Genera and Species of the Anisakidae Family and Their Geographical Distribution
Abstract
Simple Summary
Abstract
1. Introduction
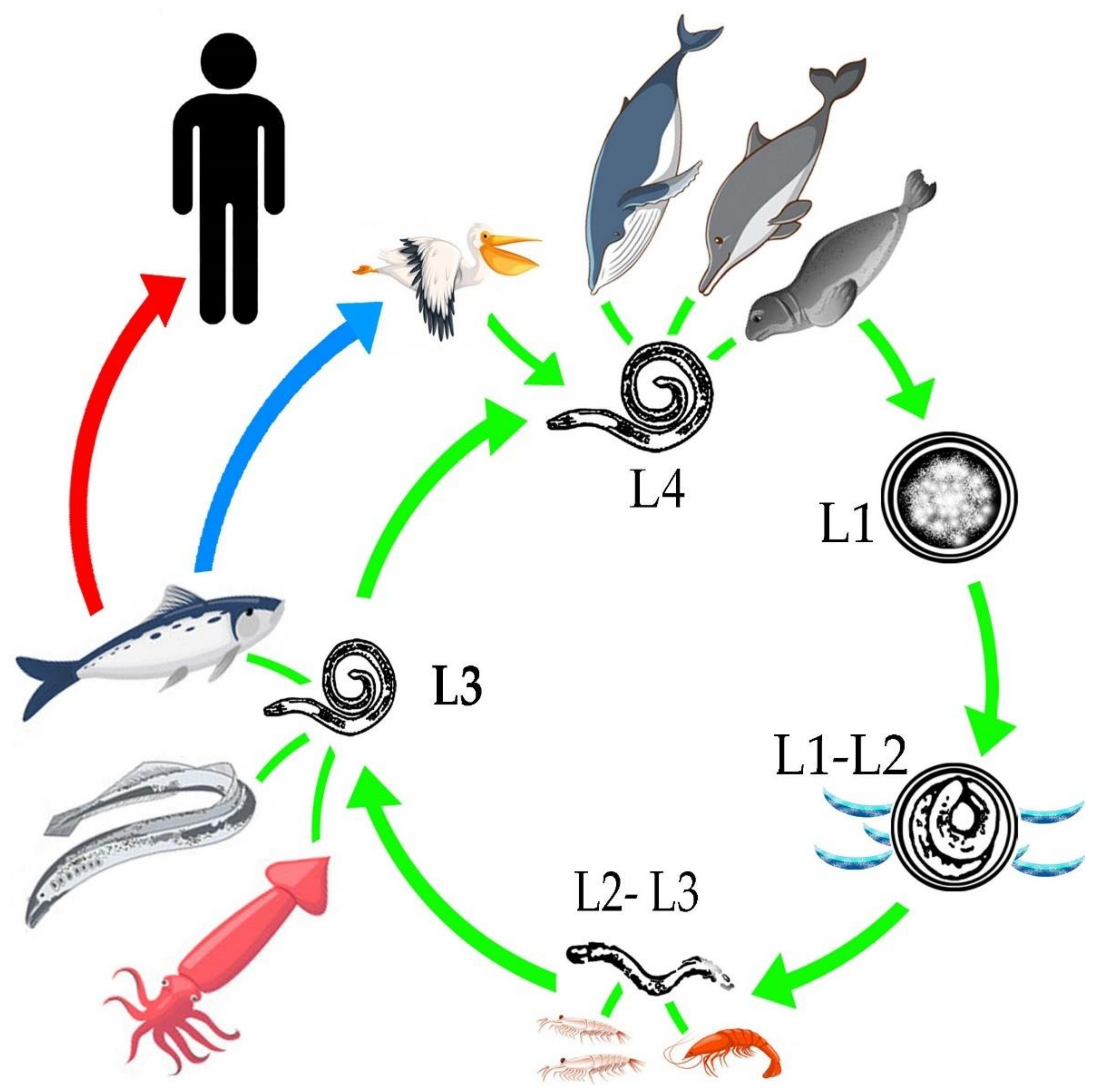
2. Life Cycle
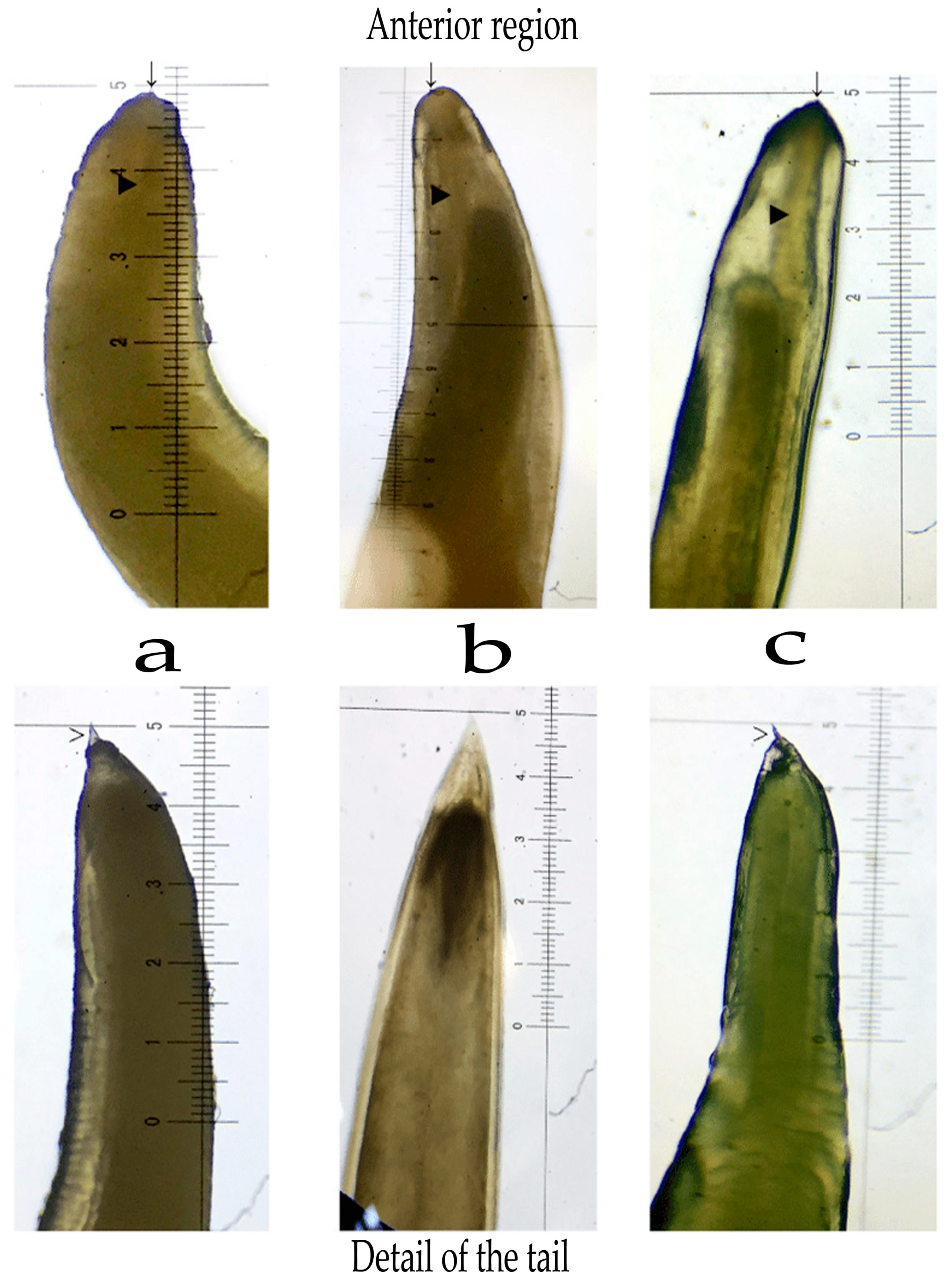
3. Overview of Morphology
4. Taxonomy Based on Molecular Evidence
5. Geographical Distribution
6. Anisakiosis/Anisakiasis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castellanos-Monroy, J.A.; Daschner, A.; Putostovrh, M.; Cuellar, C. Characteristics related to fish consumption and the risk of ichthyozoonosis in a Colombian population. Rev. Salud Pública 2020, 21, e169898. [Google Scholar]
- Monroy, G.C.; Santamaría, M.A.; Toméa, I.C.; Mayoralas, M.V.P. Gastritis aguda por anisakis. Rev. Clin. Med. Fam. 2014, 7, 56–58. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P.; Zhu, X. Description and genetic characterisation of Pulchrascaris australis n. sp. in the scalloped hammerhead shark, Sphyrna lewini (Griffin & Smith) in Australian waters. Parasitol. Res. 2020, 119, 1729–1742. [Google Scholar] [PubMed]
- Murata, R.; Suzuki, J.; Sadamasu, K.; Kai, A. Morphological and molecular characterization of Anisakis larvae (Nematoda: Anisakidae) in Beryx splendens from Japanese waters. Parasitol. Int. 2011, 60, 193–198. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Ferrantelli, V.; Costa, A.; Graci, S.; Buscemi, M.D.; Giangrosso, G.; Porcarello, C.; Palumbo, S.; Cammilleri, G. Anisakid Nematodes as Possible Markers to Trace Fish Products. Ital. J. Food Saf. 2015, 4, 4090. [Google Scholar] [CrossRef]
- Laffon–Leal, S.M.; Vidal–Martínez, V.M.; Arjona–Torres, G. ‘Cebiche’––A potential source of human anisakiasis in Mexico? J. Helminthol. 2000, 74, 151–154. [Google Scholar] [CrossRef]
- Mattiucci, S.; Paoletti, M.; Borrini, F.; Palumbo, M.; Palmieri, M.R.; Gomes, V.; Casati, A.; Nascetti, G. First molecular identification of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in a paraffin–embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect. Dis. 2011, 11, 82. [Google Scholar] [CrossRef]
- Castellanos, J.A.; Tangua, A.R.; Salazar, L. Anisakidae nematodes isolated from the flathead grey mulletfish (Mugilcephalus) of Buenaventura, Colombia. Int. J. Parasitol. Parasites. Wildl. 2017, 6, 265–270. [Google Scholar] [CrossRef]
- Grano-Maldonado, M.I.; Medina-Vera, R.A. Parasitosis, gastronomic tourism and food identities: A public health problem in Mazatlán, Sinaloa, México. Neotrop. Helminthol. 2019, 13, 203–225. [Google Scholar]
- Tokiwa, T.; Kobayashi, Y.; Ike, K.; Morishima, Y.; Sugiyama, H. Detection of Anisakid Larvae in Marinated Mackerel Sushi in Tokyo, Japan. Jpn. J. Infect. Dis. 2018, 71, 88–89. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/3/ca9229es/ca9229es.pdf (accessed on 3 October 2020).
- Ahmed, M.; Ayoob, F.; Kesavan, M.; Gumaste, V.; Khalil, A. Gastrointestinal Anisakidosis—Watch What You Eat. Cureus 2016, 8, e860. [Google Scholar] [PubMed]
- Bao, M.; Pierce, G.J.; Pascual, S.; González-Muñoz, M.; Mattiucci, S.; Mladineo, I.; Cipriani, P.; Bušelić, I.; Strachan, N.J. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci. Rep. 2017, 7, 43699. [Google Scholar] [CrossRef] [PubMed]
- Roca-Geronès, X.; Alcover, M.M.; Godínez-González, C.; González-Moreno, O.; Masachs, M.; Fisa, R.; Montoliu, I. First Molecular Diagnosis of Clinical Cases of Gastric Anisakiosis in Spain. Genes 2020, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Di Azevedo, M.I.N.; Carvalho, V.L.; Iñiguez, A.M. Integrative taxonomy of anisakid nematodes in stranded cetaceans from Brazilian waters: An update on parasite’s hosts and geographical records. Parasitol. Res. 2017, 116, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Quiazon, K.M.; Santos, M.D.; Yoshinaga, T. Anisakis species (Nematoda: Anisakidae) of Dwarf Sperm Whale Kogia sima (Owen, 1866) stranded off the Pacific coast of southern Philippine archipelago. Vet. Parasitol. 2013, 197, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Mladineo, I.; Hrabar, J.; Vrbatović, A.; Duvnjak, S.; Gomerčić, T.; Duras, M. Microbiota and gut ultrastructure of Anisakis pegreffii isolated from stranded cetaceans in the Adriatic Sea. Parasites Vectors 2019, 12, 381. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Lyons, E.T.; Spraker, T.R. Anisakids (Nematoda: Anisakidae) from stomachs of northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska: Parasitological and pathological analysis. Parasitol. Res. 2014, 113, 4463–4470. [Google Scholar] [CrossRef]
- Najda, K.; Simard, M.; Osewska, J.; Dziekońska-Rynko, J.; Dzido, J.; Rokicki, J. Anisakidae in beluga whales Delphinapterus leucas from Hudson Bay and Hudson Strait. Dis. Aquat. Organ. 2015, 115, 9–14. [Google Scholar] [CrossRef]
- D’Amelio, S.; Mathiopoulos, K.D.; Santos, C.P.; Pugachev, O.N.; Webb, S.C.; Picanço, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea) defined by polymerase-chain-reaction-based restriction fragment length polymorphism. Int. J. Parasitol. 2000, 30, 223–226. [Google Scholar] [CrossRef]
- Shamsi, S.; Suthar, J. Occurrence of Terranova larval types (Nematoda: Anisakidae) in Australian marine fish with comments on their specific identities. PeerJ 2016, 4, e1722. [Google Scholar] [CrossRef]
- Shamsi, S.; Norman, R.; Gasser, R.; Beveridge, I. Redescription and genetic characterization of selected Contracaecum spp. (Nematoda: Anisakidae) from various hosts in Australia. Parasitol. Res. 2009, 104, 1507–1525. [Google Scholar] [CrossRef]
- Pantoja, C.S.; Borges, J.N.; Santos, C.P.; Luque, J.L. Molecular and Morphological Characterization of Anisakid Nematode Larvae from the Sandperches Pseudopercis numida and Pinguipes brasilianus (Perciformes: Pinguipedidae) off Brazil. J. Parasitol. 2015, 101, 492–499. [Google Scholar] [CrossRef]
- Timi, J.T.; Paoletti, M.; Cimmaruta, R.; Lanfranchi, A.L.; Alarcos, A.J.; Garbin, L.; George-Nascimento, M.; Rodríguez, D.H.; Giardino, G.V.; Mattiucci, S. Molecular identification, morphological characterization and new insights into the ecology of larval Pseudoterranova cattani in fishes from the Argentine coast with its differentiation from the Antarctic species, P. decipiens sp. E (Nematoda: Anisakidae). Vet. Parasitol. 2014, 199, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Sugiyama, H.; Yamasaki, H.; Ramirez, C.; Rosas, R.; Mercado, R. Human Infections with Pseudoterranova cattani Nematodes, Chile. Emerg. Infect. Dis. 2015, 21, 1874–1875. [Google Scholar] [CrossRef] [PubMed]
- Tanzola, R.D.; Sardella, N.H. Terranova galeocerdonis (Thwaite, 1927) (Nematoda: Anisakidae) from Carcharias taurus (Chondrichthyes: Odontaspididae) off Argentina, with comments on some related species. Syst. Parasitol. 2006, 64, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.E.; Saad, C.D.; Cepeda, P.B.; Luque, J.L. Larvals of Terranova sp. (Nematoda: Anisakidae) parasitic in Plagioscion squamosissimus (Perciformes: Sciaenidae) from Araguaia River, State of Tocantins, Brazil. Rev. Bras. Parasitol. Vet. 2007, 16, 110–115. [Google Scholar] [PubMed]
- Mattiucci, S.; Paoletti, M.; Olivero-Verbel, J.; Baldiris, R.; Arroyo-Salgado, B.; Garbin, L.; Navone, G.; Nascetti, G. Contracaecum bioccai n. sp. from the brown pelican Pelecanus occidentalis (L.) in Colombia (Nematoda: Anisakidae): Morphology, molecular evidence and its genetic relationship with congeners from fish—Eating birds. Syst. Parasitol. 2008, 69, 101–121. [Google Scholar] [CrossRef]
- D’Amelio, S.; Cavallero, S.; Dronen, N.O.; Barros, N.B.; Paggi, L. Two new species of Contracaecum Railliet & Henry, 1912 (Nematoda: Anisakidae), C. fagerholmi n. sp. and C. rudolphii F from the brown pelican Pelecanus occidentalis in the northern Gulf of Mexico. Syst. Parasitol. 2012, 81, 1–16. [Google Scholar]
- Mattiucci, S.; Paoletti, M.; Solorzano, A.C.; Nascetti, G. Contracaecum gibsoni n. sp. and C. overstreeti n. sp. (Nematoda: Anisakidae) from the Dalmatian pelican Pelecanus crispus (L.) in Greek waters: Genetic and morphological evidence. Syst. Parasitol. 2010, 75, 207–224. [Google Scholar] [CrossRef]
- Garbin, L.E.; Diaz, J.I.; Navone, G.T. Species of Contracaecum parasitizing the Magellanic Penguin Spheniscus magellanicus (Spheniscidae) from the Argentinean Coast. J. Parasitol. 2019, 105, 222–231. [Google Scholar] [CrossRef]
- Molina-Fernández, D.; Valles-Vega, I.; Hernández-Trujillo, S.; Adroher, F.J.; Benítez, R. A scanning electron microscopy study of early development in vitro of Contracaecum multipapillatum s.l. (Nematoda: Anisakidae) from brown pelican (Pelecanus occidentalis) from the Gult of California, Mexico. Parasitol. Res. 2017, 116, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, G.Z. Occurrence of Anisakis pegreffii (Nematoda: Anisakidae) Larvae in Imported John Dory (Zeus faber) from Senegalese Coast Sold in Turkish Supermarkets. Acta Parasitol. 2019, 64, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Garbin, L.E.; Mattiucci, S.; Paoletti, M.; Diaz, J.I.; Nascetti, G.; Navone, G.T. Molecular identification and larval morphological description of Contracaecum pelagicum (Nematoda: Anisakidae) from the anchovy Engraulis anchoita (Engraulidae) and fish–eating birds from the Argentine North Patagonian Sea. Parasitol. Int. 2013, 62, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.N.; Santos, H.L.; Brandão, M.L.; Santos, E.G.; De-Miranda, D.F.; Balthazar, D.A.; Luque, J.L.; Santos, C.P. Molecular and morphological characterization of Contracaecum pelagicum (Nematoda) parasitizing Spheniscus magellanicus (Chordata) from Brazilian waters. Rev. Bras. Parasitol. Vet. 2014, 23, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ugland, K.I.; Strømnes, E.; Berland, B.; Aspholm, P.E. Growth, fecundity and sex ratio of adult whaleworm (Anisakis simplex; Nematoda, Ascaridoidea, Anisakidae) in three whale species from the North–East Atlantic. Parasitol. Res. 2004, 92, 484–489. [Google Scholar] [CrossRef]
- Smith, J.W. Anisakis simplex (Rudolphi, 1809, det. Krabbe, 1878) (Nematoda: Ascaridoidea): Morphology and morphometry of larvae from euphausiids and fish, and a review of the 1ife–history and ecology. J. Helminthol. 1983, 57, 205–224. [Google Scholar] [CrossRef]
- Gregori, M.; Roura, Á.; Abollo, E.; González, Á.F.; Pascual, S. Anisakis simplex complex (Nematoda: Anisakidae) in zooplankton communities from temperate NE Atlantic waters. J. Nat. Hist. 2015, 49, 13–14. [Google Scholar] [CrossRef]
- Køie, M.; Fagerholm, H.P. The life cycle of Contracaecum osculatum (Rudolphi, 1802) sensu stricto (Nematoda, Ascaridoidea, Anisakidae) in view of experimental infections. Parasitol. Res. 1995, 81, 481–489. [Google Scholar] [CrossRef]
- Valles-Vega, I.; Molina-Fernández, D.; Benítez, R.; Hernández-Trujillo, S.; Adroher, F.J. Early development and life cycle of Contracaecum multipapillatum s.l. from a brown pelican Pelecanus occidentalis in the Gulf of California, Mexico. Dis. Aquat. Organ. 2017, 125, 167–178. [Google Scholar] [CrossRef]
- Reyes, R.N.E.; Vega, S.V.; Gómez-de Anda, F.R.; García, R.P.B.; González, R.L.; Zepeda-Velázquez, A.P. Species of Anisakidae nematodes and Clinostomum spp. infecting lisa Mugil curema (Mugilidae) intended for human consumption in Mexico. Rev. Bras. Parasitol. Vet. 2020, 29, e017819. [Google Scholar]
- Mehrdana, F.; Bahlool, Q.Z.M.; Skov, J.; Marana, M.H.; Sindberg, D.; Mundeling, M.; Overgaard, B.C.; Korbut, R.; Strøm, S.B.; Kania, P.W.; et al. Occurrence of zoonotic nematodes Pseudoterranova decipiens, Contracaecum osculatum and Anisakis simplex in cod (Gadus morhua) from the Baltic Sea. Vet. Parasitol. 2014, 205, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Yabsley, M.; Keel, K. Anisakiasis with proventricular perforation in a greater shearwater (Puffinus gravis) off the coast of Georgia, USA. J. Zoo Wildl. Med. 2012, 43, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Berland, B. Nematodes from some Norwegian marine fishes. Sarsia 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Shamsi, S.; Ghadam, M.; Suthar, J.; Mousavi, H.E.; Soltani, M.; Mirzargar, S. Occurrence of ascaridoid nematodes in selected edible fish from the Persian Gulf and description of Hysterothylacium larval type XV and Hysterothylacium persicum n. sp. (Nematoda: Raphidascarididae). Int. J. Food Microbiol. 2016, 7, 265–273. [Google Scholar] [CrossRef]
- Mašová, S.; Baruš, V.; Seifertová, M.; Malala, J.; Jirků, M. Redescription and molecular characterisation of Dujardinascaris madagascariensis and a note on D. dujardini (Nematoda: Heterocheilidae), parasites of Crocodylus niloticus, with a key to Dujardinascaris spp. in crocodilians. Zootaxa 2014, 3893, 261–276. [Google Scholar] [CrossRef]
- Grabda, J. Studies on the life cycle and morphogenesis of Anisakis simplex (Rudolphi, 1809) (Nematoda: Anisakidae) cultured in vitro. Acta. Ichthyol. Piscat. 1976, 6, 119–141. [Google Scholar] [CrossRef]
- Baptista-Fernandes, T.; Rodrigues, M.; Castro, I.; Paixão, P.; Pinto-Marques, P.; Roque, L.; Toscano, C. Human gastric hyperinfection by Anisakis simplex: A severe and unusual presentation and a brief review. Int. J. Infect. Dis. 2017, 64, 38–41. [Google Scholar] [CrossRef]
- Wharton, D. Nematode eggshells. Parasitology 1980, 81, 447–463. [Google Scholar] [CrossRef]
- Moravec, F. Nematodes of Fresh Water Fishes of the Neotropical Region; Academia: Praha, Czech Republic, 1998; Volume 464. [Google Scholar]
- Pérez-i-García, D.; Constenla, M.; Carrassón, M.; Montero, F.E.; Soler-Membrives, A.; González–Solís, D. Raphidascaris (Raphidascaris) macrouri n. sp. (Nematoda: Anisakidae) from two deep-sea macrourid fishes in the Western Mediterranean: Morphological and molecular characterisations. Parasitol. Int. 2015, 64, 345–352. [Google Scholar] [CrossRef]
- Moravec, F.; Thatcher, V.E. Raphidascaroides brasiliensis n. sp. (Nematoda: Anisakidae), an intestinal parasite of the thorny catfish Pterodoras granulosus from Amazonia, Brazil. Syst. Parasitol. 1997, 38, 65–71. [Google Scholar] [CrossRef]
- Moravec, F.; Jirků, M. Dujardinascaris mormyropsis n. sp. (Nematoda: Anisakidae) from the osteoglossiform fish Mormyrops anguilloides (Linnaeus) (Mormyridae) in Central Africa. Syst. Parasitol. 2014, 88, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, Y.; Wang, S.; Tu, G.; Tang, X.; Wu, X. Preliminary report on the intestinal parasites and their diversity in captive Chinese alligators. Nutr. Hosp. 2014, 31, 813–819. [Google Scholar] [PubMed]
- Téllez, M.; Nifong, J. Gastric nematode diversity between estuarine and inland freshwater populations of the American alligator (Alligator mississippiensis, daudin 1802), and the prediction of intermediate hosts. Int. J. Parasitol. Parasites Wildl. 2014, 3, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Junker, K.; Boomker, J.; Govender, D.; Mutafchiev, Y. Nematodes found in Nile crocodiles in the Kruger National Park, South Africa, with redescriptions of Multicaecum agile (Wedl, 1861) (Heterocheilidae) and Camallanus kaapstaadi Southwell & Kirshner, 1937 (Camallanidae). Syst. Parasitol. 2019, 96, 381–398. [Google Scholar]
- Gamal, R.; Hassan, T. Morphological Characterization of Dujardinascaris Spp. (Nematoda: Anisakidae) from the Striped Red Mullet Mullus Surmuletus in the Mediterranean Sea. Helminthologia 2019, 56, 338–346. [Google Scholar]
- Moravec, F.; Kohn, A.; Fernandes, B.M. Two new species of the genus Goezia, G. brasiliensis sp. n. and G. brevicaeca sp. n. (Nematoda: Anisakidae), from freshwater fishes in Brazil. Folia Parasitol. 1994, 41, 271–278. [Google Scholar]
- Martins, L.M.; Yoshitoshi, E.R. A new nematode species Goezia leporini n. sp. (Anisakidae) from cultured freshwater fish Leporinus macrocephalus (Anostomidae) in Brazil. Braz. J. Biol. 2003, 63, 497–506. [Google Scholar] [CrossRef]
- El Alfi, N.M. The nematode Goezia sp. (Anisakidae) from Bagrus bayad (Osteichthyes) from Egypt. J. Egypt. Soc. Parasitol. 2005, 35, 205–211. [Google Scholar]
- Akther, M.; Alam, A.; D’Silva, J.; Bhuiyan, A.I.; Bristow, G.A.; Berland, B. Goezia bangladeshi n. sp. (Nematoda: Anisakidae) from an anadromous fish Tenualosa ilisha (Clupeidae). J. Helminthol. 2004, 78, 105–113. [Google Scholar] [CrossRef]
- Jackson, G.J.; Bier, J.W.; Payne, W.L.; Gerding, T.A.; Knollenberg, W.G. Nematodes in Fresh Market Fish of the Washington, D.C. Area. J. Food Prot. 1978, 41, 613–620. [Google Scholar] [CrossRef]
- Silva, M.T.D.; Cavalcante, P.H.O.; Camargo, A.C.A.; Chagas-Moutinho, V.A.D.; Santos, E.G.N.D.; Santos, C.P. Integrative taxonomy of Goezia spinulosa (Nematoda: Raphidascarididae) from arapaimas in the northwestern Brazil. Vet. Parasitol. 2017, 242, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.B.; Luque, J.L. An integrated phylogenetic analysis on ascaridoid nematodes (Anisakidae, Raphidascarididae), including further description and intraspecific variations of Raphidascaris (Sprentascaris) lanfrediae in freshwater fishes from Brazil. Parasitol. Int. 2017, 66, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Gasser, R.; Beveridge, I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013, 62, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Malta, L.S.; Paiva, F.; Elisei, C.; Tavares, L.E.R.; Pereira, F.B. A new species of Raphidascaris (Nematoda: Raphidascarididae) infecting the fish Gymnogeophagus balzanii (Cichlidae) from the Pantanal wetlands, Brazil and a taxonomic update of the subgenera of Raphidascaris based on molecular phylogeny and morphology. J. Helminthol. 2018, 94, e24. [Google Scholar] [CrossRef]
- Moravec, F.; Justine, J.L. New records of anisakid nematodes from marine fishes off New Caledonia, with descriptions of five new species of Raphidascaris (Ichthyascaris) (Nematoda, Anisakidae). Parasite 2020, 27, 20. [Google Scholar] [CrossRef]
- Cavallero, S.; Nadler, S.A.; Paggi, L.; Barros, N.B.; D’Amelio, S. Molecular characterization and phylogeny of anisakid nematodes from cetaceans from southeastern Atlantic coasts of USA, Gulf of Mexico, and Caribbean Sea. Parasitol. Res. 2011, 108, 781–792. [Google Scholar] [CrossRef]
- Perkins, S.L.; Martinsen, E.S.; Falk, B.G. Do molecules matter more than morphology? Promises and pitfalls in parasites. J. Parasitol. 2011, 138, 1664–1674. [Google Scholar] [CrossRef]
- D’Amelio, S.; Lombardo, F.; Pizzarelli, A.; Bellini, I.; Cavallero, S. Advances in Omic Studies Drive Discoveries in the Biology of Anisakid Nematodes. Genes 2020, 15, 801. [Google Scholar] [CrossRef]
- Mattiucci, S.; Nascetti, G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv. Parasitol. 2008, 66, 47–148. [Google Scholar]
- Ondrejicka, D.A.; Locke, S.A.; Morey, K.; Borisenko, A.V.; Hanner, R.H. Status and prospects of DNA barcoding in medically important parasites and vectors. Trends Parasitol. 2014, 30, 582–591. [Google Scholar] [CrossRef]
- Shamsi, S.; Briand, M.J.; Jean-Lou, J. Occurrence of Anisakis (Nematoda: Anisakidae) larvae in unusual hosts in Southern hemisphere. Parasitol. Int. 2017, 66, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Spröhnle-Barrera, C.; Hossen, M.D.F. Occurrence of Anisakis spp. (Nematoda: Anisakidae) in a pygmy sperm whale Kogia breviceps (Cetacea: Kogiidae) in Australian waters. Dis. Aquat. Organ. 2019, 134, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Chen, Y.; Poupa, A.; Ghadam, M.; Justine, J.-L. Occurrence of anisakid parasites in marine fishes and whales off New Caledonia. Parasitol. Res. 2018, 117, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Abaunza, P.; Damiano, S.; Garcia, A.; Santos, M.N.; Nascetti, G. Distribution of Anisakis larvae, identified by genetic markers, and their use for stock characterization of demersal and pelagic fish from European waters: An update. J. Helminthol. 2007, 81, 117–127. [Google Scholar] [CrossRef]
- Saraiva, A.; Faranda, A.; Damiano, S.; Hermida, M.; Santos, M.J.; Ventura, C.H. Six species of Anisakis (Nematoda: Anisakidae) parasites of the black scabbardfish, Aphanopus carbo from NE Atlantic waters: Genetic markers and fish biology. Parassitologia 2007, 49, 229. [Google Scholar]
- Mattiucci, S.; Paoletti, M.; Webb, S.C. Anisakis nascettii n. sp. (Nematoda: Anisakidae) from beaked whales of the southern hemisphere: Morphological description, genetic relationships between congeners and ecological data. Syst. Parasitol. 2009, 74, 199–217. [Google Scholar] [CrossRef]
- Quiazon, K.M.A. Anisakis Dujardin, 1845 infection (Nematoda: Anisakidae) in Pygmy Sperm Whale Kogia breviceps Blainville, 1838 from west Pacific region off the coast of Philippine archipelago. Parasitol. Res. 2016, 115, 3663–3668. [Google Scholar] [CrossRef]
- Cavallero, S.; Magnabosco, C.; Civettini, M.; Boffo, L.; Mingarelli, G.; Buratti, P.; Giovanardi, O.; Fortuna, C.M.; Arcangeli, G. Survey of Anisakis sp. and Hysterothylacium sp. in sardines and anchovies from the North Adriatic Sea. Int. J. Food Microbiol. 2015, 200, 18–21. [Google Scholar] [CrossRef]
- Quiazon, K.M.; Yoshinaga, T.; Ogawa, K. Distribution of Anisakis species larvae from fishes of the Japanese waters. Parasitol. Int. 2011, 60, 223–226. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, L.P.; Gibson, D.I.; Lü, L.; Xu, Z.; Li, H.T.; Ju, H.D.; Li, L. Detection of ascaridoid nematode parasites in the important marine food-fish Conger myriaster (Brevoort) (Anguilliformes: Congridae) from the Zhoushan Fishery, China. Parasites Vectors 2018, 11, 274. [Google Scholar] [CrossRef]
- Cho, J.; Lim, H.; Jung, B.K.; Shin, E.H.; Chai, J.Y. Anisakis pegreffii Larvae in Sea Eels (Astroconger myriaster) from the South Sea, Republic of Korea. Korean J. Parasitol. 2015, 53, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, J.Y.; Chen, H.X.; Ju, H.-D.; An, M.; Xu, Z.; Zhang, L.P. Survey for the presence of ascaridoid larvae in the cinnamon flounder Pseudorhombus cinnamoneus (Temminck & Schlegel) (Pleuronectiformes: Paralichthyidae). Int. J. Food Microbiol. 2017, 241, 108–116. [Google Scholar]
- Cammilleri, G.; Pulvirenti, A.; Costa, A.; Graci, S.; Collura, R.; Buscemi, M.D.; Sciortino, S.; Vitale, B.V.; Vazzana, M.; Brunone, M.; et al. Seasonal trend of Anisakidae infestation in South Mediterranean bluefish. Nat. Prod. Res. 2019, 34, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Jung, B.K.; Cho, J.; Yooyen, T.; Shin, E.H.; Chai, J.Y. Molecular diagnosis of cause of anisakiasis in humans, South Korea. Emerg. Infect. Dis. 2015, 21, 342–344. [Google Scholar] [CrossRef]
- Gaglio, G.; Battaglia, P.; Costa, A.; Cavallaro, M.; Cammilleri, G.; Graci, S.; Buscemi, M.D.; Ferrantelli, V.; Andaloro, F.; Marino, F. Anisakis spp. larvae in three mesopelagic and bathypelagic fish species of the central Mediterranean Sea. Parasitol. Int. 2018, 67, 23–28. [Google Scholar] [CrossRef]
- Kong, Q.; Fan, L.; Zhang, J.; Akao, N.; Dong, K.; Lou, D.; Ding, J.; Tong, Q.; Zheng, B.; Chen, R.; et al. Molecular identification of Anisakis and Hysterothylacium larvae in marine fishes from the East China Sea and the Pacific coast of central Japan. Int. J. Food Microbiol. 2015, 199, 1–7. [Google Scholar] [CrossRef]
- Abattouy, N.; López, A.V.; Maldonado, J.L.; Benajiba, M.H.; Martín-Sánchez, J. Epidemiology and molecular identification of Anisakis pegreffii (Nematoda: Anisakidae) in the horse mackerel Trachurus from northern Morocco. J. Helminthol. 2014, 88, 257–263. [Google Scholar] [CrossRef]
- Umehara, A.; Kawakami, Y.; Ooi, H.K.; Uchida, A.; Ohmae, H.; Sugiyama, H. Molecular identification of Anisakis type I larvae isolated from hairtail fish off the coasts of Taiwan and Japan. Int. J. Food Microbiol. 2010, 143, 161–165. [Google Scholar] [CrossRef]
- Pawlak, J.; Nadolna-Ałtyn, K.; Szostakowska, B.; Pachur, M.; Bańkowska, A.; Podolska, M. First evidence of the presence of Anisakis simplex in Crangon and Contracaecum osculatum in Gammarus sp. by in situ examination of the stomach contents of cod (Gadus morhua) from the southern Baltic Sea. Parasitology 2019, 146, 1699–1706. [Google Scholar] [CrossRef]
- Fonseca, M.C.; Knoff, M.; Felizardo, N.N.; Di Azevedo, M.I.; Torres, E.J.; Gomes, D.C.; Iñiguez, A.M.; Clemente, S.C.S. Integrative taxonomy of Anisakidae and Raphidascarididae (Nematoda) in Paralichthys patagonicus and Xystreurys rasile (Pisces: Teleostei) from Brazil. Int. J. Food Microbiol. 2016, 235, 113–124. [Google Scholar] [CrossRef]
- Palm, H.W.; Theisen, S.; Damriyasa, I.M.; Kusmintarsih, E.S.; Oka, I.B.; Setyowati, E.A.; Suratma, N.A.; Wibowo, S.; Kleinertz, S. Anisakis (Nematoda: Ascaridoidea) from Indonesia. Dis. Aquat. Organ. 2017, 123, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Poupa, A.; Justine, J.-L. Characterisation of Ascaridoid larvae from marine fish off New Caledonia, with description of new Hysterothylacium larval types XIII and XIV. Parasitol. Int. 2015, 64, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Pontes, T.; D’Amelio, S.; Costa, G.; Paggi, L. Molecular characterization of larval anisakid nematodes from marine fishes of Madeira by a PCR-based approach, with evidence for a new species. J. Parasitol. 2005, 91, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Garbin, L.; Mattiucci, S.; Paoletti, M.; González-Acuña, D.; Nascetti, G. Genetic and morphological evidences for the existence of a new species of Contracaecum (Nematoda: Anisakidae) parasite of Phalacrocorax brasilianus (Gmelin) from Chile and its genetic relationships with congeners from fish-eating birds. J. Parasitol. 2011, 97, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Turner, A.; Wassens, S. Description and genetic characterization of a new Contracaecum larval type (Nematoda: Anisakidae) from Australia. J. Helminthol. 2018, 92, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.S.; Shamsi, S. Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia. Int. J. Food Microbiol. 2019, 308, 108306. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Kania, P.W.; Mehrdana, F.; Marana, M.H.; Buchmann, K. Contracaecum osculatum and other anisakid nematodes in grey seals and cod in the Baltic Sea: Molecular and ecological links. J. Helminthol. 2018, 92, 81–89. [Google Scholar] [CrossRef]
- Horbowy, J.; Podolska, M.; Nadolna-Ałtyn, K. Increasing occurrence of anisakid nematodes in the liver of cod (Gadus morhua) from the Baltic Sea: Does infection affect the condition and mortality of fish? Fish. Res. 2016, 179, 98–103. [Google Scholar] [CrossRef]
- Pekmezci, G.Z.; Yardimci, B. On the occurrence and molecular identification of Contracaecum larvae (Nematoda: Anisakidae) in Mugil cephalus from Turkish waters. Parasitol. Res. 2019, 118, 1393–1402. [Google Scholar] [CrossRef]
- Mattiucci, S.; Sbaraglia, G.L.; Palomba, M.; Filippi, S.; Paoletti, M.; Cipriani, P.; Nascetti, G. Genetic identification and insights into the ecology of Contracaecum rudolphii A and C. rudolphii B (Nematoda: Anisakidae) from cormorants and fish of aquatic ecosystems of Central Italy. Parasitol. Res. 2020, 119, 1243–1257. [Google Scholar] [CrossRef]
- Shamsi, S.; Norman, R.; Gasser, R.; Beveridge, I. Genetic and morphological evidences for the existence of sibling species within Contracaecum rudolphii (Hartwich, 1964) (Nematoda: Anisakidae) in Australia. Parasitol. Res. 2009, 105, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Gasser, R.; Beveridge, I.; Shabani, A.A. Contracaecum pyripapillatum n. sp. (Nematoda: Anisakidae) and a description of C. multipapillatum (von Drasche, 1882) from the Australian pelican, Pelecanus conspicillatus. Parasitol. Res. 2008, 103, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.T.; Xu, Z.; Li, L. Morphological variability and molecular characterization of Mawsonascaris australis (Johnston & Mawson, 1943) (Nematoda: Ascaridoidea: Acanthocheilidae) from the brown guitarfish Rhinobatos schlegelii Muller & Henle (Rhinopristiformes: Rhinobatidae). J. Helminthol. 2018, 92, 760–764. [Google Scholar] [PubMed]
- Shamsi, S.; Dang, M.; Zhu, X.; Nowak, B. Genetic and morphological characterization of Mawsonascaris vulvolacinata n. sp. (Nematoda: Anisakidae) and associated histopathology in a wild caught cowtail stingray, Pastinachus ater. J. Fish. Dis. 2019, 42, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Mafra, C.; Mantovani, C.; Borges, J.N.; Barcelos, R.M.; Santos, C.P. Morphological and molecular diagnosis of Pseudoterranova decipiens (sensu stricto) (Anisakidae) in imported cod sold in Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 209–215. [Google Scholar] [CrossRef][Green Version]
- Quraishy, S.A.; Abdel-Gaber, R.; Dkhil, M.A.M. First record of Pseudoterranova decipiens (Nematoda, Anisakidae) infecting the red spot emperor Lethrinus lentjan in the Red Sea. Rev. Bras. Parasitol. Vet. 2019, 28, 625–631. [Google Scholar] [CrossRef]
- Podolska, M.; Pawlikowski, B.; Nadolna-Ałtyn, K.; Pawlak, J.; Komar-Szymczak, K.; Szostakowska, B. How effective is freezing at killing Anisakis simplex, Pseudoterranova krabbei, and P. decipiens larvae? An experimental evaluation of time–temperature conditions. Parasitol. Res. 2019, 118, 2139–2147. [Google Scholar] [CrossRef]
- Marcer, F.; Tosi, F.; Franzo, G.; Vetri, A.; Ravagnan, S.; Santoro, M.; Marchiori, E. Updates on Ecology and Life Cycle of Sulcascaris sulcata (Nematoda: Anisakidae) in Mediterranean Grounds: Molecular Identification of Larvae Infecting Edible Scallops. Front. Vet. Sci. 2020, 7, 64. [Google Scholar] [CrossRef]
- Nadler, S.A.; D’Amelio, S.; Dailey, M.D.; Paggi, L.; Siu, S.; Sakanari, J.A. Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from northern Pacific marine mammals. J. Parasitol. 2005, 91, 1413–1429. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P.; Zhu, X. Description and characterisation of Terranova pectinolabiata n. sp. (Nematoda: Anisakidae) in great hammerhead shark, Sphyrna mokarran (Rüppell, 1837), in Australia. Parasitol. Res. 2019, 118, 2159–2168. [Google Scholar] [CrossRef]
- Santoro, M.; Marchiori, E.; Palomba, M.; Degli, U.B.; Marcer, F.; Mattiucci, S. The Mediterranean Mussel (Mytilus galloprovincialis) as Intermediate Host for the Anisakid Sulcascaris sulcata (Nematoda), a Pathogen Parasite of the Mediterranean Loggerhead Turtle (Caretta caretta). Pathogens 2020, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Ando, K.; Usui, M.; Sugiyama, H.; Hayashi, A.; Tanemura, A.; Kato, H.; Kuriyama, N.; Kishiwada, M.; Mizuno, S.; et al. A case of hepatic anisakiasis caused by Pseudoterranova decipiens mimicking metastatic liver cancer. BMC Infec. Dis. 2018, 18, 619. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, M.; Yoshimura, H. Clinicopathologic studies on larval anisakiasis in Japan. Am. J. Trop. Med. Hyg. 1967, 16, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.N.C. Hábitos alimentarios and relación interespecífica entre la ballena azul (Balaenoptera musculus) and la ballena de aleta (B. physalus) en el suroeste del Golfo de California. Master’s Thesis, Centro Interdisciplinario de Ciencias Marinas, La paz, Baja California Sur, Mexico, 2010. [Google Scholar]
- Arizono, N.; Yamada, M.; Tegoshi, T.; Yoshikawa, M. Anisakis simplex sensu stricto and Anisakis pegreffii: Biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog. Dis. 2012, 9, 517–521. [Google Scholar] [CrossRef]
- Valiñas, B.; Lorenzo, S.; Eiras, A.; Figueiras, A.; Sanmartín, M.L.; Ubeira, F.M. Prevalence of and risk factors for IgE sensitization to Anisakis simplex in a Spanish population. Allergy 2001, 56, 667–671. [Google Scholar] [CrossRef]
- Rezapour, M.; Agarwal, N. You Are What You Eat: A Case of Nematode-Induced Eosinophilic Esophagitis. ACG Case Rep. J. 2017, 4, e13. [Google Scholar] [CrossRef]
- Amir, A.; Ngui, R.; Ismail, W.H.; Wong, K.T.; Ong, J.S.; Lim, Y.A.; Lau, Y.L.; Mahmud, R. Anisakiasis Causing Acute Dysentery in Malaysia. Am. J. Trop. Med. Hyg. 2016, 95, 410–412. [Google Scholar] [CrossRef][Green Version]
- Shimamura, Y.; Muwanwella, N.; Chandran, S.; Kandel, G.; Marcon, N. Common symptoms from an uncommon infection: Gastrointestinal anisakiasis. Can. J. Gastroenterol. Hepatol. 2016, 1–7. [Google Scholar] [CrossRef]
- Mizumura, N.; Okumura, S.; Tsuchihashi, H.; Ogawa, M.; Kawasaki, M. A Second Attack of Anisakis: Intestinal Anisakiasis Following Gastric Anisakiasis. ACG Case Rep. J. 2018, 5, e65. [Google Scholar] [CrossRef]
- Hochberg, N.S.; Hamer, H.D. Anisakidosis: Perils of the deep. Clin. Infect. Dis. 2010, 51, 806–812. [Google Scholar] [CrossRef]
- Audicana, M.T.; Fernández, C.L.; Muñoz, D.; Fernández, E.; Navarro, J.A.; Del Pozo, M.D. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J. Allergy Clin. Immunol. 1995, 96, 558–560. [Google Scholar] [CrossRef]
- Menéndez, P.; Pardo, R.; Delgado, M.; León, C. Mesenteric tumor due to chronic anisakiasis. Rev. Esp. Enferm. Dig. 2005, 107, 570–572. [Google Scholar] [CrossRef]
- Kuhn, T.; Cunze, S.; Kochmann, J.; Klimpel, S. Environmental variables and definitive host distribution: A habitat suitability modelling for endohelminth parasites in the marine realm. Sci. Rep. 2016, 10, 30246. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S. Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes 2019, 4, 9. [Google Scholar] [CrossRef]
- Højgaard, D.P. Impact of temperature, salinity and light on hatching of eggs of Anisakis simplex (Nematoda, Anisakidae), isolated by a new method, and some remarks on survival of larvae. Sarsia 1998, 83, 21–28. [Google Scholar] [CrossRef]
- Palm, H.W. Fish Parasites as Biological Indicators in a Changing World: Can We Monitor Environmental Impact and Climate Change? In Progress in Parasitology; Springer: New York, NY, USA, 2011; pp. 223–250. [Google Scholar]
- Rodríguez, H.; Bañón, R.; Ramilo, A. The hidden companion of non-native fishes in north-east Atlantic waters. J. Fish. Dis. 2019, 42, 1013–1021. [Google Scholar] [CrossRef]
- Boussellaa, W.; Neifar, L.; Goedknegt, M.A.; Thielges, D.W. Lessepsian migration and parasitism: Richness, prevalence and inten-sity of parasites in the invasive fish Sphyraena chrysotaenia compared to its native congener Sphyraena sphyraena in Tunisian coastal waters. PeerJ 2018, 6, e5558. [Google Scholar] [CrossRef]
- Shamsi, S.; Sheorey, H. Seafood-borne parasitic diseases in Australia: Are they rare or underdiagnosed? Intern. Med. J. 2018, 48, 591–596. [Google Scholar] [CrossRef]
- Fukui, S.; Matsuo, T.; Mori, N. Palatine Tonsillar Infection by Pseudoterranova azarasi. Am. J. Trop. Med. Hyg. 2020, 103, 8. [Google Scholar] [CrossRef]
- Arizono, N.; Miura, T.; Yamada, M.; Tegoshi, T.; Onishi, K. Human infection with Pseudoterranova azarasi roundworm. Emerg. Infect. Dis. 2011, 17, 555–556. [Google Scholar] [CrossRef]
- Menghi, C.I.; Gatta, C.L.; Arias, L.E.; Santoni, G.; Nicola, F.; Smayevsky, J.; Degese, M.; Krivokapich, S.J. Human infection with Pseudoterranova cattani by ingestion of “ceviche” in Buenos Aires, Argentina. Rev. Argent. Microbiol. 2020, 52, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Pesson, B.; Royant, M.; Lemoine, J.P.; Pfaff, A.W.; Abou-Bacar, A.; Yera, H.; Fréalle, E.; Dupouy-Camet, J.; Merino-Espinosa, G.; et al. Molecular diagnosis of Pseudoterranova decipiens s.s in human, France. BMC Infect. Dis. 2017, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From Obscure Infectious Worm to Inducer of Immune Hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Measures, L.; Gagnon, J.; Desbiens, C. Human intestinal anisakiosis due to consumption of raw salmon. Am. J. Surg. Pathol. 2003, 27, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.D.; Audicana, M.; Diez, J.M.; Muñoz, D.; Ansotegui, I.J.; Fernández, E.; García, M.; Etxenagusia, M.; Moneo, I.; Fernández, C.L. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy 1997, 52, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Lopata, A.L. Allergic reactions to Anisakis found in fish. Curr. Allergy Asthma Rep. 2014, 14, 455. [Google Scholar] [CrossRef]
- Zuloaga, J.; Arias, J.; Balibrea, J.L. Anisakiasis digestiva. Aspectos de interés para el cirujano. Cir. Esp. 2004, 75, 9–13. [Google Scholar] [CrossRef]
- Peláez, M.A.; Codoceo, C.M.; Montiel, P.M.; Gómez, F.S.; Castellano, G.; Herruzo, J.A.S. Anisakiasis múltiple. Rev. Esp. Enferm. Dig. 2008, 100, 581–582. [Google Scholar] [CrossRef][Green Version]
- Zullo, A.; Hassan, C.; Scaccianoce, G.; Lorenzetti, R.; Campo, S.M.; Morini, S. Gastric anisakiasis: Do not forget theclinical history! J. Gastrointest. Liver Dis. 2010, 19, 359. [Google Scholar]
- Baron, L.; Branca, G.; Trombetta, C.; Punzo, E.; Quarto, F.; Speciale, G.; Barresi, V. Intestinal anisakidosis: Histopathological findings and differential diagnosis. Pathol. Res. Pract. 2014, 210, 746–750. [Google Scholar] [CrossRef]
- Moneo, I.; Caballero, M.L.; Gómez, F.; Ortega, E.; Alonso, M.J. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J. Allergy Clin. Immunol. 2000, 106, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Anadón, A.M.; García–Bodas, E.; Romarís, F.; Iglesias, R.; Gárate, T.; Ubeira, F.M. Novel sequences and epitopes of diagnostic value derived from the Anisakis simplex Ani s 7 major allergen. Allergy 2008, 63, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E. Anisakis—Immunology of a foodborne parasitosis. Parasite Immunol. 2016, 38, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Aguirre, J.M.; Echevarria, M.A.; Martinez-Conde, R.; Pontón, J. Gingivostomatitis after eating fish parasitized by Anisakis simplex: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 437–440. [Google Scholar] [CrossRef]
- Daschner, A.; Alonso, G.; Cabanas, R.; Suárez-de-Parga, J.M.; López-Serrano, M.C. Gastroallergic anisakiasis: Border line between food allergy and parasitic disease–clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J. Allergy Clin. Immunol. 2000, 105, 176–181. [Google Scholar] [CrossRef]
- Morsy, K.; Mahmoud, B.A.; Abdel-Ghaffar, F.; Deeb, E.L.S.; Ebead, S. Pathogenic Potential of Fresh, Frozen, and Thermally Treated Anisakis spp. Type II (L3) (Nematoda: Anisakidae) after oral Inoculation in to Wistar Rats: A Histopathological Study. J. Nematol. 2017, 49, 427–436. [Google Scholar] [CrossRef]
- Ludovisi, A.; Di-Felice, G.; Carballeda-Sangiao, N.; Barletta, B.; Butteroni, C.; Corinti, S.; Marucci, G.; González-Muñoz, M.; Pozio, E.; Gómez-Morales, M.A. Allergenic activity of Pseudoterranova decipiens (Nematoda: Anisakidae) in BALB/c mice. Parasites Vectors 2017, 10, 290. [Google Scholar] [CrossRef]
- Baeza, M.L.; Conejero, L.; Higaki, Y.; Martín, E.; Pérez, C.; Infante, S.; Rubio, M.; Zubeldia, J.M. Anisakis simplex allergy: A murine model of anaphylaxis induced by parasitic proteins displays a mixed Th1/Th2 pattern. Clin. Exp. Immunol. 2005, 142, 433–440. [Google Scholar] [CrossRef]
- Sakanari, J.A. Anisakis—From the platter to the microfuge. Parasitol. Today 1990, 6, 323–327. [Google Scholar] [CrossRef]
- Shweiki, E.; Rittenhouse, D.W.; Ochoa, J.E.; Punja, V.P.; Zubair, M.H.; Baliff, J.P. Acute small-bowel obstruction from intestinal anisakiasis after the ingestion of raw clams; documenting a new method of marine-to-human parasitic transmission. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2014; Volume 1. [Google Scholar]
- Roser, D.; Stensvold, C.R. Anisakiasis Mistaken for Dientamoebiasis? Clin. Infect. Dis. 2013, 57, 1500. [Google Scholar] [CrossRef]
- Takei, H.; Powell, S.D. Intestinal anisakidosis (anisakidosis). Ann. Diagn. Pathol. 2007, 11, 350–352. [Google Scholar] [CrossRef] [PubMed]
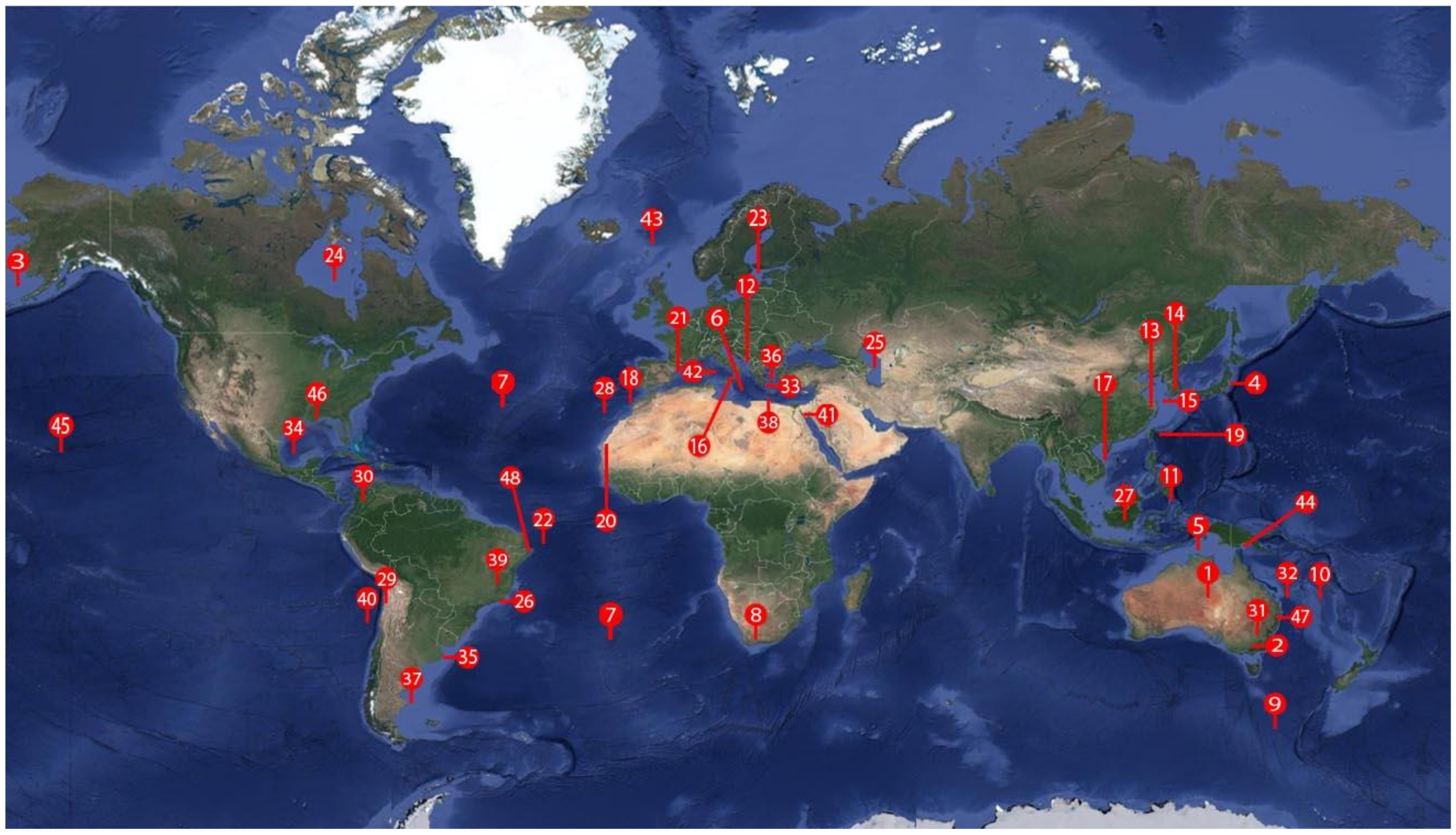
| Genus/Species | Host Common Name/Scientific Name | Map | Reference |
|---|---|---|---|
| Anisakis berlandi | Grey petrels/Procellaria cinerea Pygmy sperm whale/Kogia breviceps | 1 2 | [74,75] |
| Pygmy sperm whale/Kogia breviceps | 2 | [75] | |
| A. brevispiculata | Dwarf sperm whale/Kogia sima Pygmy sperm whale/Kogia breviceps | 2 3 | [18,75] |
| Splendid Alfonsino/Beryx splendens | 4 5 | [4,76] | |
| A. nascettii | European hake/Merluccius merluccius L. | 6 7 | [77] |
| Black Scabbardfish/Aphanopus carbo | 7 | [78] | |
| Squid/Moroteuthis ingens Ziphiid species belong to the Genus/Mesoplodon | 10 9 7 | [79] | |
| A. paggiae | Dwarf sperm whale/Kogia sima | 3 2 10 | [16,75,76] |
| Pygmy sperm whale/Kogia breviceps | 11 10 | [17,76,80] | |
| Splendid Alfonsino/Beryx splendens | 4 | [4] | |
| *** A. pegreffi | Grey petrels/Procellaria cinerea Little penguin/Eudyptulae minor | 1 | [81] |
| Sardine/Sardina pilchardus Anchovy/Engraulidae spp. | 12 | [81] | |
| Whitespotted conger/Conger myriaster | 4 | [82] | |
| Whitespotted conger/Conger myriaster | 13 | [83] | |
| Whitespotted conger/Conger myriaster | 14 | [84] | |
| Cinnamon flounder/Pseudorhombus cinnamoneus | 15 | [85] | |
| Sardine/Sardina pilchardus Silver scabbardfish/Lepidopus caudatus Electric lantern fish/Electrona risso Slender lightfish/Vinciguerria attenuate Spothead lantern fish/Diaphus metopoclampus | 16 | [86] | |
| Chum salmon/Oncorhynchus keta | 14 | [87,88] | |
| Redlip croaker/Larimichthys polyactis Atlantic mackerel/Scomber scombrus Largehead hairtail/Trichiurus lepturus | 17 | [89] | |
| Atlantic horse mackerel/Trachurus trachurus | 18 | [90] | |
| Largehead hairtail/Trichiurus lepturus | 4 19 | [91] | |
| Striped dolphin/Stenella coeruleoalba | 20 | [18] | |
| John Dory/Zeus faber | 21 | [34] | |
| A. physeteris | Common Atlantic grenadier/Nezumia aequalis Roughsnout grenadier/Trachynicus scabrus | 22 | [52] |
| Common Atlantic grenadier/Nezumia aequalis Roughsnout grenadier/Trachynicus scabrus | 16 | [88] | |
| Splendid Alfonsino/Beryx splendens | 4 | [4] | |
| *** A. simplex s.s. | Redlip croaker/Larimichthys polyactis Atlantic mackerel/Scomber scombrus Largehead hairtail/Trichurius lepturus | 18 | [89] |
| Northern fur seal/Callorhinus ursinus | 23 24 | [19] | |
| Beluga whale/Delphinapterus leucas | 25 | [20] | |
| Common Atlantic grenadier/Nezumia aequalis Roughsnout grenadier/Trachynicus scabrus | 23 25 | [52] | |
| Chum salmon/Oncorhynchus keta | 3 21 | [82] | |
| Largehead hairtail/Trichiurus lepturus | 3 21 | [91] | |
| Brown shrimp/Crangon crangon | 26 | [92] | |
| A. schupakovi | Caspian seal/Phoca caspica | 27 | [21] |
| A. typica | Sandperch/Pseudopercis semifasciata Namorado sandperch/Pseudopercis numida Brazilian sandperch/Pinguipes brasiliansus Patagonian flounder/Paralichtys patagonicus Fantail flounder/Xystreurys rasile Atlantic spotted dolphin/Stenella frontalis Fraser’s dolphin/Lagenodeplhis hosei | 28 | [16,22,93] |
| Bullet mackerel/Auxis rochei rochei | 29 | [94] | |
| Largehead hairtail/Trichiurus lepturus | 4 21 | [91] | |
| Longnose trevally/Caragoides chysophrys Bumpnose trevally/Carangoides hedlandensis Imposter Jack or Imposter Trevally/Carangoides cf talamparoides Bigeye scad/Selar crumenophtalmus Dorab wolf-herring/Chirocentrus dorab Giant herring/Elops cf hawaiensi Ornate Snapper/Pristipomoides argyrogrammicus Silver moonyfish/Monodactylus argenteus Jpanese whiptail/Pentapodus nagasakiensis Indian mackerel/Rastrelliger kanagurta Orange-spotted grouper/Epinephelus coioides Bigeye barracuda/Sphyraena forsteri Sawtooth barracuda/Sphyraena putnamae Brushtooth lizardfish/Saurida undosquamis Live sharksucker/Echeneis naucrates Areolate grouper/Epinephelus areolatus Threadfin bream/Nemipterus furcosus Bartailed goatfish/Upeneus vittatus Mackerel scad/Decapterus macarellus Scomberoides sp. | 10 | [76,95] | |
| Spinner shark/Carcharhinus brevipinna Blue-lipped sea krait/Laticauda laticaudata | 10 | [74] | |
| A. ziphidarum | Chub mackerel/Scomber japonicus | 29 30 | [21,96] |
| Atlantic grenadier/Nezumia aequalis Roughsnout grenadier/Trachynicus scabrus | 23 | [52] | |
| Electric lantern fish/Electrona risso Spothead lantern fish/Diaphus metopoclampus Slender lightfish/Vinciguerria attenuata | 16 | [88] | |
| Cetaceans | 28 | [16] | |
| Pygmy sperm whale/Kogia breviceps | 11 | [17] | |
| Black-scarbbard fish/Aphanopus carbo | 29 | [96] | |
| Contracaecum australe | Olivaceous cormorant/Phalacrocorax brasilianus | 1 | [97] |
| C. bancrofti | Great white pelican/Pelecanus onocrotalus | 31 | [23] |
| Common carp/Cyprinus carpio Carp gudgeon/Hypseleotris sp Gambusia/Gambusia holbrooki Weather loach/Misgurnus anguilicaudatus Rainbow fish/Melanotaenia fluviatilis Bony bream/Nematalosa erebi Australian smelt/Retropinna semoni | 32 | [98] | |
| C. bioccai | American brown pelican/Pelecanus occidentalis | 33 | [29] |
| C. chubutensis | Olivaceous cormorant/Phalacrocorax brasilianus | 1 | [97] |
| C. eudyptulae | Little penguin/Eudyptula minor | 31 | [23] |
| C. fagerholmi n. | American brown pelican/Pelecanus occidentalis | 34 | [30] |
| C. galeocerdonis | Elasmobranch fish | 35 | [22] |
| C. gibsoni | Dalmatian pelican/Pelecanus crispus | 36 | [31] |
| C. margolisi | Mammals of the pinniped family | 31 | [23] |
| C. mirounga | Magellanic penguin/Spheniscus Magellanicus | 37 | [32] |
| C. microcephalum | Australian pelican/Pelecanus melanoleucos | 31 | [23] |
| Pelicans/Pelecanus spp. | 31 | [23] | |
| C. multipapillatum | American brown pelican/Pelecanus occidentalis | 34 | [33] |
| C. ogmorhini | Australian fur seal/Arctocephalus pusillus doriferus New Zealand fur seal/Arctocephalus fosteri | 31 | [23] |
| Australian pilchard/Sardinops sagax Aanchovy/Engraulidae sp. | 32 | [99] | |
| *** C. osculatum | Northern fur seal/Callorhinus ursinus | 24 25 | [19,20] |
| Baltic cod/Gadus morhua Grey seal/Halichoerus grypus | 26 | [100,101] | |
| Amphipod crustacean/Gammarus spp. | 26 | [102] | |
| C. overstreeti | Flathead grey mullet/Mugil cephalus Dalmatian pelican/Pelecanus crispus | 38 | [102] |
| Pelicans/Pelecanus spp. | 36 | [31] | |
| C. pelagicum | Magellanic penguin/Spheniscus magellanicus | 39 | [35] |
| Magellanic penguin/Spheniscus magellanicus | 3 | [36] | |
| C. rudolphii A, B, C, D and E | Neotropic cormorant/Phalacrocorax brasilianus Eel/Anguilla Anguilla European seabass/Dicentrarchus labrax Mediterranean banded killifish/Aphanius fasciatus Big-scale sand smelt/Leuciscus cephalus Barbel/Barbus barbus Crucian carp/Carassius carassius | 1 | [103] |
| C. rudolphii D and E | Black cormorant/Phalacrocorax carbo Pied cormorant/Phalacrocorax varius | 1 | [104] |
| C. pyripapillatum | Australian pelican/Pelecanus conspicillatus | 2 | [105] |
| C. rudolphii F | American brown pelican/Pelecanus occidentalis | 34 | [30] |
| C. septentrionale | Neotropic cormorant/Phalacrocorax brasilianus | 1 | [97] |
| C. variegatum | Australian darter/Anhinga melanogaster Australian pelican/Pelecanus conspicillatus | 31 | [23] |
| Mawsonascaris australis | Brown guitarfish/Rhinobatos schlegelii | 4 | [106] |
| M. vulvolacinata | Cowtail stingray/Pastinachus sephen | 35 | [107] |
| Phocascaris crystophorae | Northern fur seal/Callorhinus ursinus | 25 | [17] |
| *** Pseudoterranova azarazi | Northern fur seal/Callorhinus ursinus | 25 | [19] |
| Steller’s sea lion/Eumetopias jubatus Californian sea lion/Zalophus californianus Harbor seal/Phoca vitulina richardsii Bearded seal/Erignathus barbatus | 11 | [22] | |
| P. bulbosa | White whale/Delphinapterus leucas | 24 | [20] |
| Bearded seal/Erignathus barbatus | 4 | [22] | |
| *** P. cattani | South American sea lion/Otaria flavescens (Otaria byronia) | 40 | [25] |
| South American sea lion/Otaria flavescens (Otaria byronia) | 1 | [26] | |
| South American sea lion/Otaria flavescens (Otaria byronia) | 41 | [22] | |
| *** P. decipiens (Sensu stricto) | Atlantic cod/Gadus morhua Pacific cod/Gadus macrocephalus | 42 | [108] |
| Spinner dolphin/Stenella longirostris | 28 | [16] | |
| White whale/Delphinapterus leucas | 24 | [20] | |
| Pink ear emperor/Lethrinus lentjan | 7 | [109] | |
| Californian sea lion/Zalophus californianus Harbor seal/Phoca vitulina richardsii Harbor seal/Phoca vituline Grey seal/Halichoerus grypus Hooded seal/Cystophora cristata Norhern elephant seal/Mirounga angustirostris | 7 | [22,109] | |
| *** P. krabbei | Atlantic cod/Gadus morhua Atlantic horse mackerel/Trachurus trachurus | 43 | [110,111] |
| Harbor seal/Phoca vituline Grey seal/Halichoerus grypus | 7 44 | [22] | |
| Pulchrascaris australis n. sp. | Scalloped hammerhead/Sphyrna lewini | 5 | [22] |
| P. chiloscyllii | Brownbanded bambooshark/Chiloscyllium punctatum Blacktip reef shark/Carcharinus melanopterus Gummy shark/Mustelus antarcticus Scalloped hammerhead/Sphyrna lewini Smooth hammerhead/Sphyrna zygaena Whitetip reef shark/Triaenenodon obesus | 8 45 46 | [22] |
| Terranova caballeroi | Green water snake/Nerodia cyclopion | 47 | [112] |
| T. galeocerdonis | Sand tiger shark/Carcharias taurus | 37 | [27,28] |
| Redbelly yellowtail fusilier/Caesio cuning Lowly trevally/Caranx ignobilis Shark mackerel/Grammatorcynus bicarinatus Mangrove red snapper/Lutjanus argentimaculatus Stripey sea perch/Lutjanus carponotatus | 35 | [22] | |
| Tiger shark/Galeocerdo cuvier Scalloped hammerhead/Sphyrna lewini Smooth hammerhead/S. zygaena Blacktai reef shark/Carcharinus amblyrhynchos | 43 48 49 | [22] | |
| T. pectinolabiata | Great hammerhead shark/Sphyrna mokarran | 32 31 | [113] |
| Sulcascaris sulcata | Bivalve mollusks | 20 | [111] |
| Mediterranean mussel/Mytilus galloprovincialis | 43 | [114] |
| Map | Localization/Continent | Map | Localization/Continent | Map | Localization/Continent |
|---|---|---|---|---|---|
| 1 | Australia/Oceania | 18 | North of Morocco/Africa | 35 | Coast of Argentina/South America |
| 2 | Victoria Australia/Oceania | 19 | Taiwanese waters/Asia | 36 | Aegean Sea, Turkey/Europe and Asia |
| 3 | St. Paul Island, Alaska/North America | 20 | Senegal/Africa | 37 | Argentina, Sea of Patagonia/South America |
| 4 | Japanese waters/Asia | 21 | Mediterranean coast, Spain/Europe and Africa | 38 | Alexandria City, Mediterranean Sea, Egypt/Africa |
| 5 | Water Australia/Oceania | 22 | Brazilian waters/South America | 39 | Minas Gerais/South America |
| 6 | Mediterranean sea/Europe, Africa and Asia | 23 | Baltic Sea/Europe | 40 | South east Chilean coast/South America |
| 7 | Atlantic Ocean | 24 | Hudson Bay and Hudson Strait, Canada/North America | 41 | Hurghada City, Gulf of Suez, Red Sea, Egypt/Africa |
| 8 | South Africa/African | 25 | Caspian Sea/between Europe and Asia | 42 | Tyrrhenian coast of southern Italy/Europe |
| 9 | Macquarie Island, Pacific Ocean southwest/Asia and Oceania | 26 | Rio de Janeiro, Brazil/South America | 43 | Faeroe Islands/ Europe |
| 10 | New Caledonia/Oceania | 27 | Indonesia/Asia | 44 | Halfway Island, Australia/Oceania |
| 11 | Philippine archipelago/Asia | 28 | Madeiran waters Portugal/African | 45 | Hawaii/North America |
| 12 | Northern Adriatic Sea/Europe | 29 | Chile/South America | 46 | Louisiana, USA/North America |
| 13 | Zhoushan, Zhejiang, China/Asia | 30 | Colombia/South America | 47 | Twynams Paar, Ceylon, South Australia and Queensland/Oceania |
| 14 | Republic of Korea/Asia | 31 | Southern New South Wales, Australia/Oceania | 48 | Natal, northern, Brazil/South America |
| 15 | Yellow Sea/Asia | 32 | Heron Island, Queensland. Australia/Oceania | ||
| 16 | Sicily and Messina/Italy/Europe | 33 | Greek waters/Europe | ||
| 17 | China Sea/Asia | 34 | Northern Gulf of Mexico/Central America |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ángeles-Hernández, J.C.; Gómez-de Anda, F.R.; Reyes-Rodríguez, N.E.; Vega-Sánchez, V.; García-Reyna, P.B.; Campos-Montiel, R.G.; Calderón-Apodaca, N.L.; Salgado-Miranda, C.; Zepeda-Velázquez, A.P. Genera and Species of the Anisakidae Family and Their Geographical Distribution. Animals 2020, 10, 2374. https://doi.org/10.3390/ani10122374
Ángeles-Hernández JC, Gómez-de Anda FR, Reyes-Rodríguez NE, Vega-Sánchez V, García-Reyna PB, Campos-Montiel RG, Calderón-Apodaca NL, Salgado-Miranda C, Zepeda-Velázquez AP. Genera and Species of the Anisakidae Family and Their Geographical Distribution. Animals. 2020; 10(12):2374. https://doi.org/10.3390/ani10122374
Chicago/Turabian StyleÁngeles-Hernández, Juan C., Fabian R. Gómez-de Anda, Nydia E. Reyes-Rodríguez, Vicente Vega-Sánchez, Patricia B. García-Reyna, Rafael G. Campos-Montiel, Norma L. Calderón-Apodaca, Celene Salgado-Miranda, and Andrea P. Zepeda-Velázquez. 2020. "Genera and Species of the Anisakidae Family and Their Geographical Distribution" Animals 10, no. 12: 2374. https://doi.org/10.3390/ani10122374
APA StyleÁngeles-Hernández, J. C., Gómez-de Anda, F. R., Reyes-Rodríguez, N. E., Vega-Sánchez, V., García-Reyna, P. B., Campos-Montiel, R. G., Calderón-Apodaca, N. L., Salgado-Miranda, C., & Zepeda-Velázquez, A. P. (2020). Genera and Species of the Anisakidae Family and Their Geographical Distribution. Animals, 10(12), 2374. https://doi.org/10.3390/ani10122374





