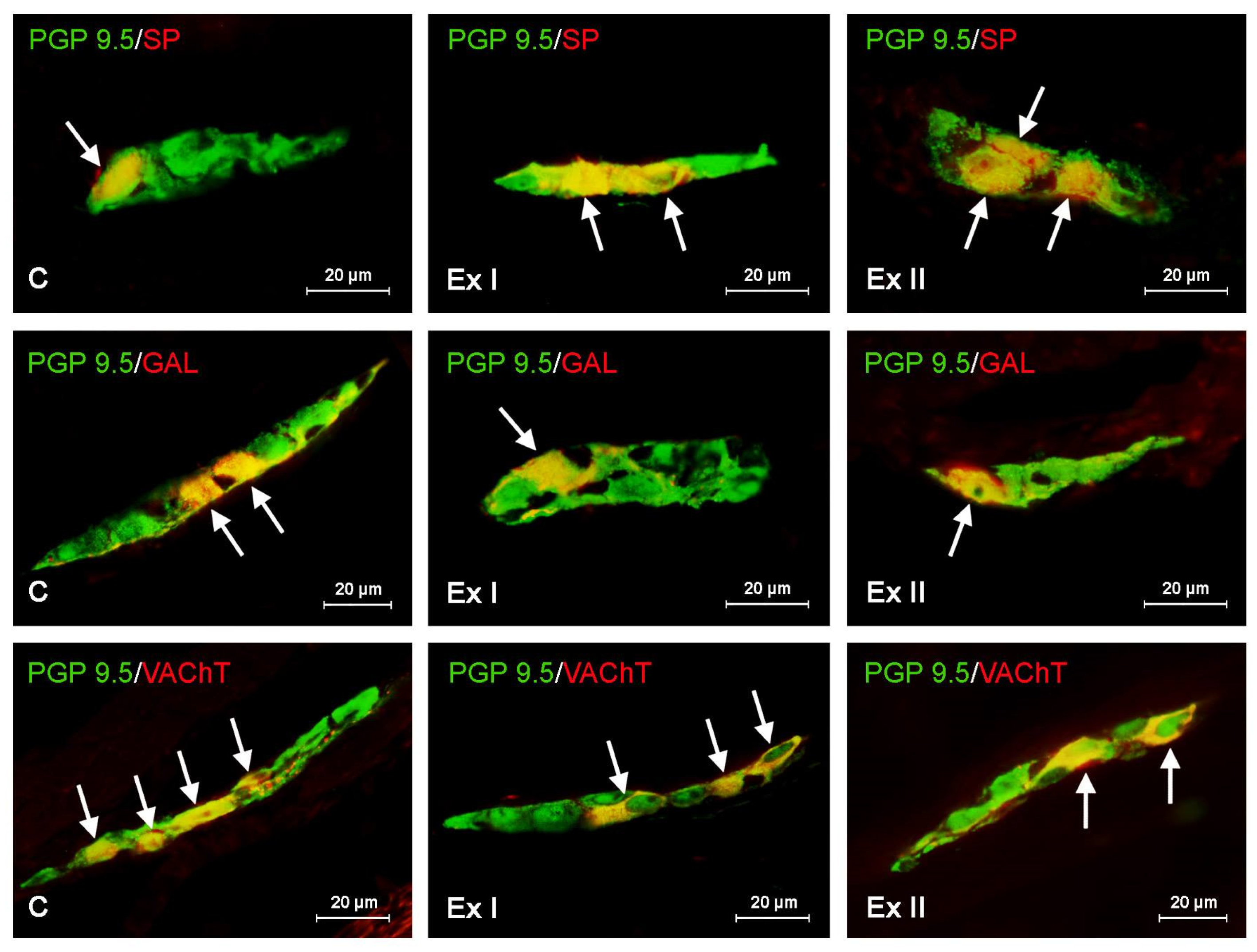
3. Results
3.1. Neurochemical Characterization of the Gastric Enteric Neurons under Physiological Conditions
3.2. The Influence of BPA on the Neurochemical Characterization of the Gastric Enteric Neurons
3.3. The Influence of BPA on the Neurochemical Characterization of the Nerve Fibers in the Mucosal and Muscular Layers of the Porcine Stomach
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staples, C.; Friederich, U.; Hall, T.; Klecka, G.; Mihaich, E.; Ortego, L.; Caspers, N.; Hentges, S. Estimating potential risks to terrestrial invertebrates and plants exposed to bisphenol A in soil amended with activated sludge biosolids. Environ. Toxicol. Chem. 2010, 29, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewska, K.; Stragierowicz, J.; Gromadzinska, J. Bisphenol A-application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Rashtian, J.; Chavkin, D.E.; Merhi, Z. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod. Biol. Endocrinol. 2019, 17, 5. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Rytel, L. The Influence of Bisphenol A (BPA) on Neuregulin 1-Like Immunoreactive Nerve Fibers in the Wall of Porcine Uterus. Int. J. Mol. Sci. 2018, 19, 2962. [Google Scholar] [CrossRef]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Koch, H.M.; Scholes, D.; Holt, V.L. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum. Reprod. 2014, 29, 2457–2464. [Google Scholar] [CrossRef]
- Neff, A.M.; Blanco, S.C.; Flaws, J.A.; Bagchi, I.C.; Bagchi, M.K. Chronic exposure of mice to bisphenol-A alters uterine fibroblast growth factor signaling and leads to aberrant epithelial proliferation. Endocrinology 2019, 160, 1234–1246. [Google Scholar] [CrossRef]
- Xu, X.; Xie, L.; Hong, X.; Ruan, Q.; Lu, H.; Zhang, Q.; Zhang, G.; Liu, X. Perinatal exposure to bisphenol-A inhibits synaptogenesis and affects the synaptic morphological development in offspring male mice. Chemosphere 2013, 91, 1073–1081. [Google Scholar] [CrossRef]
- Zhang, H.; Kuang, H.; Luo, Y.; Liu, S.; Meng, L.; Pang, Q.; Fan, R. Low-dose bisphenol A exposure impairs learning and memory ability with alterations of neuromorphology and neurotransmitters in rats. Sci. Total Environ. 2019, 697, 134036. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric. Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.C.; Johnson, S.A.; Hao, R.; Kowalczyk, A.S.; Greenberg, G.D.; Ordoñes Sanchez, E.; Laman-Maharg, A.; Trainor, B.C.; Rosenfeld, C.S. Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J. Neuroendocrinol. 2017, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, X.; Wang, D.; Wang, J.; Du, J. Effects of Bisphenol A Exposure during Pregnancy and lactation on Hippocampal Function in Newborn Rats. Int. J. Med. Sci. 2020, 17, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Musachio, E.A.S.; Araujo, S.M.; Bortolotto, V.C.; de Freitas Couto, S.; Dahleh, M.M.M.; Poetini, M.R.; Jardim, E.F.; Meichtry, L.B.; Ramborger, B.P.; Roehrs, R.; et al. Bisphenol A exposure is involved in the development of Parkinson like disease in Drosophila melanogaster. Food Chem. Toxicol. 2020, 137, 111128. [Google Scholar] [CrossRef] [PubMed]
- Sukjamnong, S.; Thongkorn, S.; Kanlayaprasit, S.; Saeliw, T.; Hussem, K.; Warayanon, W.; Hu, V.W.; Tencomnao, T.; Sarachana, T. Prenatal exposure to bisphenol A alters the transcriptome-interactome profiles of genes associated with Alzheimer’s disease in the offspring hippocampus. Sci. Rep. 2020, 10, 9487. [Google Scholar] [CrossRef]
- Sarkar, K.; Tarafder, P.; Paul, G. Bisphenol A inhibits duodenal movement ex vivo of rat through nitric oxide-mediated soluble guanylyl cyclase and α-adrenergic signaling pathways. J. Appl. Toxicol. 2016, 36, 131–139. [Google Scholar] [CrossRef]
- Qu, W.; Zhao, Z.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A suppresses proliferation and induces apoptosis in colonic epithelial cells through mitochondrial and MAPK/AKT pathways. Life Sci. 2018, 208, 167–174. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [PubMed]
- Makowska, K.; Obremski, K.; Gonkowski, S. The Impact of T-2 Toxin on Vasoactive Intestinal Polypeptide-Like Immunoreactive (VIP-LI) Nerve Structures in the Wall of the Porcine Stomach and Duodenum. Toxins 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Całka, J. Influence of acrylamide administration on the neurochemical characteristics of enteric nervous system (ENS) neurons in the porcine duodenum. Int. J. Mol. Sci. 2019, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Wattchow, D.; Brookes, S. Neuronal control in gastrointestinal disease. Eur. J. Surg. Suppl. 2002, 587, 39–46. [Google Scholar]
- Lundgren, O.; Svensson, L. The enteric nervous system and infectious diarrhea. Perspect. Med. Virol. 2003, 9, 51–67. [Google Scholar]
- Kleinschmidt, S.; Nolte, I.; Hewicker-Trautwein, M. Structural and functional changes of neuronal and glial components of the feline enteric nervous system in cats with chronic inflammatory and non-inflammatory diseases of the gastrointestinal tract. Res. Vet. Sci. 2011, 91, e129–e135. [Google Scholar] [CrossRef]
- Bassotti, G.; Villanacci, V.; Bellomi, A.; Fante, R.; Cadei, M.; Vicenzi, L.; Tonelli, F.; Nesi, G.; Asteria, C.R. An assessment of enteric nervous system and estroprogestinic receptors in obstructed defecation associated with rectal intussusception. Neurogastroenterol. Motil. 2012, 24, e155–e161. [Google Scholar] [CrossRef]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of bisphenol A (BPA). Neurogastroenterol. Motil. 2019, 31, e13580. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hu, H.Z.; Starodub, A.M.; Wood, J.D. Galanin suppresses calcium conductance and activates inwardly rectifying potassium channels in myenteric neurones from guinea-pig small intestine. Neurogastroenterol. Motil. 2001, 13, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-Gonzales, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Grob, K.; Gürtler, R.; Husøy, T.; Mennes, W.; Milana, M.R.; Penninks, A.; Roland, F.; Silano, V.; Smith, A.; Poças, M.D.F.T.; et al. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978–4599. [Google Scholar]
- Ćwiek-Ludwicka, K. Bisphenol A (BPA) in food contact materials—New scientific opinion from EFSA regarding public health risk. Rocz. Panstw. Zakl. Hig. 2015, 66, 299–307. [Google Scholar]
- Chaudhry, S.R.; Liman, M.N.P.; Peterson, D.C. Anatomy, Abdomen and Pelvis, Stomach. In StatPearls. Treasure Island (FL); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Inoue, K.; Yamaguchi, A.; Wada, M.; Yoshimura, Y.; Makino, T.; Nakazaw, H. Quantitative detection of bisphenol A and bisphenol A diglycidyl ether metabolites in human plasma by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 765, 121–126. [Google Scholar] [CrossRef]
- Tanaka, M.; Kawamoto, T.; Matsumoto, H. Distribution of 14C-bisphenol A in pregnant and newborn mice. Dent. Mater. 2010, 26, e181–e187. [Google Scholar] [CrossRef]
- Gonkowski, S. Bisphenol A (BPA)-Induced Changes in the Number of Serotonin-Positive Cells in the Mucosal Layer of Porcine Small Intestine—The Preliminary Studies. Int. J. Mol. Sci. 2020, 21, 1079. [Google Scholar] [CrossRef]
- Zalecki, M. Gastric ulcer induced changes in substance P and Nk1, Nk2, Nk3 receptors expression in different stomach localizations with regard to intrinsic neuronal system. Histochem. Cell Biol. 2019, 151, 29–42. [Google Scholar] [CrossRef]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Obremski, K.; Bulc, M.; Całka, J. The impact of low and high doses of acrylamide on the intramural neurons of the porcine ileum. Food Chem. Toxicol. 2019, 132, 110673. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Furness, J.B. Immunohistochemical localization of cholinergic markers in putative intrinsic primary afferent neurons of the guinea-pig small intestine. Cell Tissue Res. 1998, 294, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, S.; Akhtar, T.; Hameed, N.; Ashfaq, I.; Sheikh, N. In Vivo Evaluation of Histopathological Alterations and Trace Metals Estimation of the Small Intestine in Bisphenol A-Intoxicated Rats. Can. J. Gastroenterol. Hepatol. 2019, 2019, 9292316. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Dey, L.; Xie, J.T.; Aung, H.H. Gastric effects of galanin and its interaction with leptin on brain stem neuronal activity. J. Pharmacol. Exp. Ther. 2002, 301, 488–493. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S. Cocaine-and amphetamine-regulated transcript (CART) peptide in mammals gastrointestinal system—A review. Ann. Anim. Sci. 2017, 17, 3–21. [Google Scholar] [CrossRef][Green Version]
- Mokra, K.; Kocia, M.; Michałowicz, J. Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2015, 84, 79–88. [Google Scholar] [CrossRef]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 Toxin-Induced Changes in Cocaine- and Amphetamine-Regulated Transcript (CART)-Like Immunoreactivity in the Enteric Nervous System Within Selected Fragments of the Porcine Digestive Tract. Neurotox Res. 2017, 31, 136–147. [Google Scholar] [CrossRef]
- Fang, P.; Yu, M.; Wan, D.; Zhang, L.; Han, L.; Shen, Z.; Shi, M.; Zhu, Y.; Zhang, Z.; Bo, P. Regulatory effects of galanin system on development of several age-related chronic diseases. Exp. Gerontol. 2017, 95, 88–97. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, L.; Xue, Y.; Xia, Y.J. Substance P prevents 1-methyl-4-phenylpyridinium-induced cytotoxicity through inhibition of apoptosis via neurokinin-1 receptors in MES23.5 cells. Mol. Med. Rep. 2015, 12, 8085–8092. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamazaki, S.; Kumakura, S.; Someya, A.; Iseki, M.; Inada, E.; Nagaoka, I. Yokukansan, a Japanese Herbal Medicine, suppresses Substance P-induced Production of Interleukin-6 and Interleukin-8 by Human U373 MG Glioblastoma Astrocytoma Cells. Endocr. Metab. Immune Disord. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Bianchini, R.; Schlager, S.; Münz, C.; Kofler, B.; Wiesmayr, S. The neuropeptide galanin modulates natural killer cell function. Neuropeptides 2017, 64, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Obremski, K.; Gonkowski, S.; Wojtacha, P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Pol. J. Vet. Sci. 2015, 18, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Rytel, L.; Dzika, E.; Włodarczyk, A.; Kruminis-Kaszkiel, E.; Konrad, P.; Wojtkiewicz, J. Bisphenol A Causes Liver Damage and Selectively Alters the Neurochemical Coding of Intrahepatic Parasympathetic Nerves in Juvenile Porcine Models under Physiological Conditions. Int. J. Mol. Sci. 2017, 18, 2726. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach-the study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef]


| Primary Antibodies | ||||
| Antigen | Catalogue No. | Species | Working Dilution | Source |
| CART | H-003-61 | Rabbit | 1:16,000 | Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA |
| GAL | AB2233 | Guinea Pig | 1:2000 | EMD Millipore, Burlington, MA, USA |
| PGP9.5 | 7863-2004 | Mouse | 1:1000 | Bio-Rad, Hercules, CA, USA |
| SP | 8450-0505 | Rat | 1:1000 | Bio-Rad |
| VAChT | H-V007 | Rabbit | 1:2000 | Phoenix Pharmaceuticals |
| VIP | 9535-0204 | Mouse | 1:2000 | Bio-Rad |
| Secondary Antibodies | ||||
| Reagent | Working Dilution | Source | ||
| AF 488 donkey anti-mouse IgG (H + L) | 1:1000 | Thermo Fisher Scientific, Waltham, MA, USA | ||
| AF 546 goat anti-guinea pig IgG (H + L) | 1:1000 | Thermo Fisher Scientific | ||
| AF 546 goat anti-rabbit IgG (H + L) | 1:1000 | Thermo Fisher Scientific | ||
| AF 546 goat anti-rat IgG (H + L) | 1:1000 | Thermo Fisher Scientific | ||
| SP | |||
| C | Ex I | Ex II | |
| MG | 12.16 ± 0.11 | 14.54 ± 0.14 *# | 17.78 ± 0.22 *# |
| SG | 15.09 ± 0.23 | 20.57 ± 0.10 *# | 22.80 ± 0.15 *# |
| VIP | |||
| C | Ex I | Ex II | |
| MG | 14.63 ± 0.18 | 17.12 ± 0.26 *# | 18.81 ± 0.19 *# |
| SG | 17.57 ± 0.14 | 14.35 ± 0.13 *# | 13.38 ± 0.14 *# |
| GAL | |||
| C | Ex I | Ex II | |
| MG | 13.50 ± 0.18 | 18.29 ± 0.13 *# | 21.93 ± 0.16 *# |
| SG | 15.83 ± 0.12 | 20.45 ± 0.11 *# | 24.37 ± 0.16 *# |
| VAChT | |||
| C | Ex I | Ex II | |
| MG | 23.11 ± 0.19 | 20.19 ± 0.26 *# | 17.42 ± 0.22 *# |
| SG | 34.23 ± 0.23 | 31.02 ± 0.38 *# | 25.11 ± 0.22 *# |
| CART | |||
| C | Ex I | Ex II | |
| MG | 15.38 ± 0.20 | 18.25 ± 0.14 *# | 21.88 ± 0.15 *# |
| SG | 15.16 ± 0.25 | 20.49 ± 0.15 *# | 23.10 ± 0.27 *# |
| SP | |||
| C | Ex I | Ex II | |
| ML | 8.45 ± 0.19 | 10.30 ± 0.09 *# | 12.91 ± 0.09 *# |
| CML | 9.62 ± 0.10 | 10.64 ± 0.09 *# | 14.27 ± 0.09 *# |
| VIP | |||
| C | Ex I | Ex II | |
| ML | 7.92 ± 0.12 | 10.71 ± 0.10 *# | 14.47 ± 0.10 *# |
| CML | 13.27 ± 0.08 | 15.30 ± 0.10 *# | 22.68 ± 0.11 *# |
| GAL | |||
| C | Ex I | Ex II | |
| ML | 6.26 ± 0.10 | 8.96 ± 0.10 *# | 12.39 ± 0.14 *# |
| CML | 9.33 ± 0.09 | 11.47 ± 0.15 *# | 14.05 ± 0.09 *# |
| VAChT | |||
| C | Ex I | Ex II | |
| ML | 14.23 ± 0.10 | 12.96 ± 0.05 * | 12.16 ± 0.06 * |
| CML | 17.98 ± 0.10 | 14.49 ± 0.12 * | 13.66 ± 0.17 * |
| CART | |||
| C | Ex I | Ex II | |
| ML | 11.90 ± 0.07 | 13.65 ± 0.09 *# | 15.43 ± 0.07 *# |
| CML | 17.73 ± 0.18 | 19.21 ± 0.05 *# | 21.12 ± 0.09 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, K.; Gonkowski, S. Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach. Animals 2020, 10, 2445. https://doi.org/10.3390/ani10122445
Makowska K, Gonkowski S. Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach. Animals. 2020; 10(12):2445. https://doi.org/10.3390/ani10122445
Chicago/Turabian StyleMakowska, Krystyna, and Sławomir Gonkowski. 2020. "Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach" Animals 10, no. 12: 2445. https://doi.org/10.3390/ani10122445
APA StyleMakowska, K., & Gonkowski, S. (2020). Bisphenol A (BPA) Affects the Enteric Nervous System in the Porcine Stomach. Animals, 10(12), 2445. https://doi.org/10.3390/ani10122445






