A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
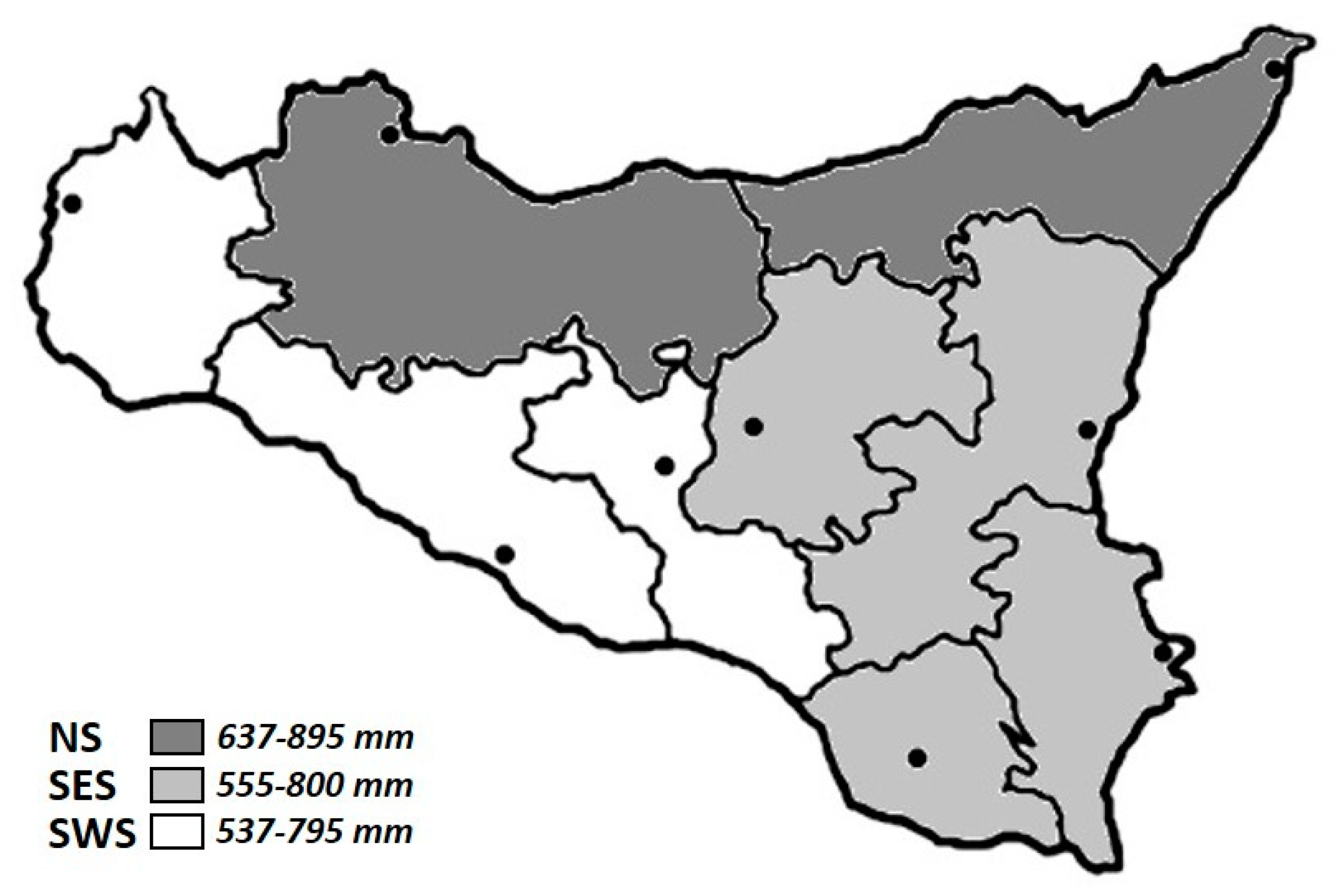
2.2. Study Area
- -
- Northern Sicily (NS): includes the Tyrrhenian side of the island, in particular Palermo and Messina districts. Rainfall is characterized by a rainy season (autumn–winter) and a dry spring–summer (rainy days per year > 70).
- -
- South-Eastern Sicily (SES): includes Catania, Syracuse, Ragusa and Enna. Rainfall is usually less frequent than in the Tyrrhenian area and the rainy days do not exceed 60.
- -
- South-Western Sicily (SWS): includes the area bordered by the Mediterranean, the Sicilian Channel and the central area: Trapani, Agrigento and Caltanissetta districts. The number of rainy days is lower than other areas (<60 days per year).
2.3. Sample
2.4. Serological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Allison, R.W.; Little, S.E. Diagnosis of rickettsial diseases in dogs and cats. Vet. Clin. Pathol. 2013, 42, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Hofmann-Lehmann, R.; Radford, A.D.; Tasker, S.; Belák, S.; Addie, D.D.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. Feline Med. Surg. 2017, 19, 542–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, C.; Ramos, C.; Coimbra, M.; Bastos, F.; Martins, A.; Pinto, P.; Nunes, M.; Vieira, M.L.; Cardoso, L.; Campino, L. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasites Vectors 2014, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebani, V.V.; Rocchigiani, G.; Nardoni, S.; Bertelloni, F.; Vasta, V.; Papini, R.A.; Verin, R.; Poli, A.; Mancianti, F. Molecular detection of tick-borne pathogens in wild red foxes (Vulpes vulpes) from Central Italy. Acta Trop. 2017, 172, 197–200. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [Green Version]
- Persichetti, M.F.; Pennisi, M.G.; Vullo, A.; Masucci, M.; Migliazzo, A.; Solano-Gallego, L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasites Vectors 2018, 20, 136. [Google Scholar] [CrossRef]
- Morganti, G.; Veronesi, F.; Stefanetti, V.; Di Muccio, T.; Fiorentino, E.; Diaferia, M.; Santoro, A.; Passamonti, F.; Gramiccia, M. Emerging feline vector-borne pathogens in Italy. Parasites Vectors 2019, 12, 193. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasites Vectors 2015, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, M.; Barlozzari, G.; Marcario, A.; Salvato, L.; Blanda, V.; De Liberato, C.; D’Agostini, C.; Torina, A.; Macrì, G. Molecular detection and characterization of spotted fever group rickettsiae in ticks from Central Italy. Ticks Tick Borne Dis. 2016, 7, 1052–1056. [Google Scholar] [CrossRef] [Green Version]
- Gray, J.; Dantas-Torres, F.; Estrada-Pena, A.; Levin, M. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013, 4, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Chalvet-Monfray, K. Impact of climate change in the epidemiology of vector-borne diseases in domestic carnivores. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parola, P.; Raoult, D. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 2001, 7, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torina, A.; Alongi, A.; Naranjo, V.; Estrada-Peña, A.; Vicente, J.; Scimeca, S.; Marino, A.M.F.; Salina, F.; Caracappa, S.; de la Fuente, J. Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a Mediterranean ecosystem. Appl. Environ. Microbiol. 2008, 74, 7578–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISPRA. Gli Indicatori del CLIMA in Italia nel 2018. 2019. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/stato-dellambiente/gli-indicatori-del-clima-in-italia-nel-2018 (accessed on 10 October 2020).
- Dipartimento Regionale dell’acqua e dei rifiuti—Osservatorio delle acque; Regione Siciliana, Palermo, Italy. Personal communication, 2020.
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 20 October 2020).
- Torina, A.; Caracappa, S. Dog tick-borne diseases in Sicily. Parassitologia 2006, 48, 145–147. [Google Scholar]
- Migliore, S.; La Marca, S.; Stabile, C.; Di Marco Lo Presti, V.; Vitale, M. A rare case of acute toxoplasmosis in a stray dog due to infection of T. gondii clonal type I: Public health concern in urban settings with stray animals? BMC Vet. Res. 2017, 13, 249. [Google Scholar] [CrossRef]
- Galluzzo, P.; Grippi, F.; Di Bella, S.; Santangelo, F.; Sciortino, S.; Castiglia, A.; Sciacca, C.; Arnone, M.; Alduina, R.; Chiarenza, G. Seroprevalence of Borrelia burgdorferi in Stray Dogs from Southern Italy. Microorganisms 2020, 8, 1688. [Google Scholar] [CrossRef]
- LAV. Randagismo: L’indagine LAV 2018. Available online: https://www.lav.it/cpanelav/js/ckeditor/kcfinder/upload/files/files/Dossier%20randagismo%202018.pdf (accessed on 28 October 2020).
- Pennisi, M.G.; Caprì, A.; Solano-Gallego, L.; Lombardo, G.; Torina, A.; Masucci, M. Prevalence of antibodies against Rickettsia conorii, Babesia canis, Ehrlichia canis, and Anaplasma phagocytophilum antigens in dogs from the Stretto di Messina area Italy. Ticks Tick Borne Dis. 2012, 3, 315–318. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Llull, J.; Osso, M.; Hegarty, B.; Breitschwerdt, E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet. Res. 2006, 37, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Vascellari, M.; Ravagnan, S.; Carminato, A.; Cazzin, S.; Carli, E.; Da Rold, G.; Lucchese, L.; Natale, A.; Otranto, D.; Capelli, G. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasites Vectors 2016, 9, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Groups | E. canis | R. conorii | ||
|---|---|---|---|---|
| S | O | S | O | |
| NS | 2327 | 773 | 1849 | 598 |
| SWS | 1866 | 374 | 380 | 307 |
| SES | 2064 | 164 | 246 | 61 |
| year | E. canis | R. conorii | ||
|---|---|---|---|---|
| No. of Sera | Positive (%) | No. of Sera | Positive (%) | |
| 2017 | 2359 | 678 (28.7) | 872 | 481 (55.2) |
| 2018 | 2553 | 775 (30.4) | 1.312 | 795 (60.6) |
| 2019 | 2656 | 785 (29.6) | 1.257 | 576 (45.1) |
| total | 7568 | 2238 (29.6) | 3441 | 1843 (53.6) |
| Groups | E. canis | R. conorii | ||
|---|---|---|---|---|
| No. of Sera | Positive (%) | No. of Sera | Positive (%) | |
| NS | 3100 | 787 (25.4) | 2.447 | 1274 (52.1) |
| SWS | 2240 | 747 (33.3) | 687 | 440 (64.1) |
| SES | 2228 | 704 (31.6) | 307 | 129 (42.0) |
| Groups | Categories | E. canis | R. conorii | ||
|---|---|---|---|---|---|
| No. of Sera | Positive (%) | No. of Sera | Positive (%) | ||
| NS | S | 2327 | 601 (25.8) | 1849 | 924 (50.0) |
| O | 773 | 186 (24.1) | 598 | 350 (58.5) | |
| SWS | S | 1866 | 626 (33.5) | 380 | 228 (60.0) |
| O | 374 | 121 (32.4) | 307 | 212 (69.1) | |
| SES | S | 2064 | 671 (32.5) | 246 | 111 (45.1) |
| O | 164 | 33 (20.1) | 61 | 18 (29.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, S.; Gargano, V.; De Maria, C.; Gambino, D.; Gentile, A.; Vitale Badaco, V.; Schirò, G.; Mira, F.; Galluzzo, P.; Vicari, D.; et al. A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019. Animals 2020, 10, 2444. https://doi.org/10.3390/ani10122444
Migliore S, Gargano V, De Maria C, Gambino D, Gentile A, Vitale Badaco V, Schirò G, Mira F, Galluzzo P, Vicari D, et al. A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019. Animals. 2020; 10(12):2444. https://doi.org/10.3390/ani10122444
Chicago/Turabian StyleMigliore, Sergio, Valeria Gargano, Claudia De Maria, Delia Gambino, Antonino Gentile, Valeria Vitale Badaco, Giorgia Schirò, Francesco Mira, Paola Galluzzo, Domenico Vicari, and et al. 2020. "A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019" Animals 10, no. 12: 2444. https://doi.org/10.3390/ani10122444
APA StyleMigliore, S., Gargano, V., De Maria, C., Gambino, D., Gentile, A., Vitale Badaco, V., Schirò, G., Mira, F., Galluzzo, P., Vicari, D., & Di Bella, S. (2020). A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019. Animals, 10(12), 2444. https://doi.org/10.3390/ani10122444





