Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Birds and Experimental Diets
2.3. Serum Biochemical Analysis
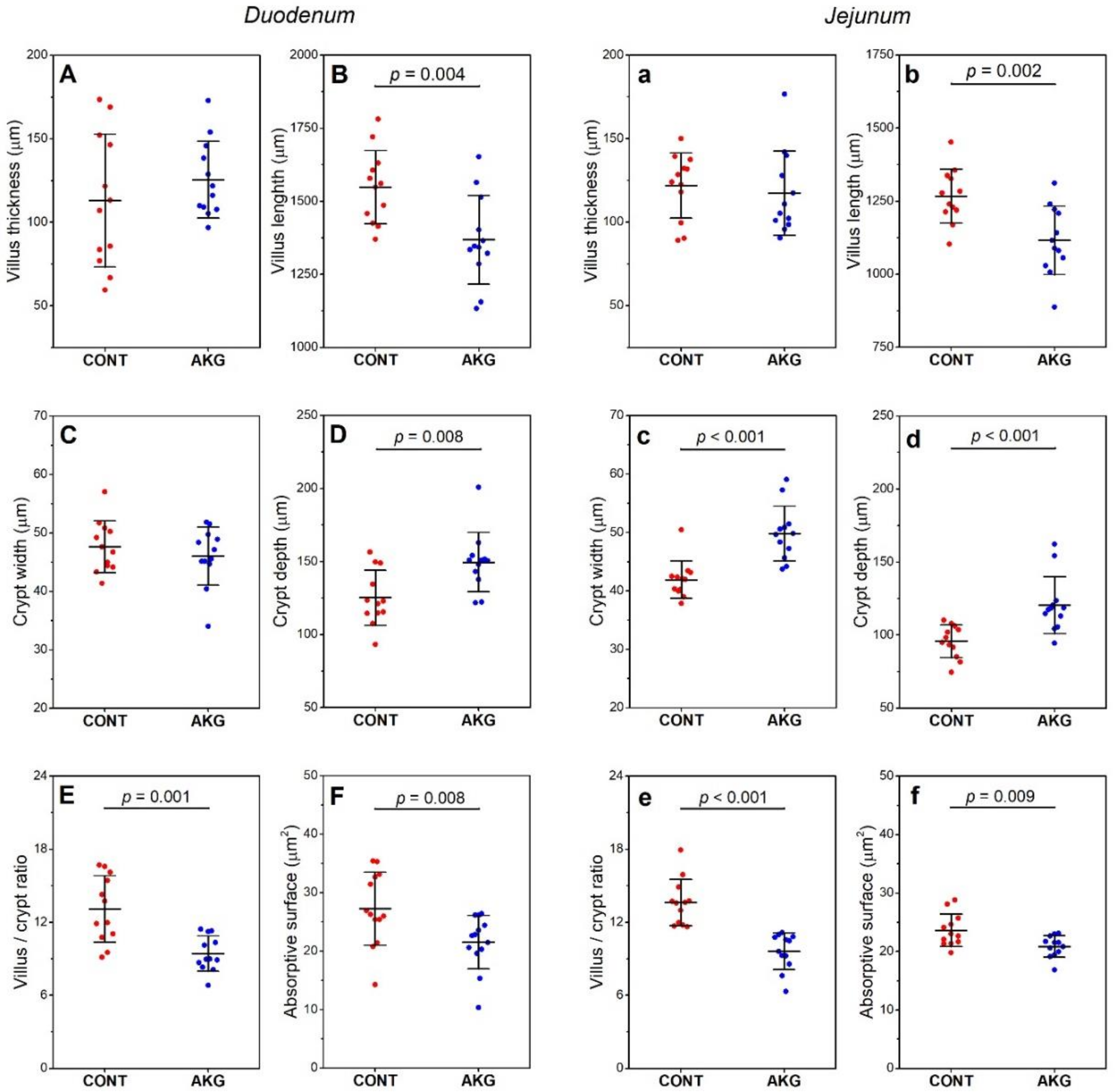
2.4. Intestine Tissue Histomorphometric Analysis
2.5. Analysis of Meat Microstructure, Physical Properties, Fatty Acid Profile and Cholesterol Content
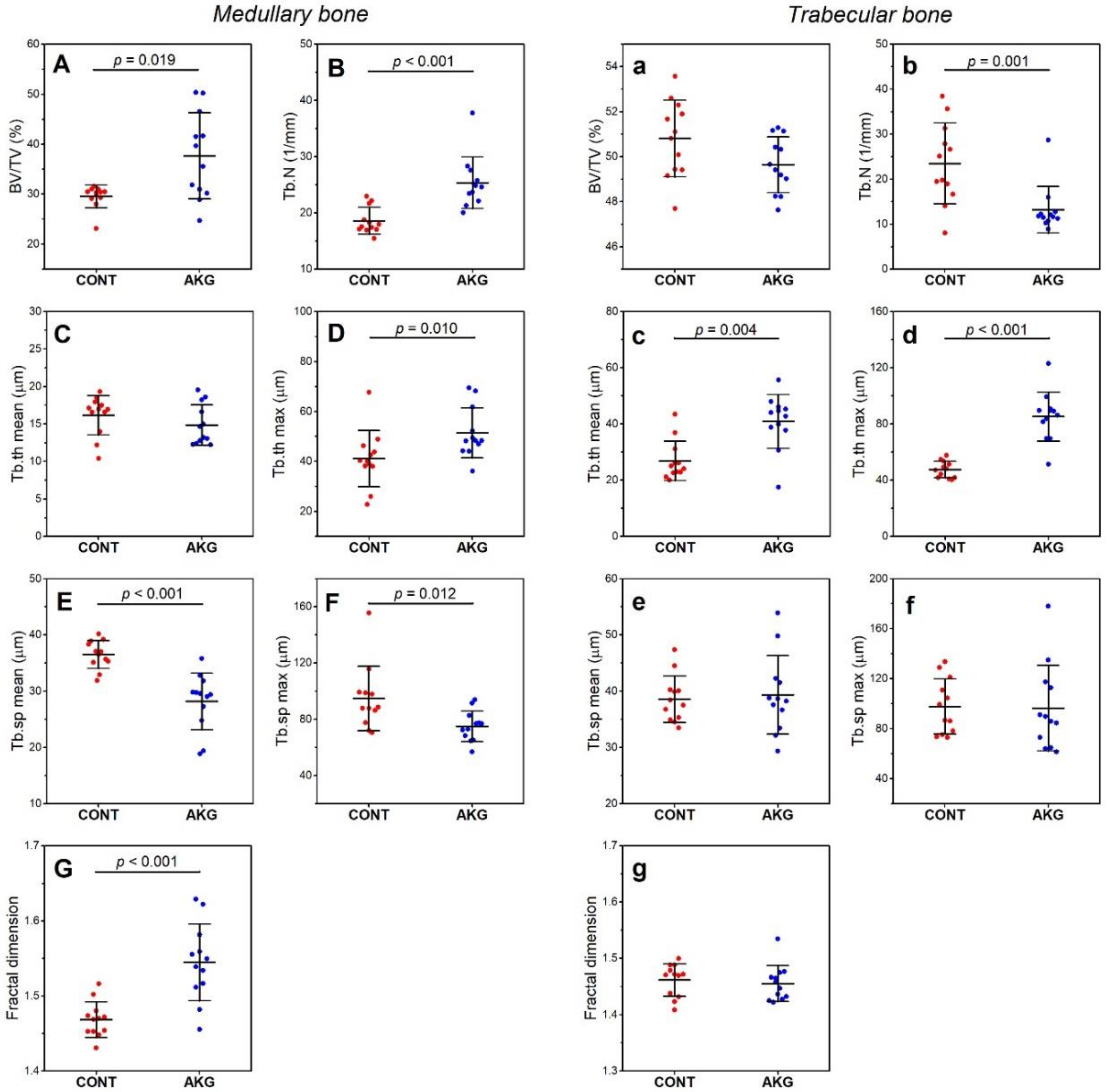
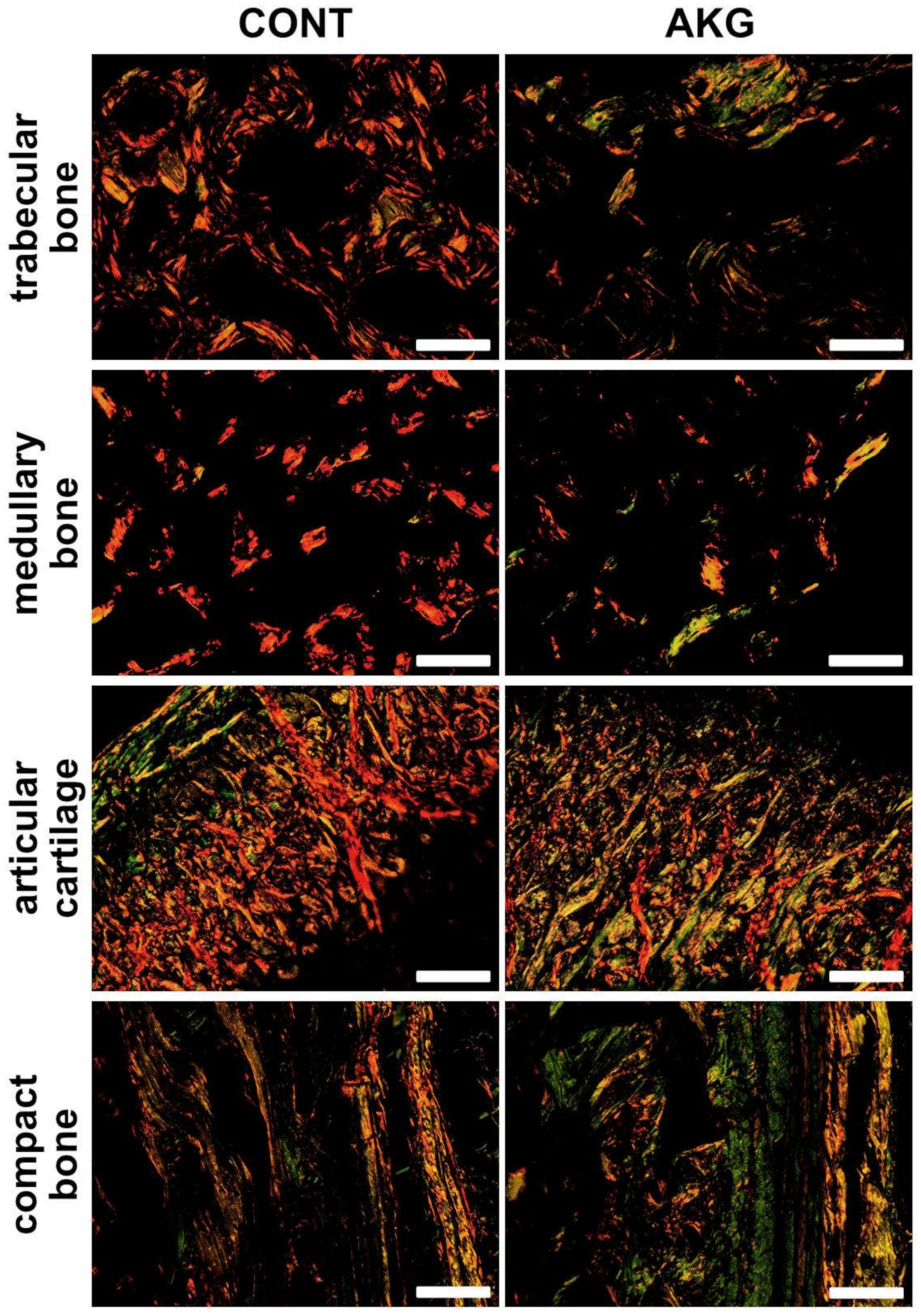
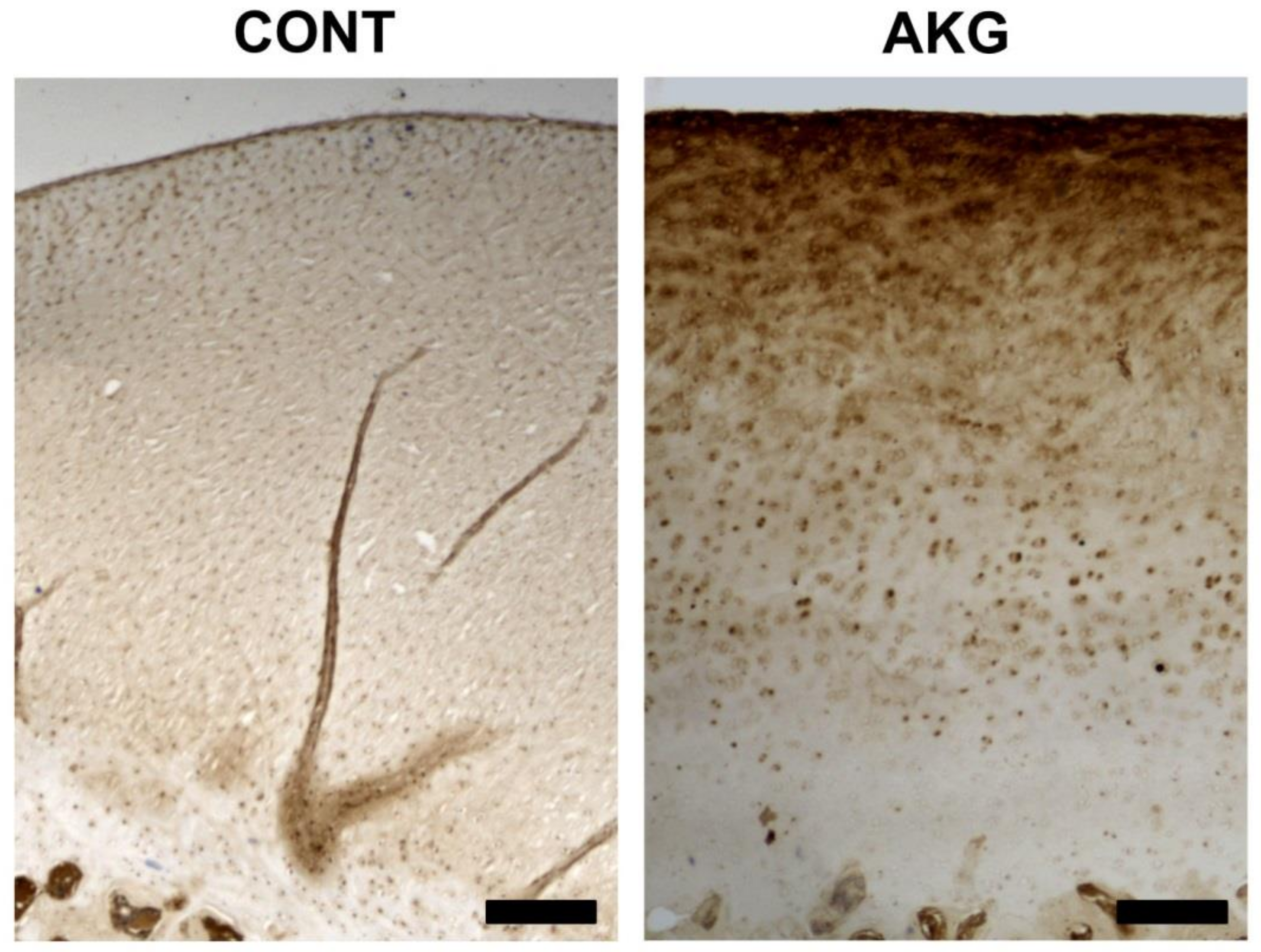
2.6. Bone and Cartilage Analysis
2.7. Statistical Analysis
3. Results
3.1. Performance
3.2. Blood Serum Biochemical Parameters
3.3. Intestinal Histomorphometry
3.4. Breast Muscle Microstructure, Physical Properties, Fatty Acid Profile and Cholesterol Content
3.5. Bone and Cartilage Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gregory, N.; Wilkins, L. Broken bones in domestic fowl: Handling and processing damage in end-of-lay battery hens. Br. Poult. Sci. 1989, 30, 555–562. [Google Scholar] [CrossRef]
- Sandilands, V. The laying hen and bone fractures. Vet. Rec. 2011, 169, 411–412. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, H.W.; Cui, L.Y.; Zhou, Z.L.; Hou, J.F. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult. Sci. 2013, 92, 1443–1453. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, D.; Świątkiewicz, S. Infectious and non-infectious factors associated with leg disorders in poultry—A review. Ann. Anim. Sci. 2017, 17, 645–669. [Google Scholar] [CrossRef]
- Olgun, O. The effect of dietary essential oil mixture supplementation on performance, egg quality and bone characteristics in laying hens. Ann. Anim. Sci. 2016, 16, 1115–1125. [Google Scholar] [CrossRef]
- Olgun, O.; Yildiz, A.O. Effects of dietary supplementation of inorganic, organic or nano zinc forms on performance, eggshell quality, and bone characteristics in laying hens. Ann. Anim. Sci. 2017, 17, 463–476. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Krawczyk, J.; Szczurek, W.; Puchała, M.; Józefiak, D. Effect of selected feed additives on egg performance and eggshell quality in laying hens fed a diet with standard or decreased calcium content. Ann. Anim. Sci. 2018, 18, 167–183. [Google Scholar] [CrossRef]
- Webster, A.B. Welfare implications of avian osteoporosis. Poult. Sci. 2004, 83, 184–192. [Google Scholar] [CrossRef]
- FAWC. Opinion on Osteoporosis and Bone Fractures in Laying Hens; Farm Animal Welfare Council: London, UK, 2010; p. 14. [Google Scholar]
- Riber, A.B.; Casey-Trott, T.M.; Herskin, M.S. The influence of keel bone damage on welfare of laying hens. Front. Vet. Sci. 2018, 5, 6. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Świątkiewicz, M.; Donaldson, J.; Puzio, I.; Muszyński, S. Is dietary 2-oxoglutaric acid effective in accelerating bone growth and development in experimentally induced intrauterine growth retarded gilts? Animals 2020, 10, 728. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Jaworka-Adamu, J.; Żmuda, A.; Rycerz, K.; Muszyński, S. The effect of HMB and 2-Ox administered during pregnancy on bone properties in primiparous and multiparous minks (Neivison vison). Bull. Vet. Inst. Pulawy 2015, 59, 563–568. [Google Scholar] [CrossRef]
- Śliwa, E.; Dobrowolski, P.; Tatara, M.R.; Piersiak, T.; Siwicki, A.; Rokita, E.; Pierzynowski, S.G. Alpha-ketoglutarate protects the liver of piglets exposed during prenatal life to chronic excess of dexamethasone from metabolic and structural changes. J. Anim. Physiol. Anim. Nutr. 2009, 93, 192–202. [Google Scholar] [CrossRef]
- Śliwa, E.; Kowalik, S.; Tatara, M.R.; Krupski, W.; Majcher, P.; Łuszczewska-Sierakowska, I.; Pierzynowski, S.G.; Studziński, T. Effect of alpha-ketoglutarate (AKG) given to pregnant sows on development of humerus and femur in newborns. Bull. Vet. Inst. Pulawy 2005, 49, 117–120. [Google Scholar]
- EFSA. Safety and efficacy of l-glutamine produced using Corynebacterium glutamicum NITE BP-02524 for all animal species. EFSA J. 2020, 18, 6075. [Google Scholar]
- Shakeri, M.; Zulkifli, I.; Soleimani, A.F.; O’Reilly, E.L.; Eckersall, P.D.; Kumari, S.; Aryani, A.A.; Abdullah, F.F. Response to dietary supplementation of L-glutamine and L-glutamate in broiler chickens reared at different stocking densities under the hot, humid tropical conditions. Poult. Sci. 2014, 93, 2700–2708. [Google Scholar] [CrossRef]
- Pierzynowski, S.; Sjodin, A. Perspectives of glutamine and its derivatives as feed additives for farm animals. J. Anim. Feed Sci. 1998, 7, 79–91. [Google Scholar] [CrossRef]
- Burrin, D.G.; Stoll, B. Metabolic fate and function of dietary glutamate in the gut. Am. J. Clin. Nutr. 2009, 90, 850S–856S. [Google Scholar] [CrossRef]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-Ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Tatara, M.R.; Śliwa, E.; Krupski, W.; Brodzki, A.; Pasternak, K. Ornithine alpha-ketoglutarate increases mineralization and mechanical properties of tibia in turkeys. Bone 2006, 39, 100–105. [Google Scholar] [CrossRef]
- Tatara, M.R.; Brodzki, A.; Krupski, W.; Śliwa, E.; Silmanowicz, P.; Majcher, P.; Pierzynowski, S.G.; Studziński, T. Effect of alpha-ketoglutarate (AKG) on bone homeostasis and plasma amino acids in turkeys. Poult. Sci. 2005, 84, 1604–1609. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Wang, L.; Ding, B.Y.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; Yin, Y.; Wu, G. Alpha-ketoglutarate and intestinal function. Front. Biosci. 2011, 16, 1186–1196. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Jungvid, H.; Fernández, J.A.; Pierzynowski, S.G. Absorption and metabolism of alpha-ketoglutarate in growing pigs. J. Anim. Physiol. Anim. Nutr. 2002, 86, 239–245. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2018/249 of 15 February 2018 Concerning the Authorisation of Taurine, Beta-Alanine, L-alanine, L-arginine, L-aspartic acid, L-histidine, D,L-isoleucine, L-leucine, L-phenylalanine, L-proline, D,L-serine, L-tyrosine, L-methionine, L-valine, L-cysteine, Glycine, Monosodium Glutamate and L-glutamic Acid as Feed Additives for all Animal Species and L-cysteine Hydrochloride Monohydrate for all Species Except Cats and Dogs. OJ L 53, 23 February 2018. pp. 1–32. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/249/oj (accessed on 15 December 2020).
- Commission Regulation (EC) 37/2010 of 22 December 2009 on Pharmacologically Active Substances and their Classification Regarding Maximum Residue Limits of Veterinary Medicinal Products in Foodstuffs of Animal Origin. OJ L 15, 20 January 2010. pp. 1–72. Available online: http://data.europa.eu/eli/reg/2010/37(1)/oj (accessed on 15 December 2020).
- Tomaszewska, E.; Dobrowolski, P.; Prost, Ł.; Hułas-Stasiak, M.; Muszyński, S.; Blicharski, T. The effect of supplementation of glutamine precursor on the growth plate, articular cartilage and cancellous bone in fundectomy-induces osteopenic bone. J. Vet. Med. Sci. 2016, 78, 563–571. [Google Scholar] [CrossRef]
- Kowalik, S.; Śliwa, E.; Tatara, M.R.; Krupski, W.; Majcher, P.; Studziński, T. Influence of alpha-ketoglutarate (AKG) on mineral density and geometrical and mechanical parameters of femora during postnatal life in piglets. Bull. Vet. Inst. Pulawy 2005, 49, 107–111. [Google Scholar]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Soltan, M.A. Influence of dietary glutamine supplementation on growth performance, small intestine morphology, immune response and some blood parameters of broiler chickens. Int. J. Poult. Sci. 2009, 8, 60–68. [Google Scholar] [CrossRef]
- Bartell, S.M.; Batal, A.B. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers. Poult. Sci. 2007, 86, 1940–1947. [Google Scholar] [CrossRef]
- Jazideh, F.; Farhoomand, P.; Daneshyar, M.; Najafi, G. The effects of dietary glutamine supplementation on growth performance and intestinal morphology of broiler chickens reared under hot conditions. Turk. J. Vet. Anim. Sci. 2014, 38, 264–270. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Wu, X.; Yin, Y.; Wu, G. Dietary alpha-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids 2010, 39, 555–564. [Google Scholar] [CrossRef]
- Puchała, M.; Krawczyk, J.; Calik, J. Influence of origin of laying hens on the quality of their carcasses and meat after the first laying period. Ann. Anim. Sci. 2014, 14, 685–696. [Google Scholar] [CrossRef]
- Smulikowska, S.; Rutkowski, A. Recommended Allowances and Nutritive Value of Feedstuffs. In Poultry Feeding Standards, 5th ed.; The Kielanowski Institute of Animal Physiology and Nutrition PAS: Jabłonna, Poland, 2018; pp. 66–74. (In Polish) [Google Scholar]
- Crisiane Zavarize, K.; Roberto Sartori, J.; Celso Pezzato, A.; Antonio Garcia, A.; Cação Cruz, V. Glutamine in diet of laying hens submitted to heat stress and thermoneutrality. Ci. Anim. Bras. 2011, 12, 400–406. [Google Scholar]
- Janssen, W.M.M.A. European Table of Energy Values for Poultry Feedstuffs, 3rd ed.; Beekbergen: Wageningen, The Netherlands, 1989; p. 24. [Google Scholar]
- Suvarna, S.K.; Layton, C.D.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kisielinski, K.; Willis, S.; Prescher, A.; Klosterhalfen, B.; Schumpelick, V. A simple new method to calculate small intestine absorptive surface in the rat. Clin. Exp. Med. 2002, 2, 131–135. [Google Scholar] [CrossRef]
- Górska, M.; Wojtysiak, D. Comparison of plasma corticosterone concentration, muscle fibre diameter, and apoptotic markers between normal and pale, soft, exudative (PSE) turkey breast muscles. Med. Wet. 2018, 74, 387–391. [Google Scholar] [CrossRef]
- CIE. Recommendations on Uniform Color Spaces-Color Difference Equations, Psychometric Color Terms; Supplement No. 2 to CIE Publication No. 15 (E-1.3.1.); Bureau Central de la Commission Internationale de l’Eclairage: Paris, France, 1978. [Google Scholar]
- Howe, J.L.; Gullett, E.A.; Usborne, W.R. Development of pink color in cooked pork. Can. Inst. Food Sci. Technol. 1982, 15, 19–23. [Google Scholar] [CrossRef]
- Sosnówka-Czajka, E.; Skomorucha, I.; Muchacka, R. Effect of organic production system on the performance and meat quality of two purebred slow-growing chicken breeds. Ann. Anim. Sci. 2017, 17, 1197–1213. [Google Scholar] [CrossRef]
- Domaradzki, P.; Florek, M.; Skałecki, P.; Litwińczuk, A.; Kędzierska-Matysek, M.; Wolanciuk, A.; Tajchman, K. Fatty acid composition, cholesterol content and lipid oxidation indices ofintramuscular fat from skeletal muscles of beaver (Castor fiber L.). Meat Sci. 2019, 150, 131–140. [Google Scholar] [CrossRef]
- Rudel, L.; Morris, M. Determination of cholesterol using o-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar]
- Muszyński, S.; Kwiecień, M.; Tomaszewska, E.; Świetlicka, I.; Dobrowolski, P.; Kasperek, K.; Jeżewska-Witkowska, G. Effect of caponization on performance and quality characteristics of long bones in Polbar chickens. Poult. Sci. 2017, 96, 491–500. [Google Scholar] [CrossRef]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 18, 403–419. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirus staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Blicharski, T.; Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Muszyński, S. A metabolite of leucine (β-hydroxy-β-methylbutyrate) given to sows during pregnancy alters bone development of their newborn offspring by hormonal modulation. PLoS ONE 2017, 12, e0179693. [Google Scholar] [CrossRef]
- Festing, M.F.W. On determining sample size in experiments involving laboratory animals. Lab. Anim. 2018, 52, 341–350. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. Bones quality indices in laying hens fed diets with a high level of DDGS and supplemented with selected feed additives. Czech J. Anim. Sci. 2014, 59, 61–68. [Google Scholar] [CrossRef]
- Wang, J.J.; Pan, T.M. Effect of red mold rice supplements on serum and egg yolk cholesterol levels of laying hens. J. Agric. Food Chem. 2003, 51, 4824–4829. [Google Scholar] [CrossRef]
- Popescu, R.G.; Voicu, S.N.; Piracalabioru, G.G.; Ciceu, A.; Gharbia, S.; Hermenean, A.; Gogergescu, S.E.; Panaite, T.D.; Dinischotu, A. Effects of dietary inclusion of bilberry and walnut leaves powder on the digestive performances and health of Tetra SL laying hens. Animals 2020, 10, 823. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Murakami, A.E.; Sakamoto, M.I.; Natali, M.R.M.; Souza, L.M.G.; Franco, J.R.G. Supplementation of glutamine and vitamin E on the morphometry of the intestinal mucosa in broiler chickens. Poult. Sci. 2007, 86, 488–495. [Google Scholar] [CrossRef]
- Sakamoto, M.I.; Murakami, A.E.; Silveira, T.G.V.; Fernandes, J.I.M.; De Oliveira, C.A.L. Influence of glutamine and vitamin E on the performance and the immune responses of broiler chickens. Braz. J. Poult. Sci. 2006, 8, 243–249. [Google Scholar] [CrossRef]
- Yi, G.F.; Allee, G.L.; Knight, C.D.; Dibnert, J.J. Impact of glutamine and oasis hatchling supplement on growth performance, small intestinal morphology, and immune response of broilers vaccinated and challenged with Eimeria maxima. Poult. Sci. 2005, 84, 283–293. [Google Scholar] [CrossRef]
- Bartell, S.M. The Effect of Supplemental Glutamine on Growth Performance, Development of the Gastrointestinal Tract, and Immune Response of Broiler Chicks. Master’s Thesis, University of Georgia, Athens, GA, USA, 2006. Available online: https://getd.libs.uga.edu/pdfs/bartell_shoshana_m_200605_ms.pdf (accessed on 15 December 2020).
- Tomaszewska, E.; Dobrowolski, P.; Wydrych, J. Postnatal administration of 2-oxoglutaric acid improves articular and growth plate cartilages and bone tissue morphology in pigs prenatally treated with dexamethasone. J. Physiol. Pharmacol. 2012, 63, 547–554. [Google Scholar]
- Tomaszewska, E.; Dobrowolski, P.; Bieńko, M.; Prost, Ł.; Szymańczyk, S.; Zdybel, A. Effects of 2-oxoglutaric acid on bone morphometry, densitometry, mechanics, and immunohistochemistry in 9-month-old boars with prenatal dexamethasone-induced osteopenia. Connect. Tissue Res. 2015, 56, 483–492. [Google Scholar] [CrossRef]
- Brun, A.; Fernández Marinone, G.; Price, E.R.; Nell, L.A.; Simões, B.M.V.; Castellar, A.; Gontero-Fourcade, M.; Cruz-Neto, A.P.; Karasov, W.H.; Caviedes-Vidal, E. Morphological bases for intestinal paracellular absorption in bats and rodents. J. Morphol. 2019, 280, 1359–1369. [Google Scholar] [CrossRef]
- Wilson, F.D.; Cummings, T.S.; Barbosa, T.M.; Williams, C.J.; Gerard, P.D.; Peebles, E.D. Comparison of two methods for determination of intestinal villus to crypt ratios and documentation of early age-associated ratio changes in broiler chickens. Poult. Sci. 2018, 97, 1757–1761. [Google Scholar] [CrossRef]
- Luquetti, B.C.; Alarcon, M.F.F.; Lunedo, R.; Campos, D.M.B.; Furlan, R.L.; Macari, M. Effects of glutamine on performance and intestinal mucosa morphometry of broiler chickens vaccinated against coccidiosis. Sci. Agric. 2016, 73, 322–327. [Google Scholar] [CrossRef]
- Domeneghini, C.; Di Giancamillo, A.; Bosi, G.; Arrighi, S. Can nutraceuticals affect the structure of intestinal mucosa? Qualitative and quantitative microanatomy in L-glutamine diet-supplemented weaning piglets. Vet. Res. Commun. 2006, 30, 331–342. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Tseng, B.J.; Tseng, S.H. Influence of growth hormone and glutamine on intestinal stem cells: A narrative review. Nutrients 2019, 11, 1941. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I. Postnatal administration of 2-oxoglutaric acid improves the intestinal barrier affected by the prenatal action of dexamethasone in pigs. Nutrition 2012, 28, 190–196. [Google Scholar] [CrossRef]
- Konturek, S. Physiology of the Gastrointestinal Tract, 2nd ed.; PZWL: Warsaw, Poland, 1985; p. 515. [Google Scholar]
- Śliwa, E. Effect of simultaneous versus apart administration of dexamethasone and alpha-ketoglutarate on growth hormone, cortisol and insulin-like growth factor-I in piglets. Bull. Vet. Inst. Pulawy 2006, 50, 205–210. [Google Scholar]
- Scanes, C.G.; Harvey, S. Hormones and growth in poultry. Poult. Sci. 1984, 63, 2062–2074. [Google Scholar] [CrossRef]
- Harvey, S.; Scanes, C.G.; Sharp, P.J. Variations in plasma growth hormone concentrations in laying hens. Br. Poult. Sci. 1979, 20, 163–166. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Wang, X.; Jiao, H.; Lin, H. Peripheral insulin doesn’t alter appetite of broiler chicks. Asian-Australas. J. Anim. Sci. 2016, 29, 1294–1299. [Google Scholar] [CrossRef]
- Shiraishi, J.; Yanagita, K.; Fukumori, R.; Sugino, T.; Fujita, M.; Kawakami, S.; McMurtry, J.P.; Bungo, T. Comparisons of insulin related parameters in commercial-type chicks: Evidence for insulin resistance in broiler chicks. Physiol. Behav. 2011, 103, 233–239. [Google Scholar] [CrossRef]
- Stout, R.W.; Buchanan, K.D.; Vallance-Owen, J. The relationship of arterial disease and glucagon metabolism in insulin-treated chickens. Atherosclerosis 1973, 18, 153–162. [Google Scholar] [CrossRef]
- Pal, L.; Grossmann, R.; Dublecz, K.; Husveth, F.; Wagner, L.; Bartos, A.; Kovacs, G.J. Effects of glucagon and insulin on plasma glucose, triglyceride, and triglyceride-rich lipoprotein concentrations in laying hens fed diets containing different types of fats. Poult. Sci. 2002, 81, 1694–1702. [Google Scholar] [CrossRef]
- Dupont, S.; Tesseraud, J.S. Insulin signaling in chicken liver and muscle. Gen. Comp. Endocrinol. 2009, 163, 52–57. [Google Scholar] [CrossRef]
- Obrzut, J.; Krawczyk, J.; Calik, J.; Świątkiewicz, S.; Pietras, M.; Utnik-Banaś, K. Meat quality of poulards obtained from three conserved breeds of hens. Ann. Anim. Sci. 2018, 18, 261–280. [Google Scholar] [CrossRef]
- Puchała, M.; Krawczyk, J.; Sokołowicz, Z.; Utnik-Banaś, K. Effect of breed and production system on physicochemical characteristics of meat from multi-purpose hens. Ann. Anim. Sci. 2015, 15, 247–261. [Google Scholar] [CrossRef]
- Śliwa, E.; Tatara, M.R.; Pierzynowski, S.G. Total cholesterol, glucose and electrolytes in piglets’ serum after alpha-ketoglutarate (AKG) and dexamethasone treatment during prenatal and neonatal life. Bull. Vet. Inst. Pulawy 2006, 50, 561–566. [Google Scholar]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Szczurek, W.; Calik, J.; Bederska-Łojewska, D.; Orczewska-Dudek, S.; Muszyński, S.; Tomaszewska, E.; Józefiak, D. Algal oil as source of polyunsaturated fatty acids in laying hens nutrition: Effect on egg performance, egg quality indices and fatty acid composition of egg yolk lipids. Ann. Anim. Sci. 2020, 20, 961–973. [Google Scholar] [CrossRef]
- Węsierska, E.; Niemczyńska, K.; Pasternak, M.; Arczewska-Włosek, A. Selected physical and chemical characteristics of eggs laid by hens fed diets with different levels of hybrid rye. Ann. Anim. Sci. 2019, 19, 1009–1020. [Google Scholar] [CrossRef]
- Gumułka, M.; Wojtysiak, D.; Kapkowska, E.; Połtowicz, K.; Rabsztyn, A. Microstructure and technological meat quality of geese from conservation flock and commercial hybrids. Ann. Anim. Sci. 2009, 9, 205–213. [Google Scholar]
- Sirotkin, A.V.; Harrath, A.H.; Grossmann, R. Metabolic status and ghrelin regulate plasma levels and release of ovarian hormones in layer chicks. Physiol. Res. 2016, 66, 85–92. [Google Scholar] [CrossRef]
- Richards, M.P.; McMurtry, J.P. The avian proghrelin system. Int. J. Pept. 2010, 2010, 749401. [Google Scholar] [CrossRef]
- Friedman-Einat, M.; Seroussi, E. Avian leptin: Bird’s-eye view of the evolution of vertebrate energy-balance control. Trends Endocrinol. Metab. 2019, 30, 819–832. [Google Scholar] [CrossRef]
- Löhmus, M.; Sundström, F.L.; Silverin, B. Chronic administration of leptin in Asian Blue Quail. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 13–22. [Google Scholar] [CrossRef]
- Jiang, S.; Cui, L.; Shi, C.; Ke, X.; Luo, J.; Hou, J. Effects of dietary energy and calcium levels on performance, egg shell quality and bone metabolism in hens. Vet. J. 2013, 198, 252–258. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteoblast physiology in normal and pathological condition. Cell Tissue Res. 2011, 342, 289–302. [Google Scholar] [CrossRef]
- Fleming, R.H. Nutritional factors affecting poultry bone health. Proc. Nutr. Soc. 2008, 67, 177–183. [Google Scholar] [CrossRef]
- Johnsson, M.; Jonsson, K.B.; Andersson, L.; Jensen, P.; Wright, D. Genetic regulation of bone metabolism in the chicken: Similarities and differences to mammalian systems. PLoS Genet. 2015, 11, e1005250. [Google Scholar] [CrossRef]
- Scanes, C.G. (Ed.) Sturkie’s Avian Physiology, 6th ed.; Academic Press: San Diego, CA, USA, 2015; p. 1056. [Google Scholar]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Szczurek, W.; Calik, J.; Krawczyk, J.; Józefiak, D. The influence of selected feed additives on mineral utilisation and bone characteristics in laying hens. Ann. Anim. Sci. 2018, 18, 781–793. [Google Scholar] [CrossRef]
- Hiyama, S.; Yokoi, M.; Akagi, Y.; Kadoyama, Y.; Nakamori, K.; Tsuga, K.; Uchida, T.; Terayama, R. Osteoclastogenesis from bone marrow cells during estrogen-induced medullary bone formation in Japanese quails. J. Mol. Histol. 2019, 50, 389–404. [Google Scholar] [CrossRef]
- Chen, Q.; Bao, N.; Yao, Q.; Li, Z.Y. Fractal dimension: A complementary diagnostic indicator of osteoporosis to bone mineral density. Med. Hypotheses 2018, 116, 136–138. [Google Scholar] [CrossRef]

| Ingredients | Content |
|---|---|
| Ingredient (g/kg) | |
| Corn | 422.10 |
| Wheat | 210.00 |
| Soybean meal | 236.00 |
| Rapeseed oil | 20.00 |
| Limestone | 90.00 |
| Monocalcium phosphate | 12.50 |
| NaCl | 3.00 |
| DL-Methionine | 1.40 |
| Vitamin-mineral premix 1 | 5.00 |
| Nutrients composition | |
| Metabolizable energy, MJ/kg 2 | 11.60 |
| Crude protein | 170.00 |
| Lys | 8.35 |
| Met | 4.10 |
| Ca | 37.00 |
| Total P | 6.15 |
| Available P | 3.90 |
| Parameter | CONT | AKG | p-Value |
|---|---|---|---|
| Initial body weight, kg | 1.80 ± 0.03 | 1.88 ± 0.04 | 0.073 |
| Final body weight, kg | 1.95 ± 0.03 | 2.05 ± 0.04 | 0.065 |
| Daily feed intake, g | 118.7 ± 1.2 | 116.6 ± 1.1 | 0.226 |
| Laying rate, % | 95.8 ± 0.45 | 96.6 ± 0.377 | 0.209 |
| Parameter | CONT | AKG | p-Value |
|---|---|---|---|
| IGF-I, ng/mL | 6.43 ± 0.33 | 7.20 ± 0.19 | 0.061 |
| OPG, pg/mL | 801 ± 35 | 696 ± 25 | 0.022 |
| GH, ng/mL | 1.99 ± 0.13 | 2.34 ± 0.19 | 0.126 |
| Insulin, uIL/mL | 8.70 ± 1.12 | 9.76 ± 0.67 | 0.426 |
| BALP, ng/mL | 1.61 ± 0.07 | 4.65 ± 0.31 | <0.001 |
| Ghrelin, pg/mL | 136 ± 8 | 184 ± 4 | <0.001 |
| IL-1β, pg/mL | 12.7 ± 1.4 | 13.9 ± 1.2 | 0.530 |
| Leptin, pg/mL | 41.0 ± 1.7 | 49.5 ± 3.4 | 0.041 |
| OC, ng/mL | 1.59 ± 0.09 | 1.25 ± 0.07 | 0.007 |
| RANKL, ng/mL | 0.541 ± 0.063 | 0.927 ± 0.104 | 0.004 |
| TNF-α, ng/mL | 19.9 ± 2.1 | 21.7 ± 1.6 | 0.489 |
| VitD, pg/mL | 312 ± 16 | 276 ± 22 | 0.210 |
| Cholesterol, mmol/L | 3.57 ± 0.08 | 3.28 ± 0.06 | 0.009 |
| Parameter | CONT | AKG | p-Value |
|---|---|---|---|
| Absolute breast muscle weight, kg | 0.190 ± 0.005 | 0.196 ± 0.004 | 0.421 |
| Breast muscle weight of BW, % | 9.76 ± 0.26 | 9.64 ± 0.16 | 0.690 |
| pH15min | 6.21 ± 0.04 | 6.20 ± 0.04 | 0.846 |
| pH24h | 5.74 ± 0.01 | 5.72 ± 0.01 | 0.153 |
| L* (Lightness) | 34.47 ± 0.27 | 36.49 ± 0.96 | 0.069 |
| a* (Redness) | 1.06 ± 0.05 | 0.92 ± 0.05 | 0.104 |
| b* (Yellowness) | 1.89 ± 0.15 | 2.31 ± 0.17 | 0.084 |
| Chroma | 2.19 ± 0.13 | 2.49 ± 0.18 | 0.194 |
| Hue angle | 30.29 ± 2.29 | 22.28 ± 1.09 | 0.012 |
| Drip loss24h, % | 0.224 ± 0.022 | 0.301 ± 0.015 | 0.009 |
| Fibre diameter, μm | 50.27 ± 1.39 | 49.59 ± 0.65 | 0.977 |
| Shear force, N | 23.62 ± 1.33 | 30.10 ± 0.91 | <0.001 |
| Cholesterol content, mg/100 g of meat | 41.31 ± 3.08 | 29.04 ± 2.54 | 0.006 |
| Fatty Acid | CONT | AKG | p-Value |
|---|---|---|---|
| C12:0 | 0.095 ± 0.007 | 0.076 ± 0.011 | 0.177 |
| C14:0 | 0.769 ± 0.021 | 0.735 ± 0.028 | 0.345 |
| C16:0 | 23.5 ± 0.2 | 23.9 ± 0.3 | 0.398 |
| C18:0 | 8.79 ± 0.21 | 9.50 ± 0.33 | 0.090 |
| C20:0 | 0.100 ± 0.007 | 0.131 ± 0.013 | 0.112 |
| C22:0 | 0.149 ± 0.038 | 0.110 ± 0.012 | 0.342 |
| C24:0 | 0.118 ± 0.008 | 0.143 ± 0.011 | 0.098 |
| ΣSFA | 33.5 ± 0.4 | 34.6 ± 0.5 | 0.118 |
| C15:0 | 0.136 ± 0.006 | 0.129 ± 0.003 | 0.381 |
| C17:0 | 0.306 ± 0.021 | 0.271 ± 0.014 | 0.175 |
| Σ OCFA | 0.442 ± 0.025 | 0.389 ± 0.020 | 0.114 |
| ΣBCFA | 0.098 ± 0.011 | 0.105 ± 0.010 | 0.645 |
| C14:1c9 | 0.065 ± 0.006 | 0.066 ± 0.009 | 0.926 |
| Σ C15:1 | 4.78 ± 0.22 | 5.12 ± 0.45 | 0.505 |
| C16:1c7 | 0.821 ± 0.024 | 0.813 ± 0.033 | 0.842 |
| C16:1c9 | 1.76 ± 0.10 | 1.72 ± 0.09 | 0.768 |
| C17:1 c9 | 1.10 ± 0.06 | 1.17 ± 0.09 | 0.490 |
| C18:1c9 | 27.8 ± 0.7 | 27.4 ± 1.0 | 0.751 |
| C18:1c11 | 2.44 ± 0.06 | 2.57 ± 0.06 | 0.132 |
| C20:1c11 | 0.269 ± 0.057 | 0.192 ± 0.010 | 0.194 |
| Σ MUFA cis | 39.0 ± 0.6 | 39.1 ± 0.8 | 0.960 |
| C18:2n-6 LA | 15.1 ± 0.4 | 14.4 ± 0.4 | 0.248 |
| C18:3n-3 ALA | 1.08 ± 0.06 | 0.94 ± 0.7 | 0.181 |
| C18:3n-6 GLA | 0.152 ± 0.013 | 0.133 ± 0.011 | 0.284 |
| C20:2n-6 | 0.151 ± 0.006 | 0.150 ± 0.012 | 0.941 |
| C20:2n9 | 0.102 ± 0.011 | 0.108 ± 0.011 | 0.717 |
| C20:3n6 | 0.413 ± 0.037 | 0.434 ± 0.030 | 0.622 |
| C20:4n-6 AA | 6.59 ± 0.41 | 6.47 ± 0.49 | 0.858 |
| C20:5n3 EPA | 0.085 ± 0.003 | 0.078 ± 0.008 | 0.707 |
| C22:4n6 | 0.675 ± 0.034 | 0.749 ± 0.061 | 0.307 |
| C22:5n6 | 0.224 ± 0.019 | 0.270 ± 0.028 | 0.188 |
| C24:1n9 | 0.118 ± 0.007 | 0.131 ± 0.011 | 0.357 |
| C22:5n-3 DPA | 0.522 ± 0.031 | 0.503 ± 0.037 | 0.699 |
| C22:6n3 DHA | 1.59 ± 0.11 | 1.48 ± 0.15 | 0.548 |
| Σ PUFA | 26.8 ± 0.6 | 25.8 ± 0.4 | 0.209 |
| Σ n-3 | 3.35 ± 0.14 | 2.97 ± 0.15 | 0.287 |
| Σ n-6 | 23.2 ± 0.5 | 22.6 ± 0.3 | 0.337 |
| Σ LC-PUFA | 10.2 ± 0.6 | 10.1 ± 0.8 | 0.946 |
| Σ LC n-6 | 8.00 ± 0.47 | 8.08 ± 0.61 | 0.918 |
| Σ LC n-3 | 2.17 ± 0.14 | 2.02 ± 0.18 | 0.533 |
| n-6/n-3 | 6.99 ± 0.29 | 7.78 ± 0.40 | 0.121 |
| PUFA/SFA | 0.799 ± 0.021 | 0.747 ± 0.013 | 0.053 |
| Parameter | CONT | AKG | p-Value |
|---|---|---|---|
| Bone weight, g | 11.87 ± 0.11 | 11.68 ± 0.25 | 0.525 |
| Tibia bone weight of BW, % | 0.610 ± 0.010 | 0.576 ± 0.016 | 0.091 |
| Bone length, mm | 121 ± 1 | 122 ± 1 | 0.547 |
| Seedor index, mg/mm | 98.1 ± 1.3 | 95.7 ± 1.7 | 0.341 |
| MRWT | 0.350 ± 0.026 | 0.363 ± 0.031 | 0.738 |
| Cortical index, % | 25.5 ± 1.4 | 26.0 ± 1.6 | 0.805 |
| CSA, mm2 | 18.2 ± 0.8 | 19.0 ± 1.2 | 0.586 |
| CSMI, mm4 | 78.8 ± 4.1 | 85.4 ± 6.6 | 0.707 |
| Yield load, N | 94 ± 4 | 98 ± 6 | 0.552 |
| Elastic work, mJ | 18.6 ± 1.5 | 19.5 ± 1.6 | 0.700 |
| Fracture load, N | 171 ± 6 | 176 ± 7 | 0.794 |
| Work to fracture, mJ | 121 ± 6 | 124 ± 7 | 0.696 |
| Stiffness, N/mm | 259 ± 9 | 270 ± 13 | 0.495 |
| Young’s modulus, GPa | 7.94 ± 0.38 | 7.54 ± 0.48 | 0.523 |
| Yield strain, % | 0.615 ± 0.021 | 0.650 ± 0.028 | 0.322 |
| Yield stress, MPa | 49.0 ± 3.4 | 48.8 ± 3.6 | 0.665 |
| Fracture strain, % | 1.92 ± 0.05 | 1.97 ± 0.07 | 0.610 |
| Fracture stress, MPa | 94.0 ± 6.3 | 87.7 ± 5.5 | 0.840 |
| BMD, g/cm2 | 0.245 ± 0.021 | 0.227 ± 0.026 | 0.611 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Świątkiewicz, S.; Arczewska-Włosek, A.; Wojtysiak, D.; Dobrowolski, P.; Domaradzki, P.; Świetlicka, I.; Donaldson, J.; Hułas-Stasiak, M.; Muszyński, S. Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study. Animals 2020, 10, 2420. https://doi.org/10.3390/ani10122420
Tomaszewska E, Świątkiewicz S, Arczewska-Włosek A, Wojtysiak D, Dobrowolski P, Domaradzki P, Świetlicka I, Donaldson J, Hułas-Stasiak M, Muszyński S. Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study. Animals. 2020; 10(12):2420. https://doi.org/10.3390/ani10122420
Chicago/Turabian StyleTomaszewska, Ewa, Sylwester Świątkiewicz, Anna Arczewska-Włosek, Dorota Wojtysiak, Piotr Dobrowolski, Piotr Domaradzki, Izabela Świetlicka, Janine Donaldson, Monika Hułas-Stasiak, and Siemowit Muszyński. 2020. "Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study" Animals 10, no. 12: 2420. https://doi.org/10.3390/ani10122420
APA StyleTomaszewska, E., Świątkiewicz, S., Arczewska-Włosek, A., Wojtysiak, D., Dobrowolski, P., Domaradzki, P., Świetlicka, I., Donaldson, J., Hułas-Stasiak, M., & Muszyński, S. (2020). Alpha-Ketoglutarate: An Effective Feed Supplement in Improving Bone Metabolism and Muscle Quality of Laying Hens: A Preliminary Study. Animals, 10(12), 2420. https://doi.org/10.3390/ani10122420






