Proteomic Analysis of Tear Film Obtained from Diabetic Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Protein Cleaning and Precipitation
2.3. MALDI Identification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nelson, R.; Reusch, C.E. Animal models of disease: Classification and etiology of diabetes in dogs and cats. J. Endocrinol. 2014, 222, T1–T9. [Google Scholar] [CrossRef]
- Miller, E.J.; Brines, C.M. Canine Diabetes Mellitus Associated Ocular Disease. Top. Companion Anim. Med. 2018, 33, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Ihle, S.L.; Webb, A.A.; McCarville, C. Keratoconjunctival effects of diabetes mellitus in dogs. Vet. Ophthalmol. 2005, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.L.; Auchincloss, J.H. Multiple Symmetric Bilateral Cranial Nerve Palsies in Patients with Unregulated Diabetes Mellitus. Arch. Intern. Med. 1950, 85, 265–271. [Google Scholar] [CrossRef]
- Muñana, K.R. Long-Term Complications of Diabetes Mellitus, Part I: Retinopathy, Nephropathy, Neuropathy. Vet. Clin. N. Am. Small Anim. Pract. 1995, 25, 715–730. [Google Scholar] [CrossRef]
- Basher, A.W.; Roberts, S.M. Ocular Manifestations of Diabetes Mellitus: Diabetic Cataracts in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 1995, 25, 661–676. [Google Scholar] [CrossRef]
- Good, K.L.; Maggs, D.J.; Hollingsworth, S.R.; Scagliotti, R.H.; Nelson, R.W. Corneal sensitivity in dogs with diabetes mellitus. Am. J. Vet. Res. 2003, 64, 7–11. [Google Scholar] [CrossRef]
- Goebbels, M. Tear secretion and tear film function in insulin dependent diabetics. Br. J. Ophthalmol. 2000, 84, 19–21. [Google Scholar] [CrossRef]
- Nepp, J.; Abela, C.; Polzer, I.; Derbolav, A.; Wedrich, A. Is There a Correlation Between the Severity of Diabetic Retinopathy and Keratoconjunctivitis Sicca? Cornea 2000, 19, 487–491. [Google Scholar] [CrossRef]
- Ozdemir, M.; Buyukbese, M.A.; Cetinkaya, A.; Ozdemir, G. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res. Clin. Pract. 2003, 59, 195–199. [Google Scholar] [CrossRef]
- Ananthi, S.; Santhosh, R.S.; Nila, M.V.; Prajna, N.V.; Lalitha, P.; Kuppamuthu, D. Comparative proteomics of human male and female tears by two-dimensional electrophoresis. Exp. Eye Res. 2011, 92, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.D.F.; Cole, N.; Van Dyk, D.; Walsh, B.J.; Diakos, P.; Almeida, D.; Torrecilhas, A.; Laus, J.L.; Willcox, M.D. Proteomic analysis of dog tears for potential cancer markers. Res. Vet. Sci. 2008, 85, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Winiarczyk, M.; Kaarniranta, K.; Winiarczyk, S.; Adaszek, Ł.; Winiarczyk, D.; Mackiewicz, J. Tear film proteome in age-related macular degeneration. Graefe Arch. Clin. Exp. Ophthalmol. 2018, 256, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Adaszek, Ł.; Banach, T.; Bartnicki, M.; Winiarczyk, D.; Łyp, P.; Winiarczyk, S. Application the mass spectrometry MALDI-TOF technique for detection of Babesia canis canis infection in dogs. Parasitol. Res. 2014, 113, 4293–4295. [Google Scholar] [CrossRef]
- Banach, T.; Adaszek, Ł.; Wyłupek, D.; Winiarczyk, M. Applicability of 2D gel electrophoresis and liquid chromatography in proteomic analysis of urine using mass spectrometry MALDI-TOF. Pol. J. Vet. Sci. 2013, 16, 587–592. [Google Scholar] [CrossRef]
- Winiarczyk, M.; Winiarczyk, D.; Banach, T.; Adaszek, L.; Madany, J.; Mackiewicz, J.; Pietras-Ożga, D.; Winiarczyk, S. Dog Tear Film Proteome In-Depth Analysis. PLoS ONE 2015, 10, e0144242. [Google Scholar] [CrossRef]
- Csősz, É.; Boross, P.; Csutak, A.; Berta, A.; Tóth, F.; Póliska, S.; Török, Z.; Tőzsér, J. Quantitative analysis of proteins in the tear fluid of patients with diabetic retinopathy. J. Proteom. 2012, 75, 2196–2204. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, P.-K.; Yoo, H.-S.; Kim, C.-W. Comparison of tear proteins between healthy and early diabetic retinopathy patients. Clin. Biochem. 2012, 45, 60–67. [Google Scholar] [CrossRef]
- Flieger, J.; Święch-Zubilewicz, A.; Sniegocki, T.; Dolar-Szczasny, J.; Flieger, J. Determination of Tryptophan and Its Major Metabolites in Fluid from the Anterior Chamber of the Eye in Diabetic Patients with Cataract by Liquid Chromotography Mass Spectrometry (LC-MS/MS). Molecules 2018, 23, 3012. [Google Scholar] [CrossRef]
- Li, K.; Chen, Z.; Duan, F.; Liang, J.; Wu, K. Quantification of tear proteins by SDS-PAGE with an internal standard protein: A new method with special reference to small volume tears. Graefe Arch. Clin. Exp. Ophthalmol. 2010, 248, 853–862. [Google Scholar] [CrossRef]
- Posa, A.; Bräuer, L.; Schicht, M.; Garreis, F.; Beileke, S.; Paulsen, F. Schirmer strip vs. capillary tube method: Non-invasive methods of obtaining proteins from tear fluid. Ann. Anat. Anat. Anz. 2013, 195, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ablamowicz, A.F.; Nichols, J.J. Concentrations of MUC16 and MUC5AC using three tear collection methods. Mol. Vis. 2017, 23, 529–537. [Google Scholar] [PubMed]
- Green-Church, K.B.; Nichols, K.K.; Kleinholz, N.M.; Zhang, L.; Nichols, J.J. Investigation of the human tear film proteome using multiple proteomic approaches. Mol. Vis. 2008, 14, 456–470. [Google Scholar] [PubMed]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, M.; Xie, L.; Liu, F.; Yan, G.; Wang, W.; Lin, A.; Zhao, F.; Chen, Y. Tear Proteomic Analysis of Patients With Type 2 Diabetes and Dry Eye Syndrome by Two-Dimensional Nano-Liquid Chromatography Coupled With Tandem Mass Spectrometry. Investig. Opthalmol. Vis. Sci. 2014, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Török, Z.; Peto, T.; Csősz, É.; Tukacs, E.; Molnár, Á.; Maros-Szabo, Z.; Berta, A.; Tőzsér, J.; Hajdu, A.; Nagy, V.; et al. Tear fluid proteomics multimarkers for diabetic retinopathy screening. BMC Ophthalmol. 2013, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Török, Z.; Peto, T.; Csősz, É.; Tukacs, E.; Molnar, A.M.; Berta, A.; Tőzsér, J.; Hajdu, A.; Nagy, V.; Domokos, B.; et al. Combined Methods for Diabetic Retinopathy Screening, Using Retina Photographs and Tear Fluid Proteomics Biomarkers. J. Diabetes Res. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- O’Kell, A.L.; Wasserfall, C.; Catchpole, B.; Davison, L.J.; Hess, R.S.; Kushner, J.A.; Atkinson, M.A. Comparative Pathogenesis of Autoimmune Diabetes in Humans, NOD Mice, and Canines: Has a Valuable Animal Model of Type 1 Diabetes Been Overlooked? Diabetes 2017, 66, 1443–1452. [Google Scholar] [CrossRef]
- O’Kell, A.L.; Wasserfall, C.H.; Henthorn, P.S.; Atkinson, M.A.; Hess, R.S. Evaluation for type 1 diabetes associated autoantibodies in diabetic and non-diabetic Australian terriers and Samoyeds. Canine Med. Genet. 2020, 7, 1–6. [Google Scholar] [CrossRef]
- Damiano, L.; Di Stefano, P.; Leal, M.P.C.; Barba, M.; Mainiero, F.; Cabodi, S.; Tordella, L.; Sapino, A.; Castellano, I.; Canel, M.; et al. p140Cap dual regulation of E-cadherin/EGFR cross-talk and Ras signalling in tumour cell scatter and proliferation. Oncogene 2010, 29, 3677–3690. [Google Scholar] [CrossRef]
- Di Stefano, P.; Damiano, L.; Cabodi, S.; Aramu, S.; Tordella, L.; Praduroux, A.; Piva, R.; Cavallo, F.; Forni, G.; Silengo, L.; et al. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007, 26, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Hou, D.; Liang, H.; Gong, F.; Wang, Y.; Yan, X.; Jiang, X.; Wang, C.; Zhang, J.; Zen, K.; et al. miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur. J. Cancer 2014, 50, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Spear, E.D.; Alford, R.F.; Babatz, T.D.; Wood, K.M.; Mossberg, O.W.; Odinammadu, K.; Shilagardi, K.; Gray, J.J.; Michaelis, S. A humanized yeast system to analyze cleavage of prelamin A by ZMPSTE24. Methods 2019, 157, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Degrouas, C.; Varlet, A.-A.; Dutour, A.; Galant, D.; Merono, F.; Bonello-Palot, N.; Bourgeois, P.; Lasbleiz, A.; Petitjean, C.; Ancel, P.; et al. Unraveling LMNA Mutations in Metabolic Syndrome: Cellular Phenotype and Clinical Pitfalls. Cells 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Columbaro, M.; Ortolani, M.; D’Apice, M.; Novelli, G.; Fini, M.; Marmiroli, S.; Scarano, E.; Maraldi, N.; et al. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur. J. Histochem. 2011, 55, e36. [Google Scholar] [CrossRef] [PubMed]
- Sieprath, T.; Corne, T.D.J.; Nooteboom, M.; Grootaert, C.; Rajkovic, A.; Buysschaert, B.; Robijns, J.; Broers, J.L.V.; Ramaekers, F.C.S.; Koopman, W.J.H.; et al. Sustained accumulation of prelamin A and depletion of lamin A/C both cause oxidative stress and mitochondrial dysfunction but induce different cell fates. Nucleus 2015, 6, 236–246. [Google Scholar] [CrossRef]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef]
- Anderson, R.G.W. The Caveolae Membrane System. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [CrossRef]
- Pelkmans, L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 295–304. [Google Scholar] [CrossRef]
- ChengWei, Y.; Wu, Q.; Hu, K.; Yang, C.; Wu, X.; Cheung, P.; Williams, K.J. Suppression of Hepatic FLOT1 (Flotillin-1) by Type 2 Diabetes Mellitus Impairs the Disposal of Remnant Lipoproteins via Syndecan-1. Arter. Thromb. Vasc. Biol. 2018, 38, 102–113. [Google Scholar] [CrossRef]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Barson, J.R.; Morganstern, I.; Leibowitz, S.F. Complementary Roles of Orexin and Melanin-Concentrating Hormone in Feeding Behavior. Int. J. Endocrinol. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hervieu, G. Melanin-concentrating hormone functions in the nervous system: Food intake and stress. Expert Opin. Ther. Targets 2003, 7, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.P.; Bittencourt, J.C.; Adamantidis, A.R. Melanin-concentrating hormone and sleep. Curr. Opin. Neurobiol. 2017, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Sanob, H.; Iwaasab, H.; Pana, J.; Sailer, A.W.; Hreniuk, D.L.; Feighner, S.D.; Palyha, O.C.; Ponga, S.-S.; Figueroa, D.J.; et al. Melanin-Concentrating Hormone Receptor Subtypes 1 and 2: Species-Specific Gene Expression. Genomics 2002, 79, 785–792. [Google Scholar] [CrossRef]
- Craige, B.; Salazar, G.; Faundez, V. Phosphatidylinositol-4-Kinase Type II Alpha Contains an AP-3–sorting Motif and a Kinase Domain That Are Both Required for Endosome Traffic. Mol. Biol. Cell 2008, 19, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wenk, M.R.; Pellegrini, L.; Onofri, F.; Benfenati, F.; De Camilli, P. Phosphatidylinositol 4-kinase type II is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 3995–4000. [Google Scholar] [CrossRef]
- Olsen, H.L.; Høy, M.; Zhang, W.; Bertorello, A.M.; Bokvist, K.; Capito, K.; Efanov, A.M.; Meister, B.; Thams, P.; Yang, S.-N.; et al. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5187–5192. [Google Scholar] [CrossRef]
- Lamia, K.A.; Peroni, O.D.; Kim, Y.-B.; Rameh, L.E.; Kahn, B.B.; Cantley, L.C. Increased Insulin Sensitivity and Reduced Adiposity in Phosphatidylinositol 5-Phosphate 4-Kinase β−/− Mice. Mol. Cell. Biol. 2004, 24, 5080–5087. [Google Scholar] [CrossRef]
- Jwa, M.; Chang, P. PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1α-mediated unfolded protein response. Nat. Cell Biol. 2012, 14, 1223–1230. [Google Scholar] [CrossRef]
- Corda, D. New Embo Member’s Review: Functional aspects of protein mono-ADP-ribosylation. EMBO J. 2003, 22, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Roles of poly(ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol. Res. 2005, 52, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Donadoni, M.L.; Gavezzotti, R.; Borella, F.; Di Giulio, A.M.; Gorio, A. Experimental diabetic neuropathy. Inhibition of protein mono-ADP-ribosylation prevents reduction of substance P axonal transport. J. Pharmacol. Exp. Ther. 1995, 274, 570–576. [Google Scholar] [CrossRef]
- Luke, M.R.; Kjer-Nielsen, L.; Brown, D.L.; Stow, J.L.; Gleeson, P.A. GRIP Domain-mediated Targeting of Two New Coiled-coil Proteins, GCC88 and GCC185, to Subcompartments of thetrans-Golgi Network. J. Biol. Chem. 2002, 278, 4216–4226. [Google Scholar] [CrossRef]
- Munro, S.; Nichols, B.J. The GRIP domain—A novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol. 1999, 9, 377–380. [Google Scholar] [CrossRef]
- Truebestein, L.; Leonard, T.A. Coiled-coils: The long and short of it. BioEssays 2016, 38, 903–916. [Google Scholar] [CrossRef]
- Wong, M.; Gillingham, A.K.; Munro, S. The golgin coiled-coil proteins capture different types of transport carriers via distinct N-terminal motifs. BMC Biol. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Blatch, G.L.; Lässle, M. The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. BioEssays 1999, 21, 932–939. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Q.; Chen, J.; Liu, Y.; Mao, Z.; Lyu, Z.; Ni, D.; Long, Y.; Ju, P.; Liu, J.; et al. The expression patterns of Tetratricopeptide repeat domain 36 (Ttc36). Gene Expr. Patterns 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Gatto, M.; Iaccarino, L.; Ghirardello, A.; Bassi, N.; Pontisso, P.; Punzi, L.; Shoenfeld, Y.; Doria, A. Serpins, Immunity and Autoimmunity: Old Molecules, New Functions. Clin. Rev. Allergy Immunol. 2013, 45, 267–280. [Google Scholar] [CrossRef]
- Fadini, G.P.; Albiero, M.; Millioni, R.; Poncina, N.; Rigato, M.; Scotton, R.; Boscari, F.; Brocco, E.; Arrigoni, G.; Villano, G.; et al. The molecular signature of impaired diabetic wound healing identifies serpinB3 as a healing biomarker. Diabetologia 2014, 57, 1947–1956. [Google Scholar] [CrossRef] [PubMed]

| Variable | Healthy Subject n = 13 | Patients with Diabetes n = 15 |
|---|---|---|
| Age, Y | ||
| Mean (±SD) Range | 10.8 ± 1.8 8–13 | 10.3 ± 2.8 7–16 |
| Sex | ||
| Male Female | 7 6 | 7 8 |
| Weight, kg | ||
| Mean (±SD) Range | 23.1 ± 7.7 10–37 | 13.9 ± 8.6 5.4–38 |
| Duration of diabetes, y | ||
| Mean (±SD) Duration | 0 0 | 1.2 ± 0.65 0.5–2 |
| Blood glucose, mmol/L | ||
| Mean (±SD) Range | 5.9 ± 0.2 5.6–6.2 | 17.8 ± 6 8.3–29.7 |
| Retinopathy | 0 | 5 |
| Cataract | 0 | 9 |
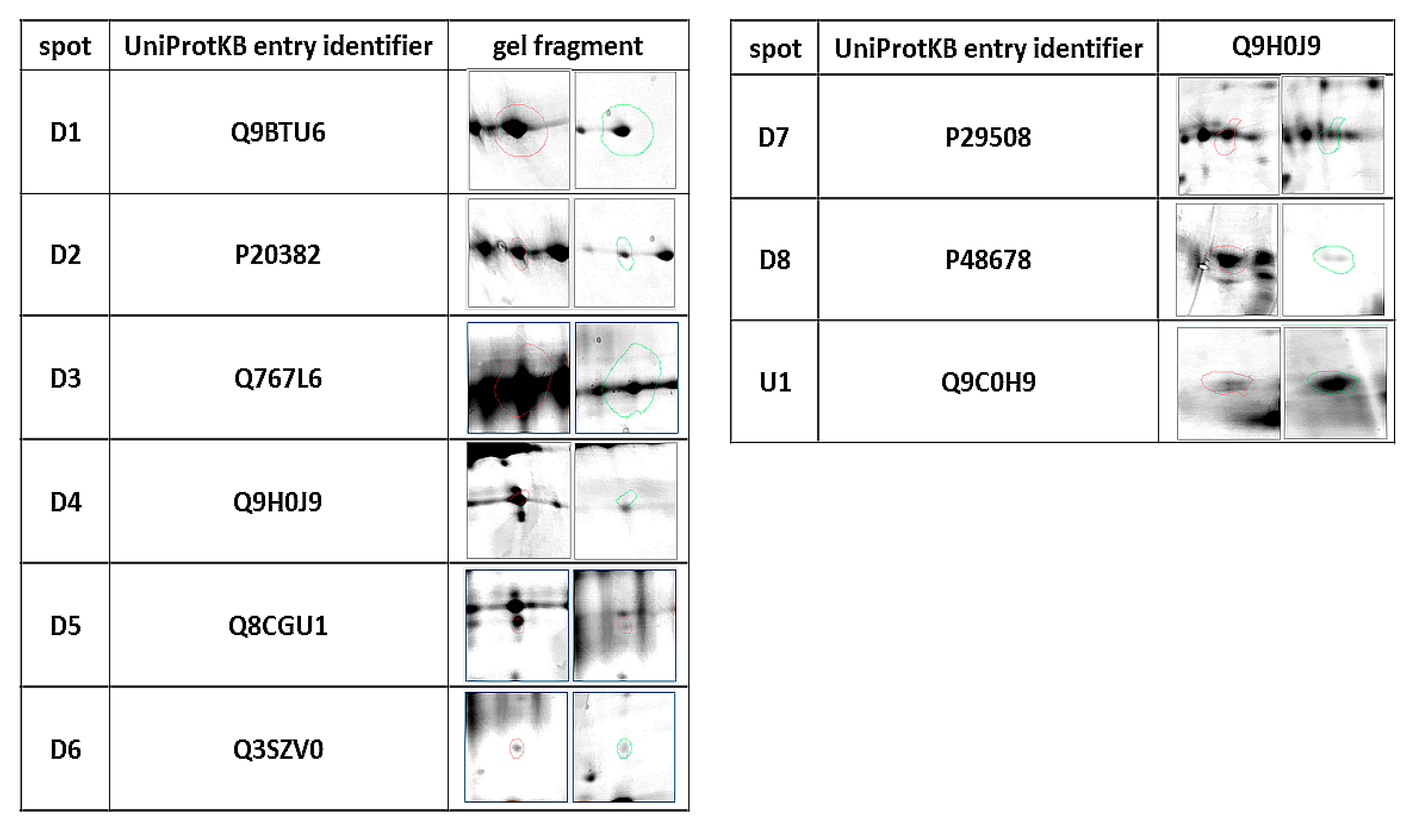
| ID | Protein | Accession Number (UniProtKB) | Species | Score | Match | MW (kDa) * | pI * | Modif. | Seq. Cov (%) | Rt ** | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | Phosphatidylinositol 4-kinase type 2-alpha | Q9BTU6 | H. sapiens | 74 | 11 | 54.388 | 8.51 | C, O | 26 | 0.437 | 0.032 |
| D2 | Pro-MCH | P20382 | H. sapiens | 75 | 6 | 18.781 | 6.74 | C, O | 38 | 0.350 | 0.04 |
| D3 | Flotillin-1 | Q767L6 | Sus scrofa | 65 | 10 | 47.558 | 7.66 | C, A, O | 24 | 0.491 | 0.03 |
| D4 | Protein mono-ADP ribosyltransferase | Q9H0J9 | H. sapiens | 66 | 11 | 80.496 | 8.84 | C, A, O | 23 | 0.403 | 0.03 |
| D5 | GRIP and coiled-coil domain-containing protein 2 | Q8CGU1 | M. musculus | 90 | 21 | 195.294 | 5.07 | C, O | 14 | 0.252 | 0.03 |
| D6 | Tetratricopeptide repeat protein 36 | Q3SZV0 | B. taurus | 81 | 11 | 20.660 | 5.09 | C, O | 40 | 0.232 | 0.02 |
| D7 | Serpin B3 | P29508 | H. sapiens | 71 | 14 | 44.594 | 6.35 | C | 38 | 0.530 | 0.04 |
| D8 | Prelamin A/C, | P48678 | M. musculus | 72 | 18 | 74.478 | 6.54 | C, O | 31 | 0.170 | 0.001 |
| U1 | SRC kinase signaling inhibitor 1 | Q9C0H9 | H. Sapiens | 66 | 15 | 127.198 | 9.39 | C, O | 15% | 2.289 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarczyk, D.; Winiarczyk, M.; Winiarczyk, S.; Michalak, K.; Adaszek, Ł. Proteomic Analysis of Tear Film Obtained from Diabetic Dogs. Animals 2020, 10, 2416. https://doi.org/10.3390/ani10122416
Winiarczyk D, Winiarczyk M, Winiarczyk S, Michalak K, Adaszek Ł. Proteomic Analysis of Tear Film Obtained from Diabetic Dogs. Animals. 2020; 10(12):2416. https://doi.org/10.3390/ani10122416
Chicago/Turabian StyleWiniarczyk, Dagmara, Mateusz Winiarczyk, Stanisław Winiarczyk, Katarzyna Michalak, and Łukasz Adaszek. 2020. "Proteomic Analysis of Tear Film Obtained from Diabetic Dogs" Animals 10, no. 12: 2416. https://doi.org/10.3390/ani10122416
APA StyleWiniarczyk, D., Winiarczyk, M., Winiarczyk, S., Michalak, K., & Adaszek, Ł. (2020). Proteomic Analysis of Tear Film Obtained from Diabetic Dogs. Animals, 10(12), 2416. https://doi.org/10.3390/ani10122416






