Microstructure of the Surface of the Tongue and Histochemical Study of the Lingual Glands of the Lowland Tapir (Tapirus terrestris Linnaeus, 1758) (Perissodactyla: Tapiridae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of the Tongue
2.2. Histological and Histochemical Analysis
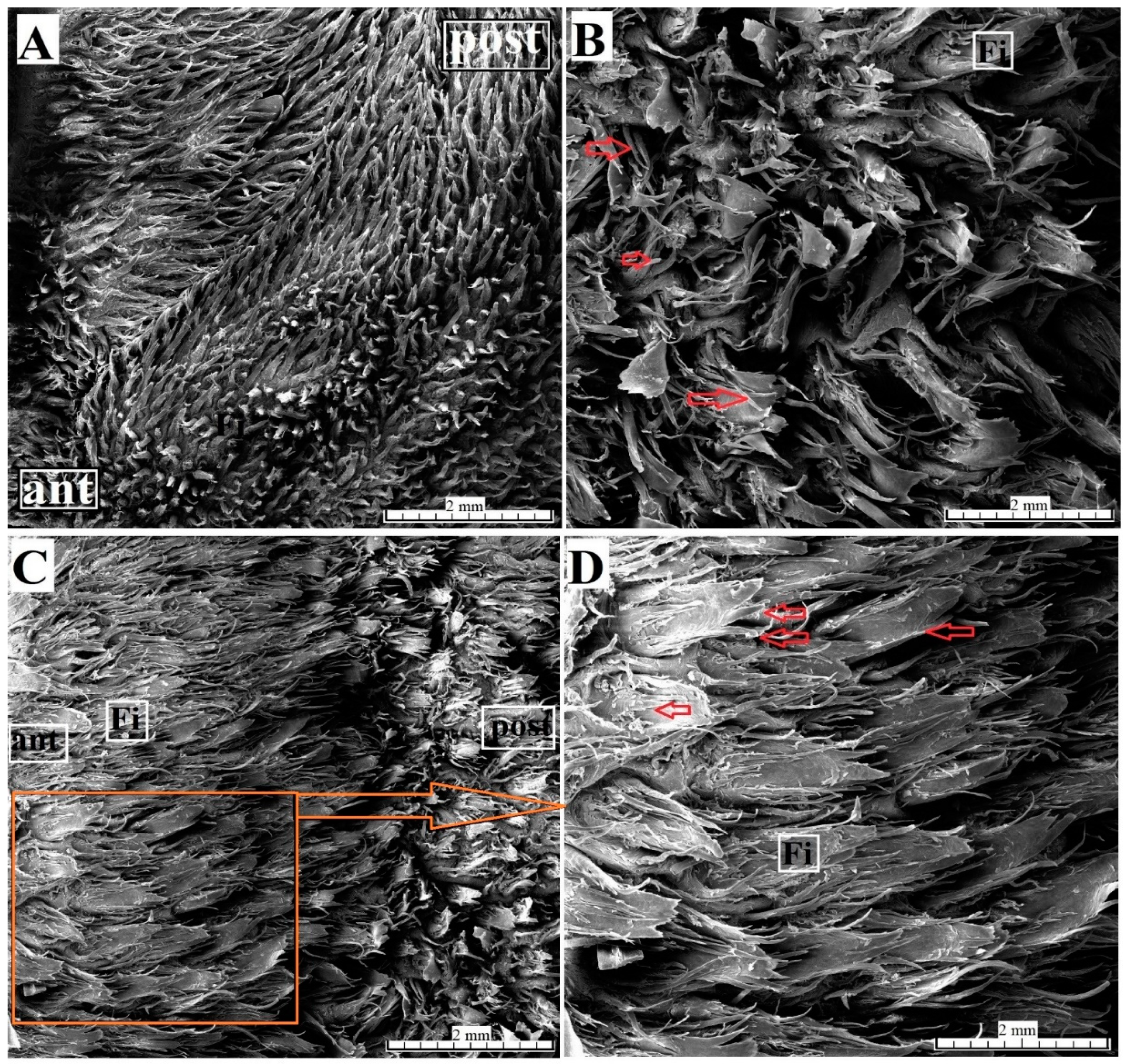
2.3. Ultrastructural Analysis—Scanning Electron Microscopy
3. Results
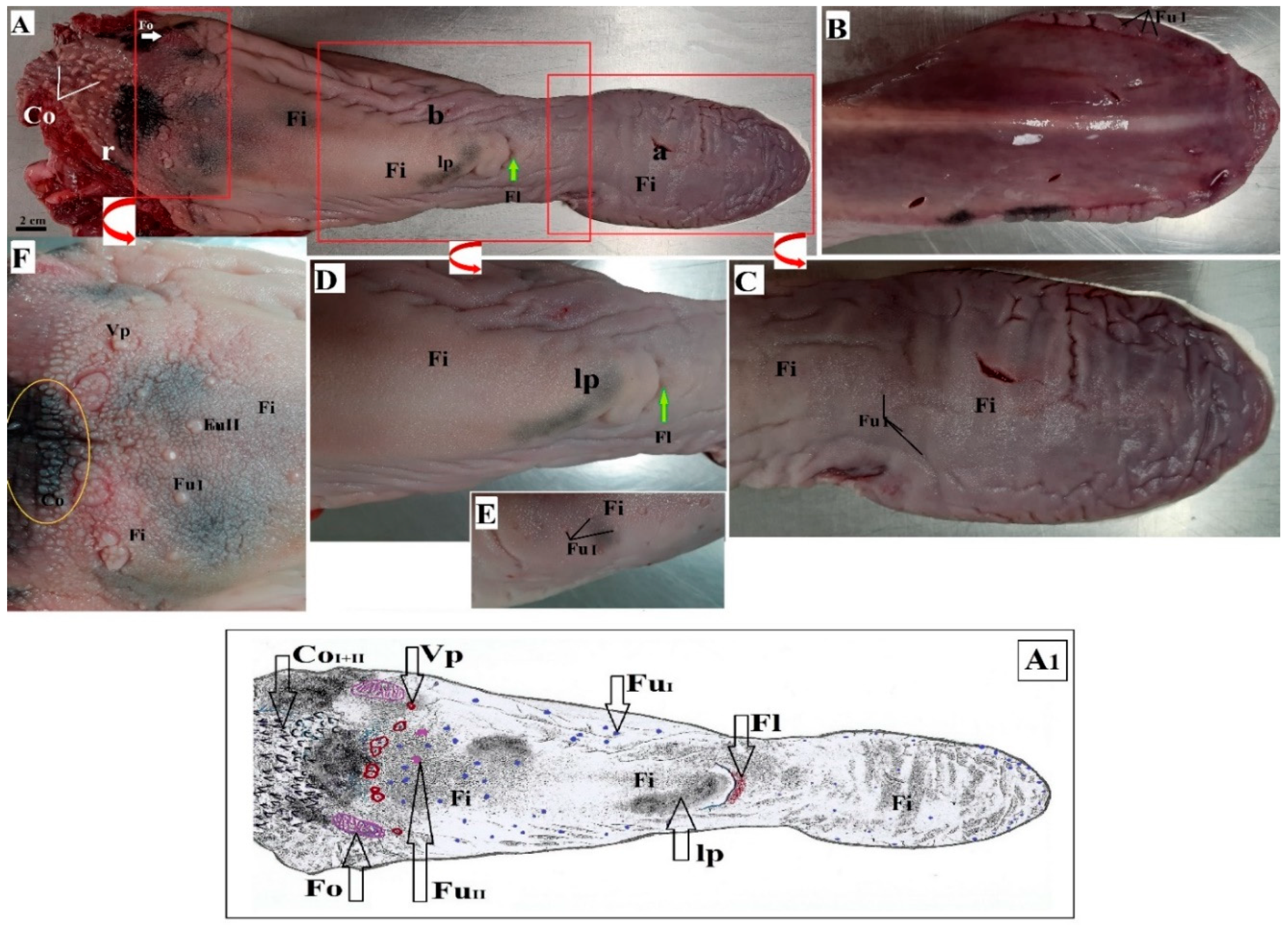
3.1. Macroscopic Findings of the Surface of the Tongue
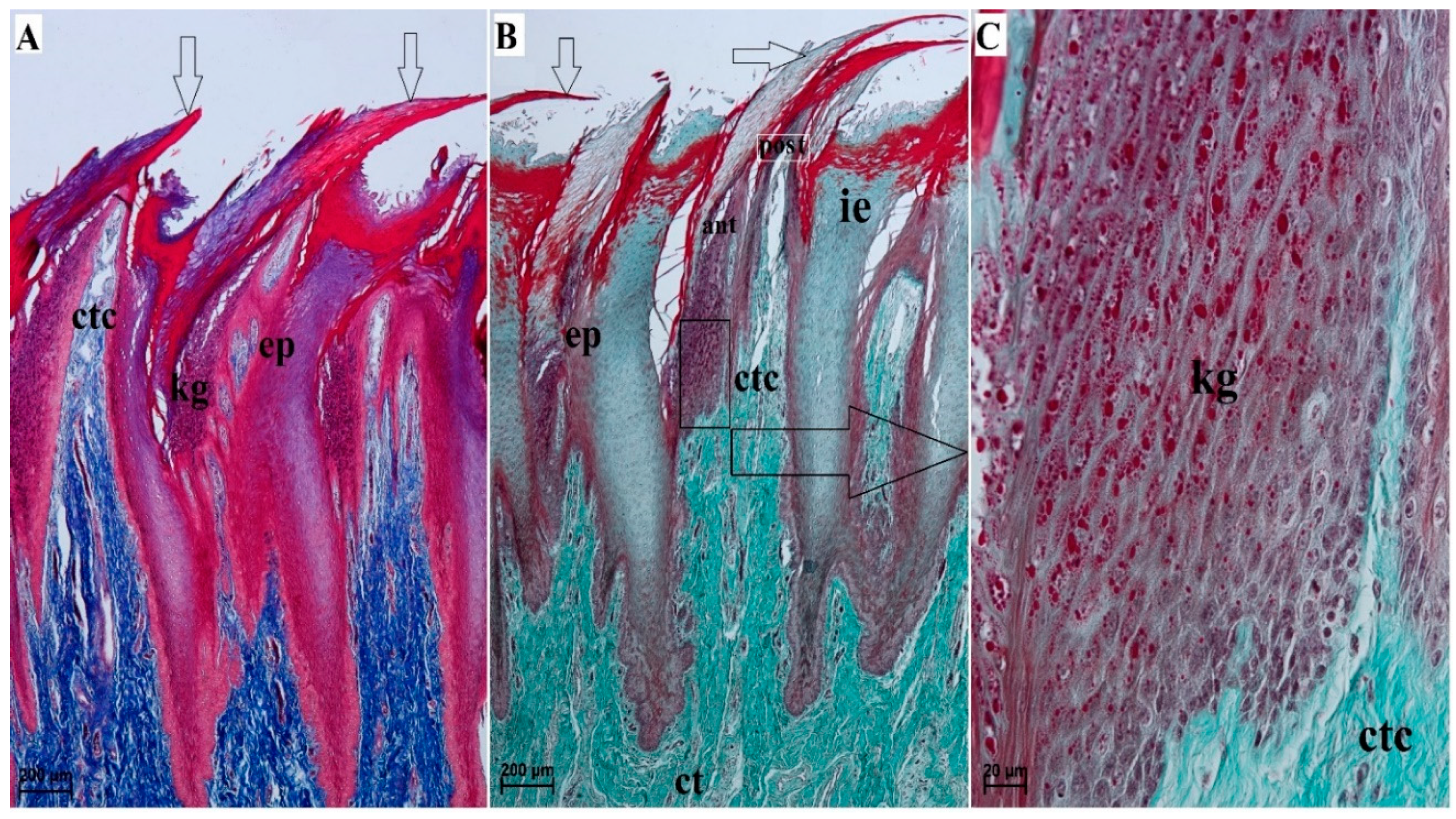
3.2. Lingual Papillae—Histological Analysis and SEM Observations
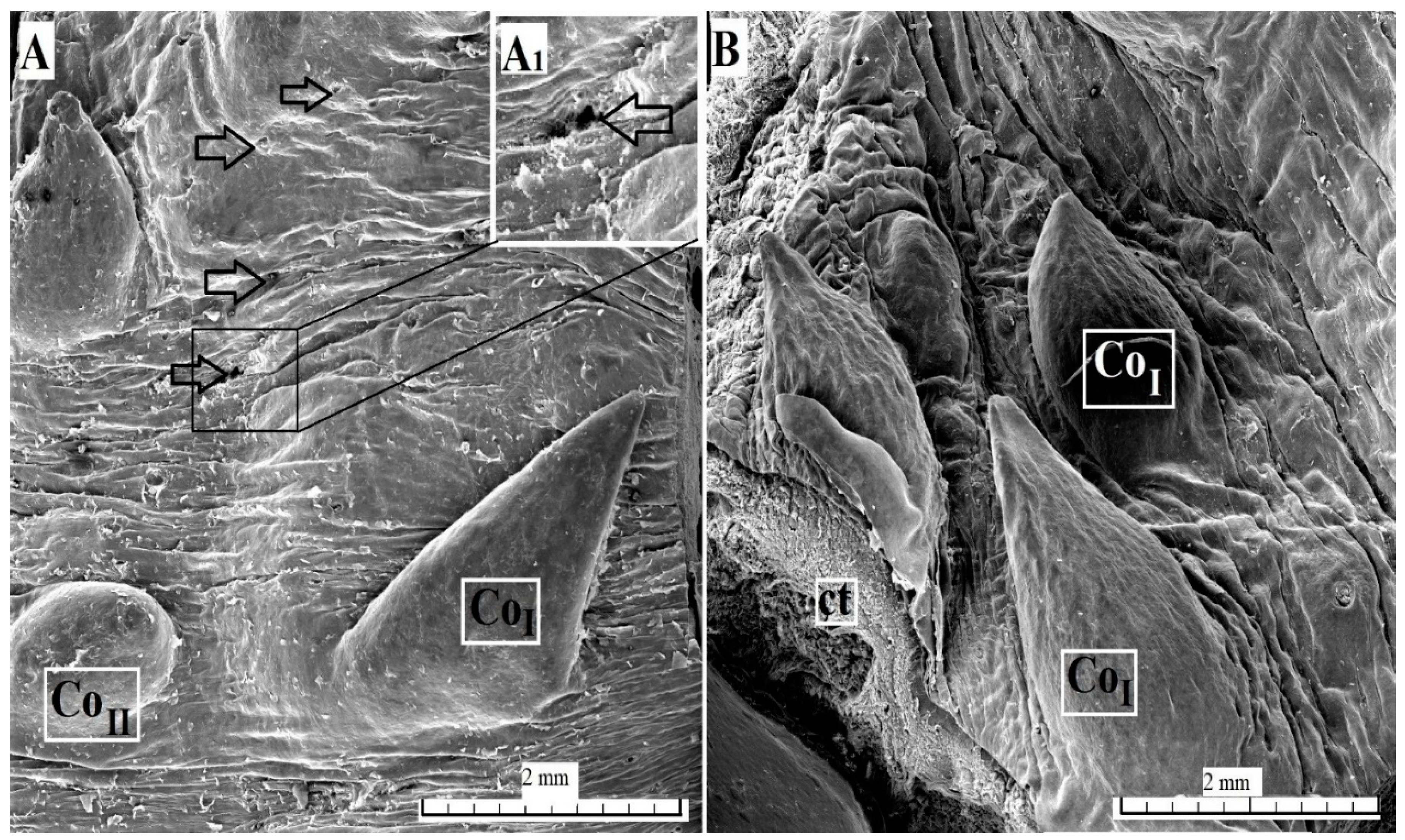
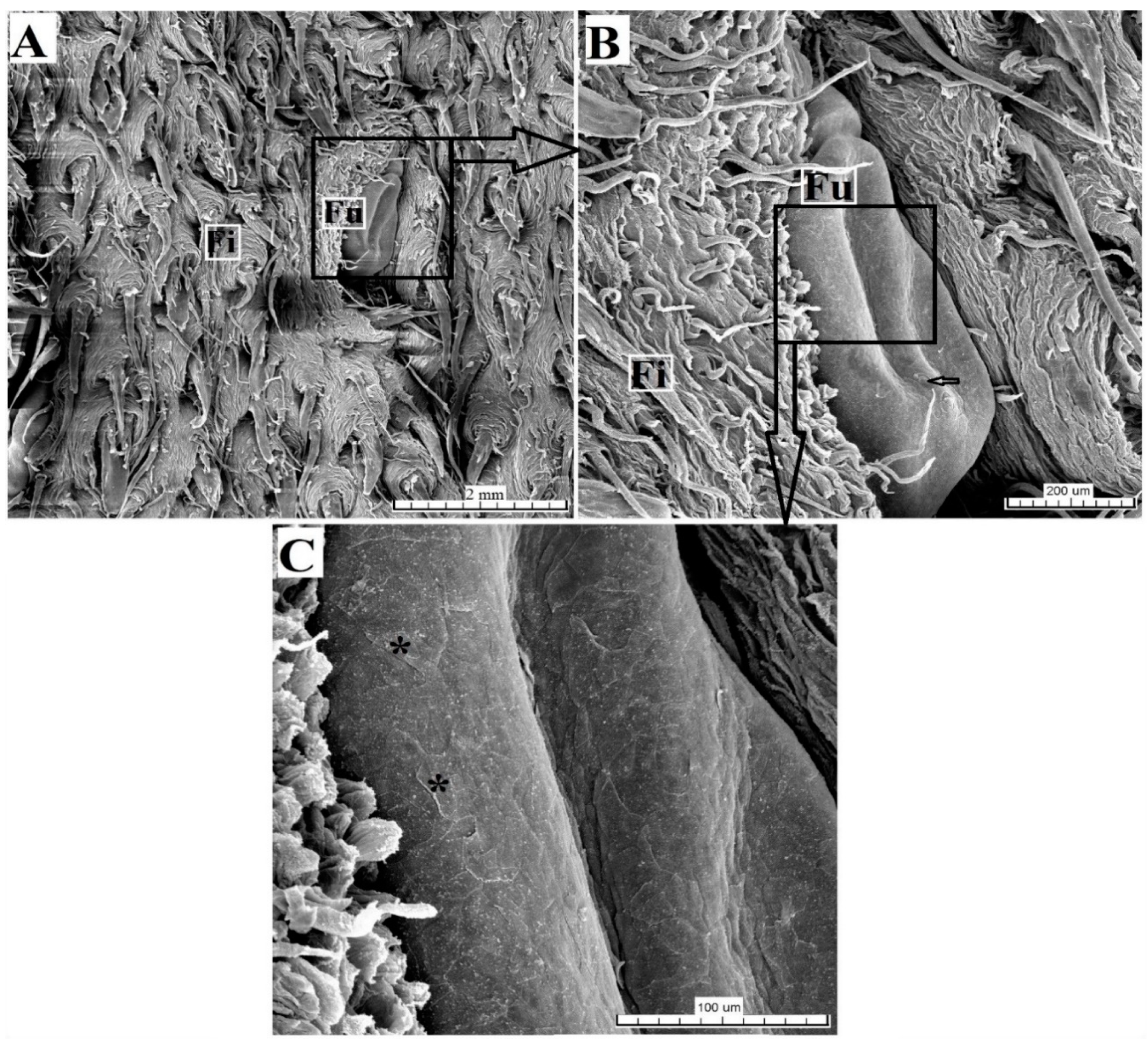
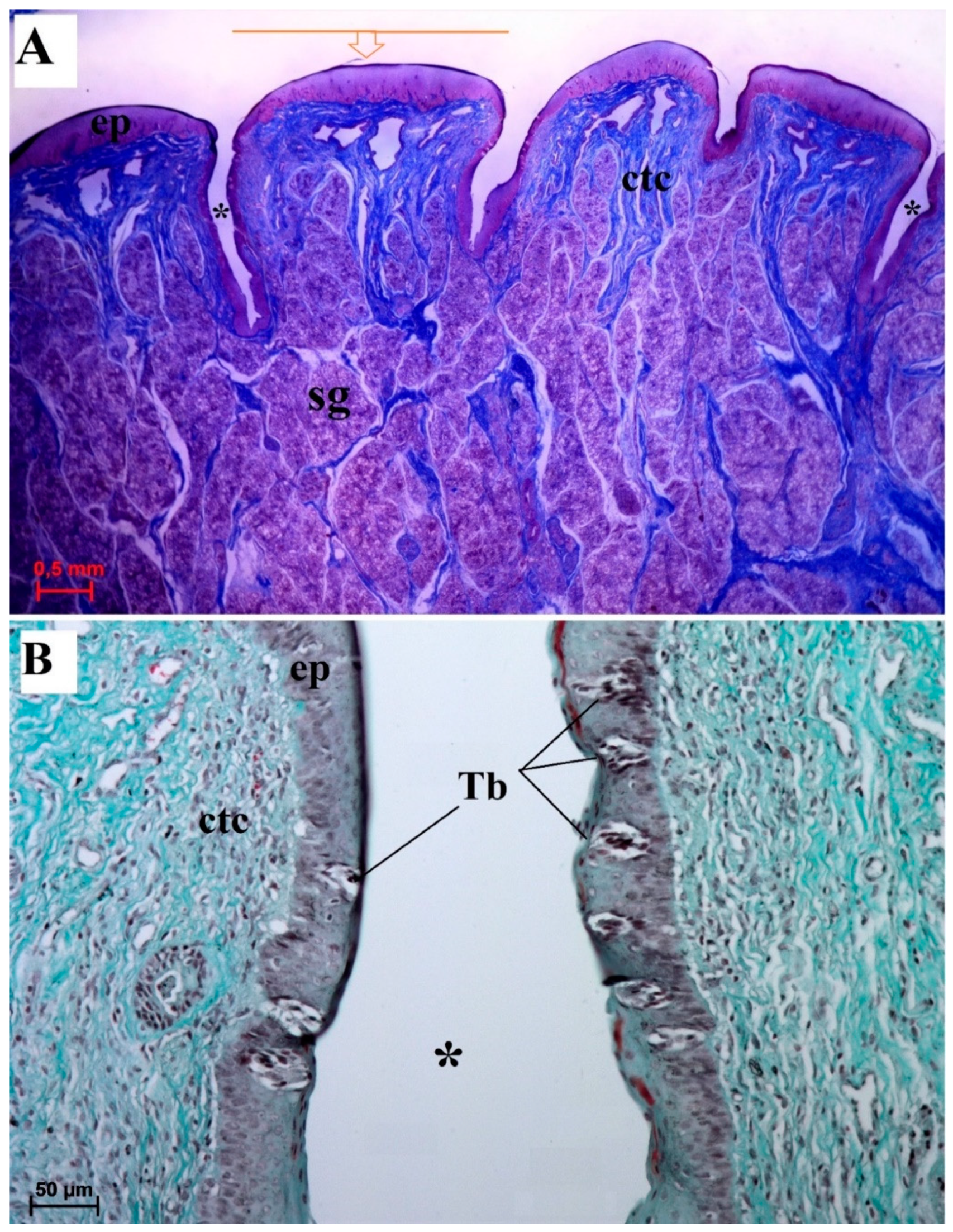
3.2.1. Filiform Papillae
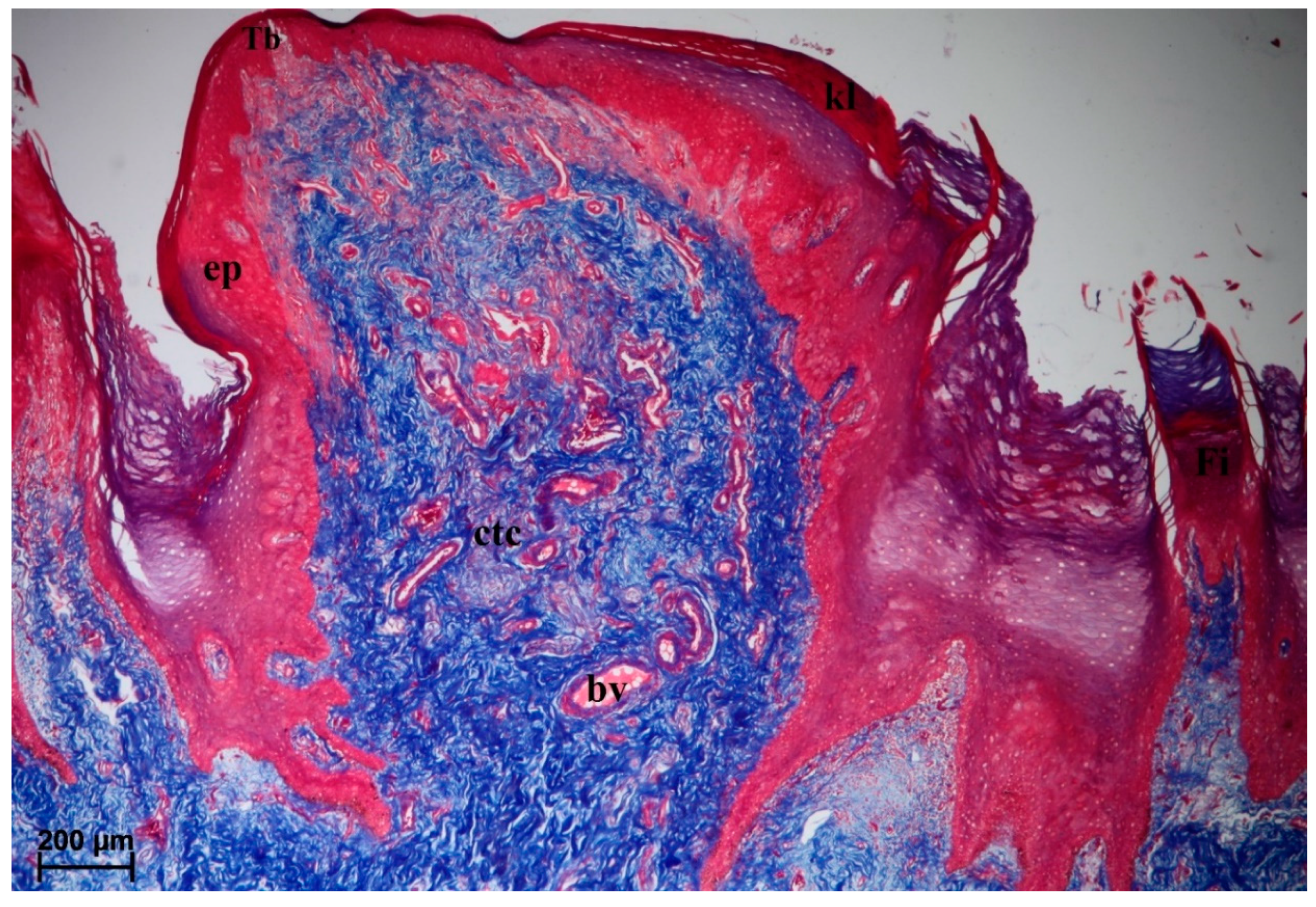
3.2.2. Conical Papillae
3.2.3. Fungiform Papillae
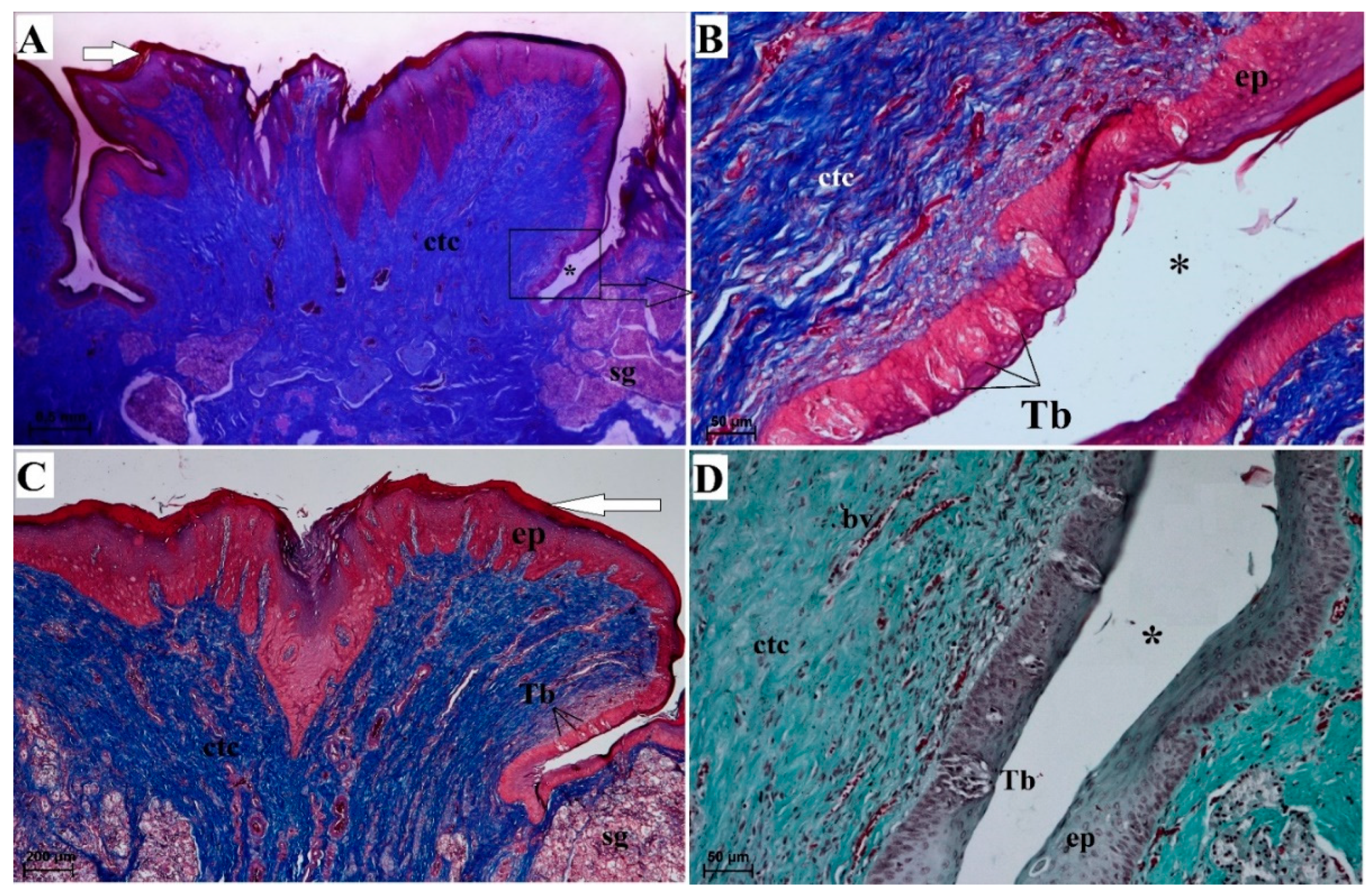
3.2.4. Vallate Papillae
3.2.5. Foliate Papillae
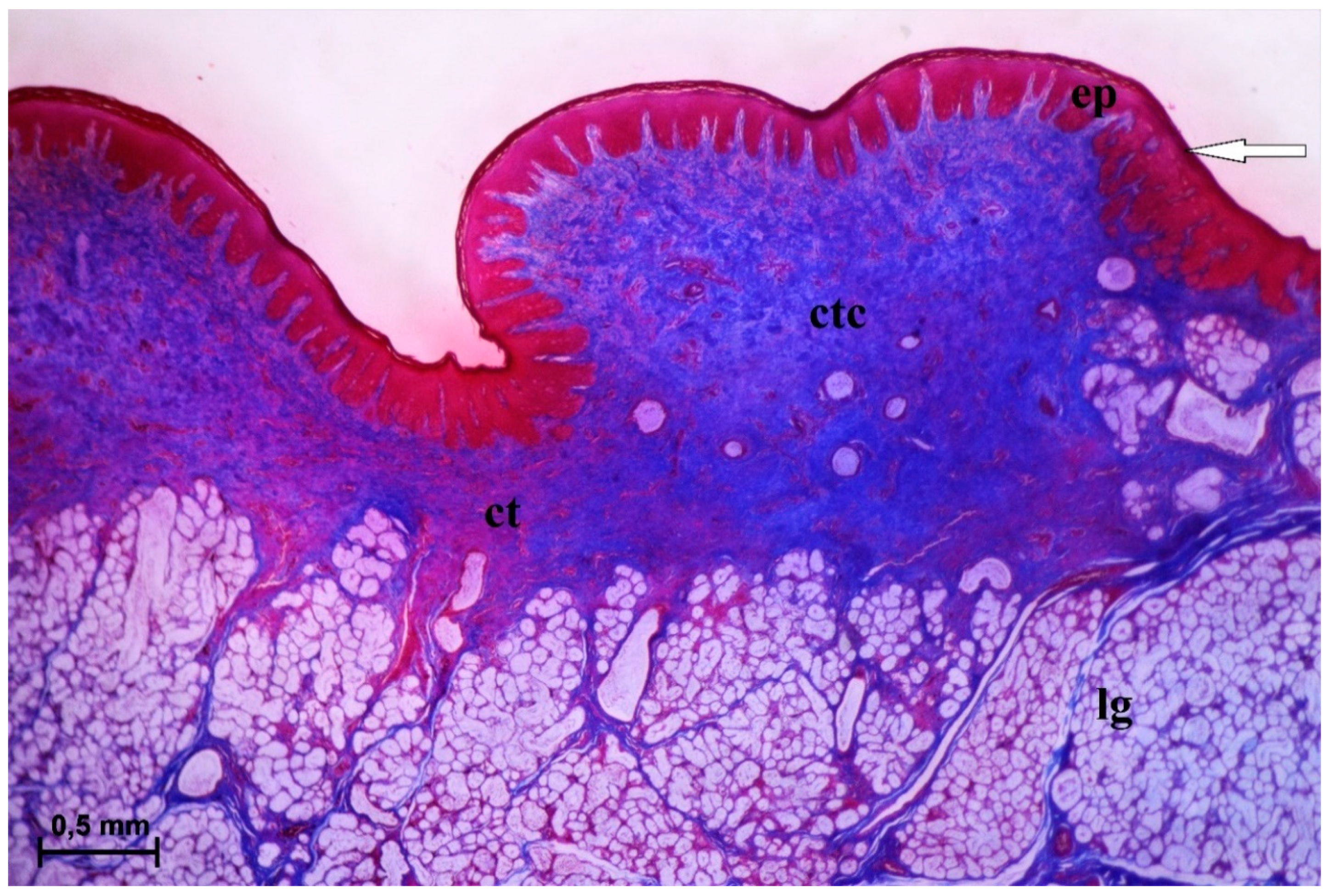
3.2.6. Lingual Glands—Histochemical Analysis
4. Discussion
4.1. The Tapiridae Tongue Versus the Tongue of Equidae and Rhinocerotidae—Similarities and Differences
4.2. The Tongue of the Tapirus Terrestris in Relation to a Diverse Diet in Chosen Mammals
4.2.1. Folivorous and Graminivorous Diet
4.2.2. Frugivorous Diet
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordeiro, J.L.; Fragoso, J.M.; Crawshaw, D.; Oliveira, L.F. Lowland tapir distribution and habitat loss in South America. PeerJ 2016, 13, e2456. [Google Scholar] [CrossRef][Green Version]
- García, M.J.; Medici, E.P.; Naranjo, E.J.; Novarino, W.; Leonardo, R.S. Distribution, habitat and adaptability of the genus Tapirus. Integr. Zool. 2012, 7, 346–355. [Google Scholar]
- Varela, D.; Flesher, K.; Cartes, J.L.; de Bustos, S.; Chalukian, S.; Ayala, G.; Richard-Hansen, C. Tapirus Terrestris. The IUCN Red List of Threatened Species. 2019, p. e.T21474A45174127. Available online: https://www.iucnredlist.org/species/21474/45174127 (accessed on 4 December 2020).
- Tobler, M.W.; Janovec, J.P.; Cornejo, F. Frugivory and seed dispersal by the lowland tapir Tapirus Terrestris in the Peruvian Amazon. Biotropica 2010, 42, 215–222. [Google Scholar] [CrossRef]
- Chalukian, S.C.; de Bustos, M.S.; Lizárraga, R.L. Diet of lowland tapir (Tapirus terrestris) in El Rey National Park, Salta, Argentina. Integr. Zool. 2013, 8, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Freeland, W.J.; Janzen, D.H. Strategies in herbivory by mammals: The role of plant secondary compounds. Am. Nat. 1974, 108, 269–289. [Google Scholar] [CrossRef]
- Coley, P.D.; Lokvam, J.; Rudolph, K.; Bromberg, K.; Sackett, T.E.; Wright, L.; Brenes-Arguedas, T.; Dvorett, D.; Ring, S.; Clark, A.; et al. Divergent defensive strategies of young leaves in two species of Inga. Ecology 2005, 86, 2633–2643. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; Silva, A.; Campos-Arceiz, A.; Zavada, M.S. On tapir ecology, evolution and conservation: What we know and future perspectives-part II. Integr. Zool. 2013, 8, 1–3. [Google Scholar] [CrossRef]
- Hibert, F.; Sabatier, D.; Andrivot, J.; Scotti-Saintagne, C.; Gonzalez, S.; Prévost, M.-F.; Grenand, P.; Chave, J.; Caron, H.; Richard-Hansen, C. Botany, genetics and ethnobotany: A crossed investigation on the elusive tapir’s diet in French Guiana. PLoS ONE 2011, 6, e25850. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.R.; Bobrowiec, P.E.D.; Sanaiotti, T.M.; Gribel, R. Seed germination from lowland tapir (Tapirus terrestris) fecal samples collected during the dry season in the Northern Brazilian Amazon. Integr. Zool. 2013, 8, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hibert, F.; Taberlet, P.; Chave, J.; Scotti-Saintagne, C.; Sabatier, D.; Richard-Hansen, C. Unveiling the diet of elusive rainforest herbivores in next generation sequencing era? The tapir as a case study. PLoS ONE 2013, 8, e60799. [Google Scholar] [CrossRef]
- Bizerril, M.X.; Rodrigues, F.H.; Hass, A. Fruit consumption and seed dispersal of Dimorphandra mollis Benth. (Leguminosae) by the lowland tapir in the cerrado of Central Brazil. Braz. J. Biol. 2005, 65, 407–413. [Google Scholar] [CrossRef] [PubMed]
- O’Farrill, G.; Galetti, M.; Campos-Arceiz, A. Frugivory and seed dispersal by tapirs: An insight on their ecological role. Integr. Zool. 2013, 8, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Witmer, L.M.; Sampson, S.C.; Solounias, N. The proboscis of tapirs (Mammalia: Perissodactyla): A case study in novel narial anatomy. J. Zool. 1999, 249, 249–267. [Google Scholar] [CrossRef]
- Milewski, A.V.; Dierenfeld, E.S. Structural and functional comparison of the proboscis between tapirs and other extant and extinct vertebrates. Integr. Zool. 2013, 8, 84–94. [Google Scholar] [CrossRef]
- Hagen, K.; Müller, D.W.H.; Wibbelt, G.; Ochs, A.; Hatt, J.-M.; Clauss, M. The macroscopic intestinal anatomy of a lowland tapir (Tapirus terrestris). Eur. J. Wildl. Res. 2014, 61, 171–176. [Google Scholar] [CrossRef]
- Abd-Elnaeim, M.M.; Zayed, A.E.; Leiser, R. Morphological characteristics of the tongue and its papillae in the donkey (Equus asinus): A light and scanning electron microscopical study. Ann. Anat. 2002, 184, 473–480. [Google Scholar] [CrossRef]
- Can, M.; Atalgin, Ş.H.; Aydin, M.F. Scanning electron microscopic studies of the lingual papillae in the English horse. Acta Vet. 2016, 66, 257–264. [Google Scholar] [CrossRef]
- Emura, S. Morphology of the lingual papillae of the Chapman’s zebra (Equus quagga chapmani). Okajimas Folia Anat. Jpn. 2018, 95, 15–18. [Google Scholar] [CrossRef]
- Emura, S.; Tamada, A.; Hayakawa, D.; Chen, H.; Shoumura, S. Morphology of the dorsal lingual papillae in the black rhinoceros (Diceros bicornis). Anat Histol. Embryol. 2000, 29, 371–374. [Google Scholar] [CrossRef]
- Jackowiak, H.; Jerbi, H.; Skieresz-Szewczyk, K.; Prozorowska, E. LM and SEM studies on tongue and lingual papillae in the donkey (Equus asinus). In Microscopy and Imaging Science: Practical Approaches to Applied Research and Education, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2017; pp. 216–222. [Google Scholar]
- Jackowiak, H.; Frackowiak, H.; Yoshimura, K.; Kumakura, M.; Kobayashi, K. Comparative morphological study on the tongue and lingual papillae of horses (Perissodactyla) and selected ruminantia (Artiodactyla). Ital. J. Anat. Embryol. 2005, 110, 55–63. [Google Scholar]
- Cave, A.J.E. Observations on rhinoceros tongue morphology. J. Zool. 1977, 181, 265–284. [Google Scholar] [CrossRef]
- Sonntag, C.F. The comparative anatomy of the tongues of the mammalia, VII: Cetacea, Sirenia and Ungulata. Proc. Zool. Soc. Lond. 1922, 92, 639–657. [Google Scholar] [CrossRef]
- Sheenan, D.C.; Hrapchak, B.B. Theory and Practice Histotechnology, 2nd ed.; CV Mosby: St. Louis, MO, USA, 1984; pp. 164–167. [Google Scholar]
- Munakata, H.; Isemur, M.; Yosizawa, Z. An application of the high-iron diamine staining for detection of sulfated glycoproteins (glycopeptides) in electrophoresis on cellulose acetate membrane. Tohoku J. Exp. Med. 1985, 145, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.S.; Henson, J.G. Methods for localizing mucosubstances in epithelial and connective tissue. In Methods and Achievements in Experimental Pathology; Jasmin, E., Bajusz, G., Eds.; Karger Press: Basel, Switzerland, 1967; pp. 78–112. [Google Scholar]
- Cizek, P.; Hamouzova, P.; Jekl, V.; Kvapil, P.; Tichy, F. Light and scanning electron microscopy of the tongue of a degu (Octodon degus). Anat. Sci. Int. 2017, 92, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Nomina Anatomica Veterinaria. Prepared by International Committee on Veterinary Gross Anatomical Nomenclature, 6th ed.; The Editorial Committee: Hanover, Germany; Ghent, Belgium; Columbia, MO, USA; Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Nomina Histologica Veterinaria. Submitted by the International Committee on Veterinary Histological Nomenclature to the World Association of Veterinary Anatomists, 1st ed.; WAVA: Arlington, VA, USA, 2017. [Google Scholar]
- Pfeiffer, C.J.; Levin, M.; Lopes, M.A.F. Ultrastructure of the horse tongue: Further observations on the lingual integumentary architecture. Anat. Histol. Embryol. 2000, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, C.A.; de Paz, P.; Sandoval, J.; Fernandez, J.G. Comparative scanning electron microscopic study of the lingual papillae in two species of domestic mammals (Equus caballus and Bos taurus). I. Gustatory papillae. Acta Anta 1988, 132, 120–123. [Google Scholar] [CrossRef]
- Gargiulo, A.M.; Pedini, V.; Ceccarelli, P.; Lorvik, S. A Lectin histochemical study of gustatory (von Ebner’s) glands of the horse tongue. Anat. Hist. Embryol. 1995, 24, 123–126. [Google Scholar] [CrossRef]
- Adnyane, I.K.M.; Zuki, A.B.; Noordin, M.M.; Agungpriyono, S. Morphological study of the lingual papillae in the barking deer, Muntiakus muntijak. Anat. Histol. Embryol. 2011, 40, 73–77. [Google Scholar] [CrossRef]
- Emura, S.; Tamada, A.; Hayakawa, D.; Chen, H.; Yano, R.; Shoumura, S. Morphology of the dorsal lingual papillae in the blackbuck (Antilope cervicapra). Okajimas Folia Anat. Jpn. 1999, 74, 247–253. [Google Scholar] [CrossRef]
- Emura, S.; Tamada, A.; Hayakawa, D.; Chen, H.; Shoumura, S. Morphology of the dorsal lingual papillae in the Barbary sheep, Ammotragus lervia. Okajimas Folia Anat. Jpn. 2000, 77, 39–45. [Google Scholar] [CrossRef]
- Emura, S.; Okumura, T.; Chen, H. Morphology of the dorsal lingual papillae in the sitatunga. Okajimas Folia Anat. Jpn. 2011, 88, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Agungpriyono, S.; Yamada, J.; Kitamura, N.; Nisa, C.; Sigit, K.; Yamamoto, Y. Morphology of the dorsal lingual papillae in the lesser mouse deer, Tragulus javanicus. J. Anat. 1995, 187, 635–640. [Google Scholar] [PubMed]
- Emura, S.; Okumura, T.; Chen, H. Morphology of the lingual papillae in the giraffe. Okajimas Folia Anat. Jpn. 2013, 89, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Emura, S.; Ohsawa, S. Morphology of the lingual papillae of the bharal (Pseudois nayaur). Okajimas Folia Anat. Jpn. 2019, 96, 27–30. [Google Scholar] [CrossRef]
- Harem, M.K.; Harem, I.; Sari, E.K.; Aydin, M.F.; Aydın, M.F. Light and scanning electron microscopic study of the dorsal lingual papillae of the goitered gazelle (Gazelle subgutturosa). J. Anim. Vet. Adv. 2011, 10, 1906–1913. [Google Scholar] [CrossRef]
- Erdoğan, S.; Pérez, W. Anatomical and scanning electron microscopic studies of the tongue and lingual papillae in the chital deer (Axisaxis, Erxleben, 1777). Acta Zool. 2013, 95, 1–9. [Google Scholar]
- Erdoğan, S.; Pérez, W. Anatomical and scanning electron microscopic studies of the tongue in the pampas deer (Cervidae: Ozotoceros bezoarticus, Linnaeus 1758). Microsc. Res. Tech. 2013, 76, 1025–1034. [Google Scholar] [CrossRef]
- Butendieck, E.; Vargas, L. Presence and distribution of lingual papillae in alpaca (Lama pacos). Arch. Med. Vet. 1998, 30, 29–36. [Google Scholar] [CrossRef]
- Goździewska-Harłajczuk, K.; Klećkowska-Nawrot, J.; Janeczek, M.; Zawadzki, M. Morphology of the lingual and buccal papillae in alpaca (Vicugna pacos)-light and scanning electron microscopy. Anat. Histol. Embryol. 2015, 44, 345–360. [Google Scholar] [CrossRef]
- Eerdunchaolu, D.V.; Takehana, K.; Yamamoto, E.; Kobayashi, A.; Cao, G.; Baiyin; Ueda, H.; Tangkawattana, P. Characteristics of dorsal lingual papillae of the Bactrian camel (Camelus bactrianus). Anat. Histol. Embryol. 2001, 30, 147–151. [Google Scholar] [CrossRef]
- El Sharaby, A.A.; Alsafy, M.; El-Gendy, S.A.; Wakisaka, S. Morphological characteristics of the vallate papillae of the one-humped camel (Camelus dromedarius). Anat. Histol. Embryol. 2012, 41, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, S.; Sağsöz, H. Papillary architecture and functional charakterization of mucosubstances in the sheep tongue. Anat. Rec. 2018, 301, 1320–1335. [Google Scholar] [CrossRef] [PubMed]
- Goździewska-Harłajczuk, K.; Klećkowska-Nawrot, J.; Hamouzová, P.; Čižek, P. Microstructure of the tongue surface and lingual glands of the Sulawesi bear cuscus, Ailurops ursinus (Marsupialia: Phalangeridae)–a light and scanning electron microscopic study. Acta. Zool. 2020. in review. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kumakura, M.; Yoshimura, K.; Nonaka, K.; Murayama, T.; Henneberg, M. Comparative morphological study of the lingual papillae and their connective tissue cores of the koala. Anat. Embryol. 2003, 206, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.J.; Pícoli, L.C.; Guimarães, J.P.; Motoyama, A.A.; Miglino, M.A.; Watanabe, L.-S. Characteristics of filiform, fungiform and vallate papillae and surface of interface epithelium-connective tissue of the maned sloth tongue mucosa (Bradypus torquatus, Iliger, 1811): Light and scanning electron microscopy study. Anat. Histol. Embryol. 2009, 38, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Emura, S.; Hayakawa, D.; Chen, H.; Shoumura, S.; Atoji, Y.; Agungpriyono, S. SEM study on the dorsal lingual surface of the lesser dog-faced fruit bat, Cynopterus brachyotis. Okajimas Folia Anat. Jpn. 2001, 78, 123–128. [Google Scholar] [CrossRef][Green Version]
- Emura, S.; Hayakawa, D.; Chen, H.; Shoumura, S.; Atoji, Y.; Wijayanto, H. SEM study on the dorsal lingual surface of the large flying fox, Pteropus vampyrus. Okajimas Folia Anat. Jpn. 2002, 79, 113–119. [Google Scholar] [CrossRef][Green Version]
- Emura, S.; Okumura, T.; Chen, H. Morphology of the lingual papillae in the Egyptian rousette bat (Rousettus aegyptiacus). Okajimas Folia Anat. Jpn. 2012, 89, 61–66. [Google Scholar] [CrossRef][Green Version]


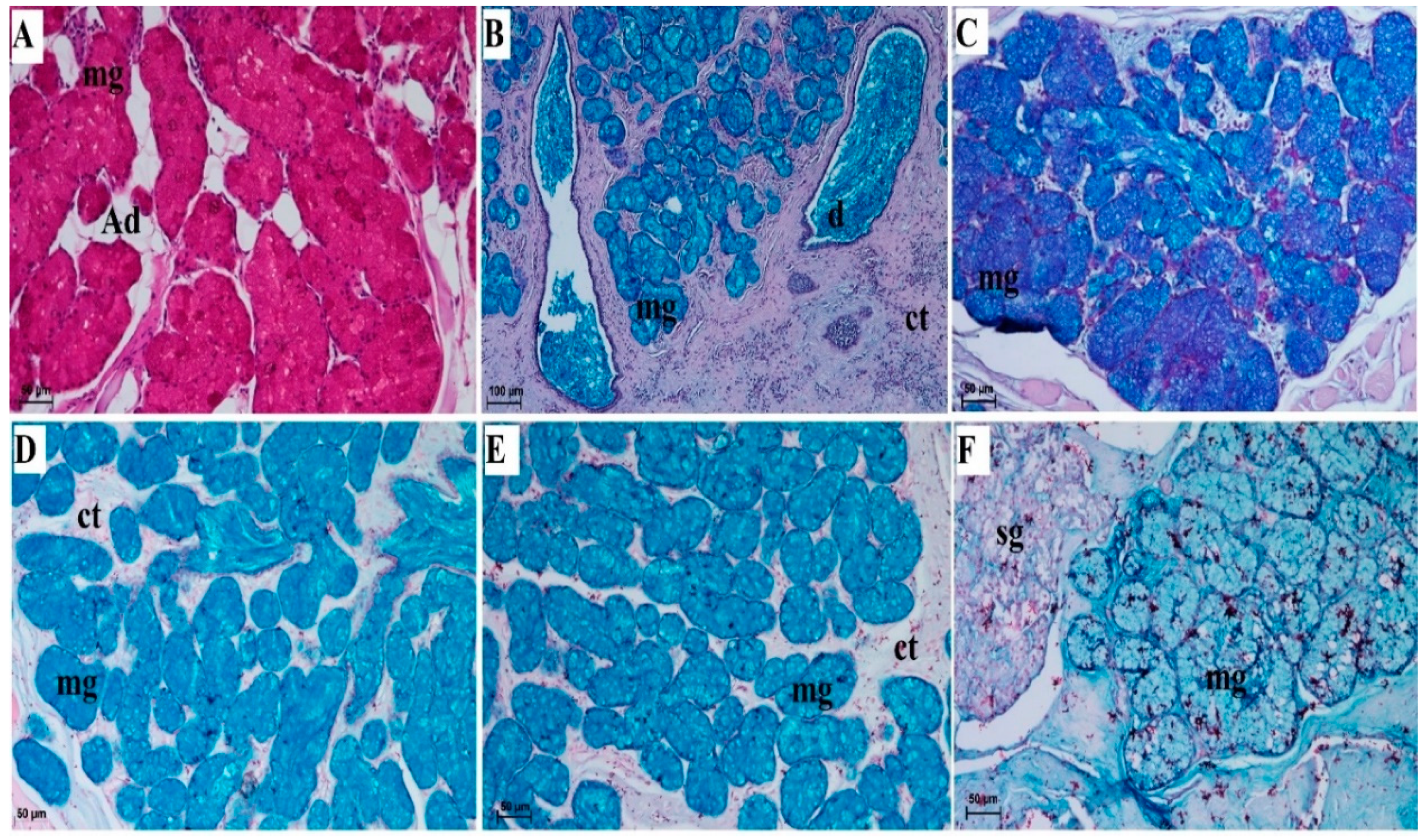
| Lingual Glands | Von Ebner’s Glands Beneath the Vallate Papillae and Foliate Papillae | Mucoserous Glands from Root of the Tongue | |
|---|---|---|---|
| Staining Method | |||
| PAS | - | +++ cells of mucous acini + cells of duct | |
| PAS-AB pH 2.5 | + cells of serous acini: bright magenta | +++ cells of mucous acini: blue ++ cells of duct: blue + cells of serous acini: bright magenta | |
| AB pH 1.0 | - | +++ cells of mucous acini + cells of duct | |
| AB pH 2.5 | - | +++ cells of mucous acini + cells of duct | |
| HDI | - | + cells of mucous acini | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goździewska-Harłajczuk, K.; Hamouzová, P.; Klećkowska-Nawrot, J.; Barszcz, K.; Čížek, P. Microstructure of the Surface of the Tongue and Histochemical Study of the Lingual Glands of the Lowland Tapir (Tapirus terrestris Linnaeus, 1758) (Perissodactyla: Tapiridae). Animals 2020, 10, 2297. https://doi.org/10.3390/ani10122297
Goździewska-Harłajczuk K, Hamouzová P, Klećkowska-Nawrot J, Barszcz K, Čížek P. Microstructure of the Surface of the Tongue and Histochemical Study of the Lingual Glands of the Lowland Tapir (Tapirus terrestris Linnaeus, 1758) (Perissodactyla: Tapiridae). Animals. 2020; 10(12):2297. https://doi.org/10.3390/ani10122297
Chicago/Turabian StyleGoździewska-Harłajczuk, Karolina, Pavla Hamouzová, Joanna Klećkowska-Nawrot, Karolina Barszcz, and Petr Čížek. 2020. "Microstructure of the Surface of the Tongue and Histochemical Study of the Lingual Glands of the Lowland Tapir (Tapirus terrestris Linnaeus, 1758) (Perissodactyla: Tapiridae)" Animals 10, no. 12: 2297. https://doi.org/10.3390/ani10122297
APA StyleGoździewska-Harłajczuk, K., Hamouzová, P., Klećkowska-Nawrot, J., Barszcz, K., & Čížek, P. (2020). Microstructure of the Surface of the Tongue and Histochemical Study of the Lingual Glands of the Lowland Tapir (Tapirus terrestris Linnaeus, 1758) (Perissodactyla: Tapiridae). Animals, 10(12), 2297. https://doi.org/10.3390/ani10122297






