Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Fish and Rearing Conditions
2.3. Experimental Setup
2.4. Sample Collection
2.5. Tissue Samples Preparation and the Measurement of the Antioxidative Indices
2.6. Transcriptomic Expression Analysis of Antioxidant Genes
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
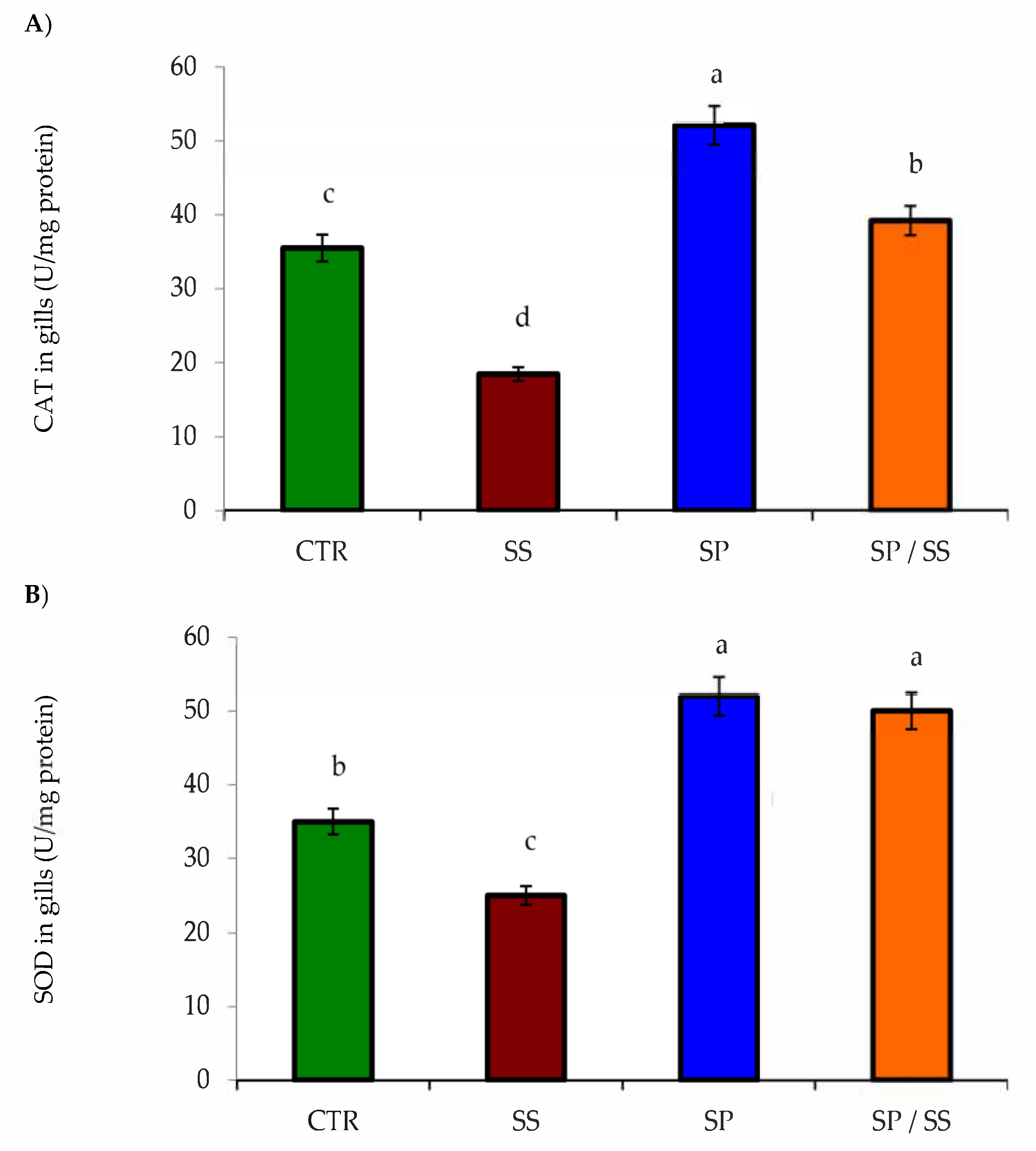
3.2. Muscle, Gills, Liver, and Kidney Antioxidation Capacity
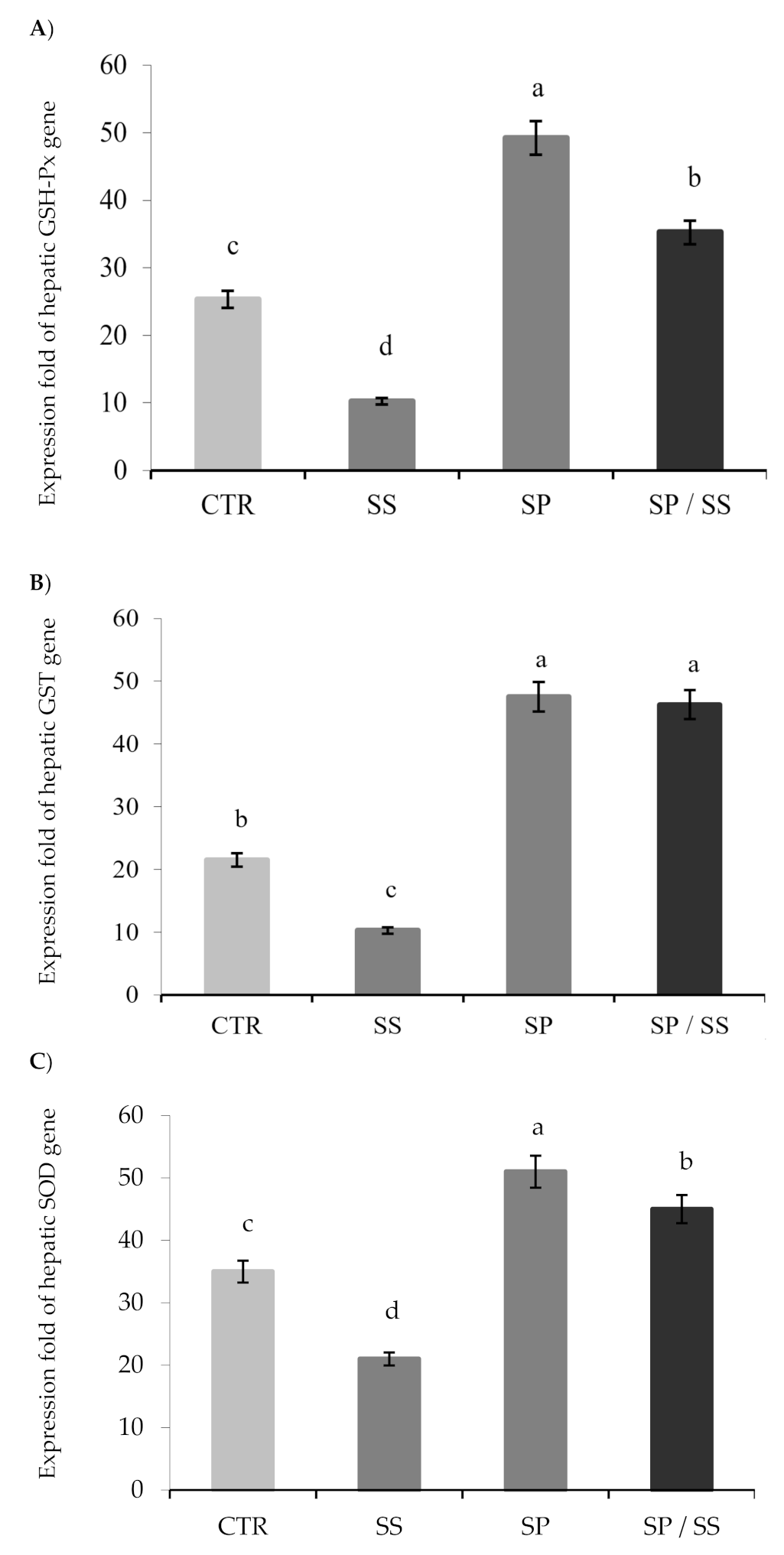
3.3. Expression of Hepatic Antioxidant Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghelichpour, M.; Mirghaed, A.T.; Dawood, M.A.; Hoseinifar, S.H.; Van Doan, H. Alteration of haematological and antioxidant parameters in common carp (Cyprinus carpio) fed olive (Olea europea) leaf extract after exposure to Danitol®. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Dawood, M.A.; Ahmadifar, M.; Moghadam, M.S.; Abarghouei, S.; Hedayati, A. Effects of polystyrene microparticles on inflammation, antioxidant enzyme activities, and related gene expression in Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 1–8. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy Metal Pollution in Aquatic Ecosystems and its Phytoremediation using Wetland Plants: An ecosustainable approach. Int. J. Phytoremediation 2008, 10, 133–160. [Google Scholar] [CrossRef]
- Wang, N.; Consbrock, R.A.; Ingersoll, C.G.; Hardesty, D.K.; Brumbaugh, W.G.; Hammer, E.; Bauer, C.R.; Mount, D.R. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ. Toxicol. Chem. 2015, 35, 115–127. [Google Scholar] [CrossRef]
- Awed, E.; Sadek, K.; Soliman, M.; Khalil, R. Biochemical alterations in serum biomarkers of Nile tilapia (Oreochromis niloticus) exposed to sodium sulphate and Spirulina platensis. Damanhour J. Vet. Sci. 2020, 4, 1–6. [Google Scholar]
- Sánchez-Muros, M.; García-Rejón, L.; Lupiáñez, J.; Higuera, M. Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout, Oncorhynchus mykiss (Walbaum): Adaptive response to a high-protein/non-carbohydrate diet and starvation of glucose 6-phosphate dehydrogenase activity. Aquac. Nutr. 1995, 1, 213–220. [Google Scholar] [CrossRef]
- Ali, M.; Majid, M.; Hussain, I.; Kali, S.; Naz, T.; Niazi, M.B.K.; Khan, M.R.A.; Zafar, M.I. Chlorpyrifos mediated oxidative damage and histopathological alterations in freshwater fish Oncorhynchus mykiss in Northern Pakistan. Aquac. Res. 2020, 51, 4583–4594. [Google Scholar] [CrossRef]
- Dawood, M.A.; El-Shamaa, I.S.; Abdel-Razik, N.I.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Soliman, A.A.; Paray, B.A.; Abdelkhalek, N. The effect of mannanoligosaccharide on the growth performance, histopathology, and the expression of immune and antioxidative related genes in Nile tilapia reared under chlorpyrifos ambient toxicity. Fish Shellfish. Immunol. 2020, 103, 421–429. [Google Scholar] [CrossRef]
- Dawood, M.A.; Abdo, S.E.; Gewaily, M.S.; Moustafa, E.M.; Saadallah, M.S.; Abdel-Kader, M.F.; Hamouda, A.H.; Omar, A.A.; Alwakeel, R.A. The influence of dietary β-glucan on immune, transcriptomic, inflammatory and histopathology disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 98, 301–311. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, X.; Huang, J.; Chen, C.; Chen, Z.; Liu, L.; Zhang, G.; Yang, J.; Zhang, Z.; Zhang, Z.; et al. Fenpropathrin, a Widely Used Pesticide, Causes Dopaminergic Degeneration. Mol. Neurobiol. 2016, 53, 995–1008. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Naiel, M.A.; Dawood, M.A.; Abdel-Latif, H.M. Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 228, 105624. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Raieni, R.F.; Dadar, M.; Yilmaz, S.; Dawood, M.A.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2020, 1–34. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Abdel-Latif, H.M.; Dawood, M.A. The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish Shellfish. Immunol. 2020, 106, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Van Doan, H.; Ahmed, H.A.; Dawood, M.A.; Abdel-Latif, H.M. Oregano (Origanum vulgare), St John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 2020, 18, 100445. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and an-ti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.E.-D.H.; El-Sayed, Y.S.; El-Far, A.H. Hepatoprotective efficacy of Spirulina platensis against lead-induced oxidative stress and genotoxicity in catfish; Clarias gariepinus. Ecotoxicol. Environ. Saf. 2017, 143, 344–350. [Google Scholar] [CrossRef]
- Khalil, S.R.; Reda, R.M.; Awad, A. Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish Shellfish. Immunol. 2017, 67, 119–128. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; Elbaghdady, H.A.M.; Zahran, E. Arsenic-induced genotoxicity in Nile tilapia (Oreochromis niloticus); the role of Spirulina platensis extract. Environ. Monit. Assess. 2015, 187, 751. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Sayed, A.E.-D.H. Ameliorative effect of Spirulina platensis against lead nitrate–induced cytotoxicity and genotoxicity in catfish Clarias gariepinus. Environ. Sci. Pollut. Res. 2019, 26, 20610–20618. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- FAO. Aquaculture Department the State of World Fisheries and Aquaculture; Food and Agriculture Organization of The United Nations: Rome, Italy, 2018; p. 2430. [Google Scholar]
- Abdel-Latif, H.M.; Soliman, A.A.; Sewilam, H.; Almeer, R.; Van Doan, H.; Alagawany, M.; Dawood, M.A. The influence of raffinose on the growth performance, oxidative status, and immunity in Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 18, 100457. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer: Boston, MA, USA, 1998; p. 700. ISBN 1461554071. [Google Scholar]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Dawood, M.A.; Elbadawy, M.; Aleya, L.; Alkahtani, S. Spirulina platensis Reduced Oxidative Damage Induced by Chlorpyrifos Toxicity in Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 473. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: London, UK, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Koracevic, D.; Harris, G.; Rayner, A.; Blair, J.; Watt, B. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Varela-Valencia, R.; Gómez-Ortiz, N.; Oskam, G.; De Coss, R.; Rubio-Piña, J.; Del Río-García, M.; Albores-Medina, A.; Zapata-Perez, O. The effect of titanium dioxide nanoparticles on antioxidant gene expression in tilapia (Oreochromis niloticus). J. Nanoparticle Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Vieira, C.A.S.C.; Vieira, J.S.; Bastos, M.S.; Zancanela, V.; Barbosa, L.T.; Gasparino, E.; Del Vesco, A.P. Expression of genes related to antioxidant activity in Nile tilapia kept under salinity stress and fed diets containing different levels of vitamin C. J. Toxicol. Environ. Heal. Part A 2017, 81, 20–30. [Google Scholar] [CrossRef]
- Wang, L.; Liang, X.; Liao, W.-Q.; Lei, L.-M.; Han, B.-P. Structural and functional characterization of microcystin detoxification-related liver genes in a phytoplanktivorous fish, Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 216–227. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Dos Santos, N.M.; Calejo, M.; Fontainhas-Fernandes, A.; Sousa, M. Copper toxicity in gills of the teleost fish, Oreochromis niloticus: Effects in apoptosis induction and cell proliferation. Aquat. Toxicol. 2009, 94, 219–228. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative c t method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.; Abdel-Tawwab, M.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. Benefits of Dietary Butyric Acid, Sodium Butyrate, and Their Protected Forms in Aquafeeds: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 421–448. [Google Scholar] [CrossRef]
- Dawood, M.A.; Abdel-Tawwab, M.; Abdel-Latif, H.M. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev. Aquac. 2020, 12, 2511–2526. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.S.; Soliman, A.A.; Shukry, M.; Amer, A.A.; Younis, E.M.; Abdel-Warith, A.-W.A.; Van Doan, H.; Saad, A.H.; Aboubakr, M.; et al. Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada). Mar. Drugs 2020, 18, 611. [Google Scholar] [CrossRef] [PubMed]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2018, 11, 1367–1378. [Google Scholar] [CrossRef]
- Berntssen, M.H.; Lundebye, A.-K.; Hamre, K. Tissue lipid peroxidative responses in Atlantic salmon (Salmo salar L.) parr fed high levels of dietary copper and cadmium. Fish Physiol. Biochem. 2000, 23, 35–48. [Google Scholar] [CrossRef]
- Dawood, M.A. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2020, 13, 642–663. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Atli, G.; Canli, M. Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol. Environ. Saf. 2010, 73, 1884–1889. [Google Scholar] [CrossRef]
- Upasani, C.D.; Balaraman, R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytotherapy Res. 2003, 17, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Arun, N.; Gupta, S.; Singh, D. Antimicrobial and antioxidant property of commonly found microalgae Spirulina platensis, Nostoc muscorum and Chlorella pyrenoidosa against some pathogenic bacteria and fungi. Int. J. Pharm. Sci. Res. 2012, 3, 4866. [Google Scholar]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2018, 10, 334–350. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina–From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, K.T.; Cho, J.G.; Lee, J.W.; Ryu, T.-K.; Yoon, J.H.; Lee, S.H.; Duong, C.N.; Eom, I.C.; Kim, P.J.; et al. Oxidative stress in juvenile common carp (Cyprinus carpio) exposed to TiO2 nanoparticles. Mol. Cell. Toxicol. 2012, 8, 357–366. [Google Scholar] [CrossRef]
- Federici, G.; Shaw, B.J.; Handy, R. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 2007, 84, 415–430. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Ahmed, N.F.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Khafaga, A.F.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A. Moringa Oleifera Leaf Extract Repairs the Oxidative Misbalance following Sub-Chronic Exposure to Sodium Fluoride in Nile Tilapia Oreochromis niloticus. Animals 2020, 10, 626. [Google Scholar] [CrossRef]
- Dawood, M.A.; Abdel-Razik, N.I.; Gewaily, M.S.; Sewilam, H.; Paray, B.A.; Soliman, A.A.; Abdelhiee, E.Y.; Aboubakr, M.; Van Doan, H.; El-Sabagh, M.; et al. β-Glucan improved the immunity, hepato-renal, and histopathology disorders induced by chlorpyrifos in Nile tilapia. Aquac. Rep. 2020, 18, 100549. [Google Scholar] [CrossRef]


| Ingredients | % on a DM Basis |
|---|---|
| Yellow corn meal | 28.0 |
| Fish meal | 25.5 |
| Soyabean meal | 21.5 |
| Wheat bran | 9.5 |
| Corn gluten meal | 2.0 |
| Rice bran | 7.3 |
| Gelatin | 2.0 |
| Fish oil | 3.0 |
| Mineral mixture a | 0.5 |
| Vitamin premix b | 0.5 |
| Di-calcium phosphate | 0.2 |
| Chemical Proximate Analysis | |
| Crude protein | 31.78% |
| Crude fiber | 5.66% |
| Ether extract | 7.15% |
| Ash | 8.14% |
| Gross energy (GE) c | 18.46 kcal/g |
| Genes | Accession No. | Sequence Primers | References |
|---|---|---|---|
| SOD | JF801727.1 | Forward: 5′-CTCCAGCCTGCCCTCAA-3′ | [31] |
| Reverse: 5′-TCCAGAAGATGGTGTGGTTAATGTG-3′ | |||
| GST | EU234530.1 | Forward: 5′-TAATGGGAGAGGGAAGATGG-3′ | [33] |
| Reverse: 5′-CTCTGCGATGTAATTCAGGA-3′ | |||
| GSH-Px | NM_001279711.1 | Forward: 5′-CGCCGAAGGTCTCGTTATT-3′ | [32] |
| Reverse: 5′-TCCCTGGACGGACACTT-3′ | |||
| β-actin | EU887951.1 | Forward: 5′-CAATGAGAGGTTCCGTTGC-3′ | [34] |
| Reverse: 5′-AGGATTCCATACCAAGGAAGG-3′ |
| Groups | IBW (g) | FBW (g) | WG (%) | SGR (%/Day) |
|---|---|---|---|---|
| Control | 40.38 ± 0.01 | 83.75 ± 4.94 | 107.42 ± 12.08 | 1.21 ± 0.10 |
| SS | 40.47 ± 0.51 | 84.25 ± 3.28 | 108.25 ± 8.99 | 1.22 ± 0.07 |
| SP | 40.21 ± 0.22 | 85.00 ± 5.28 | 111.52 ± 14.04 | 1.24 ± 0.11 |
| SP/SS | 40.19 ± 0.34 | 83.25 ± 1.37 | 107.15 ± 3.02 | 1.21 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awed, E.M.; Sadek, K.M.; Soliman, M.K.; Khalil, R.H.; Younis, E.M.; Abdel-Warith, A.-W.A.; Van Doan, H.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate. Animals 2020, 10, 2423. https://doi.org/10.3390/ani10122423
Awed EM, Sadek KM, Soliman MK, Khalil RH, Younis EM, Abdel-Warith A-WA, Van Doan H, Dawood MAO, Abdel-Latif HMR. Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate. Animals. 2020; 10(12):2423. https://doi.org/10.3390/ani10122423
Chicago/Turabian StyleAwed, Eman M., Kadry M. Sadek, Magdy K. Soliman, Riad H. Khalil, Elsayed M. Younis, Abdel-Wahab A. Abdel-Warith, Hien Van Doan, Mahmoud A.O. Dawood, and Hany M.R. Abdel-Latif. 2020. "Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate" Animals 10, no. 12: 2423. https://doi.org/10.3390/ani10122423
APA StyleAwed, E. M., Sadek, K. M., Soliman, M. K., Khalil, R. H., Younis, E. M., Abdel-Warith, A.-W. A., Van Doan, H., Dawood, M. A. O., & Abdel-Latif, H. M. R. (2020). Spirulina platensis Alleviated the Oxidative Damage in the Gills, Liver, and Kidney Organs of Nile Tilapia Intoxicated with Sodium Sulphate. Animals, 10(12), 2423. https://doi.org/10.3390/ani10122423







