Alleviation of the Adverse Effect of Dietary Carbohydrate by Supplementation of Myo-Inositol to the Diet of Nile Tilapia (Oreochromis niloticus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation and Experimental Fish
2.2. Sample Collection and Chemical Analysis
2.3. Methods of Measurement
2.3.1. Growth Performance and Body Composition
2.3.2. Proximate Composition
2.3.3. Histological Analysis
2.3.4. Biochemical Indicators
2.3.5. Gene Expression Analysis
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
3.1. Growth Performance and Morphometric Parameters
3.2. Whole-Body Proximate Composition
3.3. Parameters of Glycogen Content in Serum, Liver and Muscle
3.4. Histology and Vacuolization of the Cytoplasm Area in the Liver
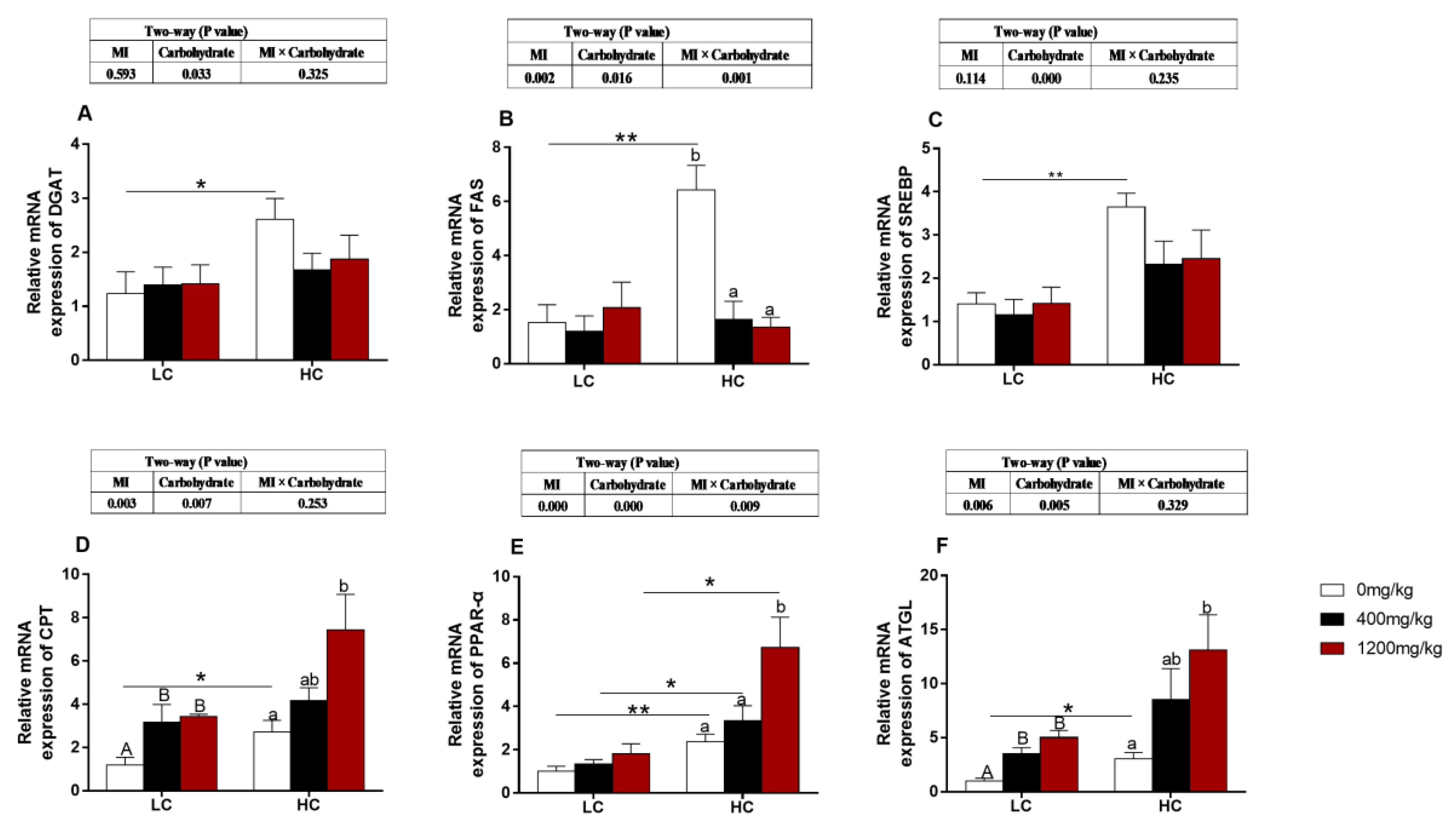
3.5. The Expression of Genes Related to Lipid Metabolism
3.6. The Expression of Carbohydrate-Metabolism-Related Genes
3.7. Serum Lipid Contents and Liver TG Content Parameters
3.8. Immune-Related and Antioxidative Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Wang, A.; Li, Z.; Zhang, J.; Sang, C.; Chen, N. Antioxidant defenses and non-specific immunity at enzymatic and transcriptional levels in response to dietary carbohydrate in a typical carnivorous fish, hybrid grouper (Epinephelus fuscoguttatus female symbol × E. lanceolatus male symbol). Fish Shellfish Immun. 2020, 100, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sang, C.; Wang, A.; Zhang, J.; Chen, N. Effects of dietary carbohydrate sources on growth performance, glycogen accumulation, insulin signaling pathway and hepatic glucose metabolism in largemouth bass, Micropterus salmoides. Aquaculture 2019, 513, 734390. [Google Scholar] [CrossRef]
- Liu, D.; Deng, K.; Sampath, W.; Gu, Z.; Pan, M.; Zhang, Y.; Zhang, W.; Mai, K. Responses of glucosensing system to glucose in Japanese flounder Paralichthys olivaceus fed diets with different carbohydrate content. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 232, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, A.; Abellan, E.; Arizcun, M.; Cardenete, G.; Morales, A.E.; Hidalgo, M.C. Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 203, 17–23. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, C.-T.; Qiao, F.; Wang, X.-D.; Qin, J.G.; Du, Z.-Y.; Chen, L.-Q. Gemfibrozil improves lipid metabolism in Nile tilapia Oreochromis niloticus fed a high-carbohydrate diet through peroxisome proliferator activated receptor-α activation. Gen. Comp. Endocr. 2020, 296, 113537. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Remo, S.C.; Wang, B.K.; Shi, H.J.; Zhang, L.; Liu, J.D.; Li, X.F. Feeding restriction alleviates high carbohydrate diet-induced oxidative stress and inflammation of Megalobrama amblycephala by activating the AMPK-SIRT1 pathway. Fish Shellfish Immun. 2019, 92, 637–648. [Google Scholar] [CrossRef]
- Khosravi, S.; Lim, S.-J.; Rahimnejad, S.; Kim, S.-S.; Lee, B.-J.; Kim, K.-W.; Han, H.-S.; Lee, K.-J. Dietary myo-inositol requirement of parrot fish, Oplegnathus fasciatus. Aquaculture 2015, 436, 1–7. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A.; Wang, X.; Huang, Z. Myo-inositol enhances the low-salinity tolerance of turbot (Scophthalmus maximus) by modulating cortisol synthesis. Biochem. Biophys. Res. Commun. 2020, 526, 913–919. [Google Scholar] [CrossRef]
- Bathena, S.P.; Huang, J.; Epstein, A.A.; Gendelman, H.E.; Boska, M.D.; Alnouti, Y. Rapid and reliable quantitation of amino acids and myo-inositol in mouse brain by high performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 893-894, 15–20. [Google Scholar] [CrossRef]
- Villalba, H.; Shah, K.; Albekairi, T.H.; Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Potential role of myo-inositol to improve ischemic stroke outcome in diabetic mouse. Brain Res. 2018, 1699, 166–176. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition-a review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef]
- Buccafusca, R.; Venditti, C.P.; Kenyon, L.C.; Johanson, R.A.; Van Bockstaele, E.; Ren, J.; Pagliardini, S.; Minarcik, J.; Golden, J.A.; Coady, M.J.; et al. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: Myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol. Genet. Metab. 2008, 95, 81–95. [Google Scholar]
- Peres, H.; Lim, C.; Klesius, P.H. Growth, chemical composition and resistance to Streptococcus iniae challenge of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of dietary inositol. Aquaculture 2004, 235, 423–432. [Google Scholar] [CrossRef]
- Wang, X.; Kultz, D. Osmolality/salinity-responsive enhancers (OSREs) control induction of osmoprotective genes in euryhaline fish. Proc. Natl. Acad. Sci. USA 2017, 114, E2729–E2738. [Google Scholar] [CrossRef]
- Shimada, M.; Ichigo, Y.; Shirouchi, B.; Takashima, S.; Inagaki, M.; Nakagawa, T.; Hayakawa, T. Treatment with myo-inositol attenuates binding of the carbohydrate-responsive element-binding protein to the ChREBP-beta and FASN genes in rat nonalcoholic fatty liver induced by high-fructose diet. Nutr. Res. 2019, 64, 49–55. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A. Transcriptome analysis provides insights into the effects of myo-inositol on the turbot Scophthalmus maximus. Fish Shellfish Immun. 2020, 106, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Lin, Z.; Liu, S.; Wang, C.; Wang, N.; Lei, Y.; Zhu, J.; Wang, X.; Qin, J.G.; Chen, L. Effects of myo-inositol on growth performance, body composition, antioxidant status, non-specific immunity and lipid metabolism of juvenile Chinese mitten crab (Eriocheir sinensis). Aquacult. Nutr. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Magouz, F.I.; Dawood, M.A.O.; Salem, M.F.I.; El-Ghandour, M.; Van Doan, H.; Mohamed, A.A.I. The role of a digestive enhancer in improving the growth performance, digestive enzymes activity, and health condition of Nile tilapia (Oreochromis niloticus) reared under suboptimal temperature. Aquaculture 2020, 526, 735388. [Google Scholar] [CrossRef]
- Han, S.-L.; Wang, J.; Li, L.-Y.; Lu, D.-L.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. The regulation of rapamycin on nutrient metabolism in Nile tilapia fed with high-energy diet. Aquaculture 2020, 520, 734975. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Qi, C.; Li, E.; Du, Z.; Qin, J.G.; Chen, L. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Schmitt, V.H.; Schmitt, C.; Hollemann, D.; Weinheimer, O.; Mamilos, A.; Kirkpatrick, C.J.; Brochhausen, C. Tissue expansion of lung bronchi due to tissue processing for histology—A comparative analysis of paraffin versus frozen sections in a pig model. Pathol. Res. Pract. 2019, 215, 152396. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, Q.; Zhou, B.; Wang, D.; Wu, R. Advantages of infrared transflection micro spectroscopy and paraffin-embedded sample preparation for biological studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immun. 2020, 102, 336–349. [Google Scholar] [CrossRef]
- Machado, M.; Castro, C.; Oliva-Teles, A.; Costas, B. Interactive effects of dietary vegetable oil and carbohydrate incorporation on the innate immune response of European seabass (Dicentrarchus labrax) juveniles subjected to acute stress. Aquaculture 2019, 498, 171–180. [Google Scholar] [CrossRef]
- Aparecida de Franca, S.; Pavani Dos Santos, M.; Nunes Queiroz da Costa, R.V.; Froelich, M.; Buzelle, S.L.; Chaves, V.E.; Giordani, M.A.; Pereira, M.P.; Colodel, E.M.; Marlise Balbinotti Andrade, C.; et al. Low-protein, high-carbohydrate diet increases glucose uptake and fatty acid synthesis in brown adipose tissue of rats. Nutrition 2014, 30, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.L.; Pereira, M.P.; Buzelle, S.L.; Dos Santos, M.P.; de Franca, S.A.; Baviera, A.M.; Andrade, C.M.; Garofalo, M.A.; Kettelhut Ido, C.; Chaves, V.E.; et al. A low-protein, high-carbohydrate diet increases de novo fatty acid synthesis from glycerol and glycerokinase content in the liver of growing rats. Nutr. Res. 2013, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhuang, Z.; Yin, P.; Chen, X.; Zhang, Y.; Tian, L.; Niu, J.; Liu, Y. Changes in growth performance, haematological parameters, hepatopancreas histopathology and antioxidant status of pacific white shrimp (Litopenaeus vannamei) fed oxidized fish oil: Regulation by dietary myo-inositol. Fish Shellfish Immun. 2019, 88, 53–64. [Google Scholar] [CrossRef] [PubMed]
- L’Abbate, S.; Nicolini, G.; Forini, F.; Marchetti, S.; Di Lascio, N.; Faita, F.; Kusmic, C. Myo-inositol and d-chiro-inositol oral supplementation ameliorate cardiac dysfunction and remodeling in a mouse model of diet-induced obesity. Pharmacol. Res. 2020, 159, 105047. [Google Scholar] [CrossRef]
- Blind, R.D. Structural analyses of inositol phosphate second messengers bound to signaling effector proteins. Adv. Biol. Regul. 2020, 75, 100667. [Google Scholar] [CrossRef]
- Foster, S.R.; Dilworth, L.L.; Thompson, R.K.; Alexander-Lindo, R.L.; Omoruyi, F.O. Effects of combined inositol hexakisphosphate and inositol supplement on antioxidant activity and metabolic enzymes in the liver of streptozotocin-induced type 2 diabetic rats. Chem. Biol. Interact. 2017, 275, 108–115. [Google Scholar] [CrossRef]
- Lete, M.G.; Tripathi, A.; Chandran, V.; Bankaitis, V.A.; McDermott, M.I. Lipid transfer proteins and instructive regulation of lipid kinase activities: Implications for inositol lipid signaling and disease. Adv. Biol. Regul. 2020, 78, 100740. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zhang, Y.; Fu, M.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. Cystathionine gamma-lyase/H2S system suppresses hepatic acetyl-CoA accumulation and nonalcoholic fatty liver disease in mice. Life Sci. 2020, 252, 117661. [Google Scholar] [CrossRef] [PubMed]
- Salie, M.J.; Thelen, J.J. Regulation and structure of the heteromeric acetyl-CoA carboxylase. Biochim. Biophys. Acta. 2016, 1861, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Nikroo, H.; Hosseini, S.R.A.; Fathi, M.; Sardar, M.A.; Khazaei, M. The effect of aerobic, resistance, and combined training on PPAR-alpha, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol. Behav. 2020, 227, 113149. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Y.; Wu, Y.; Xu, M.; Sun, Z.; Ye, C. Cloning and characterization of carnitine palmitoyltransferase Iα (CPT1α) from obscure puffer (Takifugu obscurus), and its gene expression in response to different lipid sources. Aquacult. Rep. 2020, 18, 100424. [Google Scholar] [CrossRef]
- Shi, X.C.; Sun, J.; Yang, Z.; Li, X.X.; Ji, H.; Li, Y.; Chang, Z.G.; Du, Z.Y.; Chen, L.Q. Molecular characterization and nutritional regulation of carnitine palmitoyltransferase (CPT) family in grass carp (Ctenopharyngodon idellus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 203, 11–19. [Google Scholar] [CrossRef]
- Ayisi, C.L.; Yamei, C.; Zhao, J.-L. Genes, transcription factors and enzymes involved in lipid metabolism in fin fish. Agri Gene 2018, 7, 7–14. [Google Scholar] [CrossRef]
- Yan, X.; Qin, C.; Deng, D.; Yang, G.; Feng, J.; Lu, R.; Wang, G.; Nie, G. Regulation of glucose and lipid metabolism by insulin and glucagon in vivo and in vitro in common carp Cyprinus carpio L. Aquacult. Rep. 2020, 18, 100427. [Google Scholar] [CrossRef]
- Lu, R.-H.; Jia, S.-Z.; Yang, F.; Qin, C.-B.; Zhang, Y.-R.; Meng, X.-L.; Yan, X.; Feng, J.-C.; Nie, G.-X. The function of miR-122 in the lipid metabolism and immunity of grass carp (Ctenopharyngodon idellus). Aquacult. Rep. 2020, 17, 100401. [Google Scholar] [CrossRef]
- Wang, T.; Wei, Q.; Liang, L.; Tang, X.; Yao, J.; Lu, Y.; Qu, Y.; Chen, Z.; Xing, G.; Cao, X. OSBPL2 Is Required for the Binding of COPB1 to ATGL and the Regulation of Lipid Droplet Lipolysis. iScience 2020, 23, 101252. [Google Scholar] [CrossRef]
- Li, S.; Sang, C.; Zhang, J.; Li, Z.; Chen, N. Molecular cloning, expression profiling of adipose triglyceride lipase (ATGL) and forkhead box O1 (FoxO1), and effects of dietary carbohydrate level on their expression in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Aquaculture 2018, 492, 103–112. [Google Scholar] [CrossRef]
- Genazzani, A.D. Inositol as putative integrative treatment for PCOS. Reprod. Biomed. Online 2016, 33, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Vong, C.T.; Tseng, H.H.L.; Kwan, Y.W.; Lee, S.M.; Hoi, M.P.M. G-protein coupled receptor 55 agonists increase insulin secretion through inositol trisphosphate-mediated calcium release in pancreatic beta-cells. Eur. J. Pharmacol. 2019, 854, 372–379. [Google Scholar] [CrossRef]
- Croze, M.L.; Vella, R.E.; Pillon, N.J.; Soula, H.A.; Hadji, L.; Guichardant, M.; Soulage, C.O. Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. J. Nutr. Biochem. 2013, 24, 457–466. [Google Scholar] [CrossRef]
- Gu, Z.; Mu, H.; Shen, H.; Deng, K.; Liu, D.; Yang, M.; Zhang, Y.; Zhang, W.; Mai, K. High level of dietary soybean oil affects the glucose and lipid metabolism in large yellow croaker Larimichthys crocea through the insulin-mediated PI3K/AKT signaling pathway. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 231, 34–41. [Google Scholar] [CrossRef]
- Kim, J.N.; Han, S.N.; Kim, H.K. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2014, 34, 723–731. [Google Scholar] [CrossRef]
- Osada-Oka, M.; Hashiba, Y.; Akiba, S.; Imaoka, S.; Sato, T. Glucose is necessary for stabilization of hypoxia-inducible factor-1alpha under hypoxia: Contribution of the pentose phosphate pathway to this stabilization. FEBS Lett. 2010, 584, 3073–3079. [Google Scholar] [CrossRef]
- Rodrigues, J.; Branco, V.; Lu, J.; Holmgren, A.; Carvalho, C. Toxicological effects of thiomersal and ethylmercury: Inhibition of the thioredoxin system and NADP(+)-dependent dehydrogenases of the pentose phosphate pathway. Toxicol. Appl. Pharmacol. 2015, 286, 216–223. [Google Scholar] [CrossRef]
- Ma, A.; Cui, W.; Wang, X.; Zhang, W.; Liu, Z.; Zhang, J.; Zhao, T. Osmoregulation by the myo-inositol biosynthesis pathway in turbot Scophthalmus maximus and its regulation by anabolite and c-Myc. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110636. [Google Scholar] [CrossRef]
- Olavarria, V.H.; Figueroa, J.E.; Mulero, V. Prolactin-induced activation of phagocyte NADPH oxidase in the teleost fish gilthead seabream involves the phosphorylation of p47phox by protein kinase C. Dev. Comp. Immun. 2012, 36, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Landzhov, B.; Yakimova, K. Cafeteria diet-induced obesity reduces leptin-stimulated NADPH-diaphorase reactivity in the hypothalamic arcuate nucleus of rats. Acta Histochem. 2020, 122, 151616. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.T.; Rossi, I.C.; Kucharski, L.C.; Da Silva, R.S. Hepatopancreas gluconeogenesis and glycogen content during fasting in crabs previously maintained on a high-protein or carbohydrate-rich diet. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, J.; Gong, G.; Xue, M.; Dong, Y.; Wu, X.; Wang, X.; Chen, C.; Liang, X.; Qin, Y. Gluconeogenesis during starvation and refeeding phase is affected by previous dietary carbohydrates levels and a glucose stimuli during early life in Siberian sturgeon (Acipenser baerii). Anim. Nutr. 2017, 3, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S.J.; Naser, F.; Mahieu, N.G.; McKenzie, L.D.; Dunn, G.P.; Chheda, M.G.; Patti, G.J. Consumption of NADPH for 2-HG Synthesis Increases Pentose Phosphate Pathway Flux and Sensitizes Cells to Oxidative Stress. Cell Rep. 2018, 22, 512–522. [Google Scholar] [CrossRef] [PubMed]
- de Freitas-Silva, L.; Rodriguez-Ruiz, M.; Houmani, H.; da Silva, L.C.; Palma, J.M.; Corpas, F.J. Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J. Plant Physiol. 2017, 218, 196–205. [Google Scholar] [CrossRef]
- Wasylenko, T.M.; Ahn, W.S.; Stephanopoulos, G. The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab. Eng. 2015, 30, 27–39. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Tsujita, N.; Kawai, T.; Goto, M.; Cheng, B.; Zeng, W.; Shimada, M. Itaconate regulates the glycolysis/pentose phosphate pathway transition to maintain boar sperm linear motility by regulating redox homeostasis. Free Radic. Biol. Med. 2020, 159, 44–53. [Google Scholar] [CrossRef]
- Cui, W.; Ma, A.; Huang, Z.; Liu, Z.; Yang, K.; Zhang, W. myo-inositol facilitates salinity tolerance by modulating multiple physiological functions in the turbot Scophthalmus maximus. Aquaculture 2020, 527, 735451. [Google Scholar] [CrossRef]
- Fitria, P.D.; Amin, M.; Lokapirnasari, W.P.; Lamid, M. Supplementation of fermented coffee-peel flour to increase high-density lipoprotein (HDL) cholesterol, docosahexaenoic acids (DHA) and eicosapentaenoic acids (EPA) deposition in tilapia fillet. Biocatal. Agric. Biotechnol. 2020, 24, 101502. [Google Scholar] [CrossRef]
- Zitnanova, I.; Oravec, S.; Janubova, M.; Konarikova, K.; Dvorakova, M.; Laubertova, L.; Kralova, M.; Simko, M.; Muchova, J. Gender differences in LDL and HDL subfractions in atherogenic and nonatherogenic phenotypes. Clin. Biochem. 2020, 79, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, Z.; Zhong, H.; Yin, Q.; Xiao, J.; Wang, F.; Zhou, Y.; Luo, Y. Regulation of triglyceride synthesis by estradiol in the livers of hybrid tilapia (Oreochromis niloticus female symbol × O. aureus male symbol). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 238, 110335. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Niu, J. Lipid metabolism and plasma metabolomics of juvenile largemouth bass Micropterus salmoides were affected by dietary oxidized fish oil. Aquaculture 2020, 522, 110335. [Google Scholar] [CrossRef]
- Liu, M.; Wallmon, A.; Wallin, R.; Saldeen, T. Effects of stable fish oil and simvastatin on plasma lipoproteins in patients with hyperlipidemia. Nutr. Res. 2003, 23, 1027–1034. [Google Scholar] [CrossRef]
- Zenimaru, Y.; Takahashi, S.; Takahashi, M.; Yamada, K.; Iwasaki, T.; Hattori, H.; Imagawa, M.; Ueno, M.; Suzuki, J.; Miyamori, I. Glucose deprivation accelerates VLDL receptor-mediated TG-rich lipoprotein uptake by AMPK activation in skeletal muscle cells. Biochem. Biophys. Res. Commun. 2008, 368, 716–722. [Google Scholar] [CrossRef]
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol. Med. 2020. [Google Scholar] [CrossRef]
- Sousa, L.C.; Moromizato, B.S.; de Almeida, V.D.N.S.; Miasaki, C.T.; Takahashi, L.S.; Biller, J.D. There is more than one way of feeding carnivorous fish: Surubim (Pseudoplatystoma reticulatum × P. corruscans) are able to cope with carbohydrates rich diets, but there is a trade-off between growth and immunity. Anim. Feed Sci. Technol. 2020, 262, 114382. [Google Scholar] [CrossRef]
- Kaushal, N.; Gupta, M.; Kulshreshtha, E. Hempseed (Cannabis sativa) lipid fractions alleviate high-fat diet-induced fatty liver disease through regulation of inflammation and oxidative stress. Heliyon 2020, 6, e04422. [Google Scholar] [CrossRef]
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. Epigallocatechin gallate diminishes cigarette smoke-induced oxidative stress, lipid peroxidation, and inflammation in human bronchial epithelial cells. Life Sci. 2020, 259, 118260. [Google Scholar] [CrossRef]
- Xavier, W.d.S.; Leclercq, E.; Carvalho, P.L.P.F.; Vicente, I.S.T.; Guimarães, M.G.; Rodrigues, E.J.D.; Milanezi, R.C.; Barbé, F.; Sartori, M.M.P.; Pezzato, L.E.; et al. The putative effect of a SOD-rich melon pulp-concentrate on growth performance and antioxidant status of Nile tilapia (Oreochromis niloticus) under heat/dissolved oxygen-induced stress. Aquaculture 2020, 529, 735669. [Google Scholar] [CrossRef]
- Wang, C.; Liang, Y.; Fang, Y.; Chang, X. Effects of cyclical short-term food deprivation and refeeding on compensatory growth and gene expression of SOD, GPX and HSP70 in Schizothorax wangchiachii. Fish Shellfish Immun. 2019, 94, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Yu, P.; Yin, Y.; Zhang, Y.; Lu, Y.; Mao, Q.; Li, Y. Effect of selenium-rich Bacillus subtilis against mercury-induced intestinal damage repair and oxidative stress in common carp. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 239, 108851. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immun. 2020, 98, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Soliman, A.A.; Sewilam, H.; Almeer, R.; Van Doan, H.; Alagawany, M.; Dawood, M.A.O. The influence of raffinose on the growth performance, oxidative status, and immunity in Nile tilapia (Oreochromis niloticus). Aquacult. Rep. 2020, 18, 100457. [Google Scholar] [CrossRef]
- Yan, S.; Meng, Z.; Tian, S.; Teng, M.; Yan, J.; Jia, M.; Li, R.; Zhou, Z.; Zhu, W. Neonicotinoid insecticides exposure cause amino acid metabolism disorders, lipid accumulation and oxidative stress in ICR mice. Chemosphere 2020, 246, 125661. [Google Scholar] [CrossRef]
- Chen, S.-J.; Gan, L.; Guo, Y.-C.; Tian, L.-X.; Liu, Y.-J. Changes in growth performance, aflatoxin B1 residues, immune response and antioxidant status of Litopenaeus vannamei fed with AFB1-contaminated diets and the regulating effect of dietary myo-inositol supplementation. Food Chem. 2020, 324, 126888. [Google Scholar] [CrossRef]
- Lin, J.D.; Lin, P.Y.; Chen, L.M.; Fang, W.H.; Lin, L.P.; Loh, C.H. Serum glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) levels in children and adolescents with intellectual disabilities. Res. Dev. Disabil. 2010, 31, 172–177. [Google Scholar] [CrossRef]
- Li, S.A.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary myo-inositol deficiency decreased intestinal immune function related to NF-kappaB and TOR signaling in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immun. 2018, 76, 333–346. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.; Gao, Y.; Yin, P.; Tian, L.; Niu, J.; Liu, Y. Exposure to acute ammonia stress influences survival, immune response and antioxidant status of pacific white shrimp (Litopenaeus vannamei) pretreated with diverse levels of inositol. Fish Shellfish Immun. 2019, 89, 248–256. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, J.; Lin, L.; Li, Y.; Liu, X.; Wang, Z. Study of a noninvasive detection method for the high-temperature stress response of the large yellow croaker (Larimichthys crocea). Aquacult. Rep. 2020, 18, 100514. [Google Scholar] [CrossRef]



| Ingredients | Content (g/kg Dry Basis) | |||||
|---|---|---|---|---|---|---|
| Casein (Vitamin-Free) | 320 | 320 | 320 | 320 | 320 | 320 |
| Gelatin | 80 | 80 | 80 | 80 | 80 | 80 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 | 70 |
| Corn starch | 300 | 450 | 300 | 450 | 300 | 450 |
| Myo-inositol c (mg/kg diet) | 0 | 0 | 0.4 | 0.4 | 1.2 | 1.2 |
| Vitamin premix a | 5 | 5 | 5 | 5 | 5 | 5 |
| Mineral premix b | 5 | 5 | 5 | 5 | 5 | 5 |
| Ca(H2PO4)2 | 15 | 15 | 15 | 15 | 15 | 15 |
| Carboxymethyl cellulose | 25 | 25 | 25 | 25 | 25 | 25 |
| Cellulose | 175.75 | 27.75 | 175.35 | 27.35 | 176.55 | 26.55 |
| Phagostimulant | 2 | 2 | 2 | 2 | 2 | 2 |
| BHT | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Proximate composition | ||||||
| Moisture | 100.5 | 105.6 | 100.3 | 102.3 | 106.8 | 103.9 |
| Crude protein | 372.2 | 379.8 | 375.5 | 377.4 | 376.5 | 378.4 |
| Total lipid | 69.5 | 69.6 | 68.3 | 68.7 | 68.5 | 69.3 |
| Ash | 28.82 | 28.76 | 28.73 | 30.18 | 30.16 | 29.11 |
| Gene | Position | Primer Sequence | Length | Tm | Product Size (bp) |
|---|---|---|---|---|---|
| GK | Forward | GTCATCAACCTGATGCGGGA | 20 | 60.18 | 163 |
| Reverse | ACCTGTCACGGAAACATGGG | 20 | 59.75 | ||
| PK | Forward | GCTAACCAAGACTGGCAGGT | 20 | 59.96 | 438 |
| Reverse | TGGAGGGATTCGTGGAGTCT | 20 | 59.96 | ||
| G6Pase | Forward | GGATGCTAATGGGCCTGGTC | 20 | 59.78 | 169 |
| Reverse | CAGCTACCAGTGTGCCTGTAA | 21 | 59.60 | ||
| G6PDH | Forward | TCCAGAACCTCATGGTGCTT | 20 | 60.18 | 312 |
| Reverse | GGCTCCTTGAAGGTAAGGACG | 21 | 59.69 | ||
| MIPS | Forward | CGTCCTACGAGGGAACCTCT | 20 | 60.39 | 179 |
| Reverse | GCAGAGTCTTTGCACGGAATA | 21 | 58.65 | ||
| IMPA1 | Forward | ATAAGCCGGGAAGCAGTCTC | 20 | 59.53 | 132 |
| Reverse | GTGTTTGGTCGTTCGATGGTG | 21 | 60.07 | ||
| CPT | Forward | GTGGGCGTCCAACTATGTCA | 20 | 59.04 | 251 |
| Reverse | TACGCTCGTATTGGGCTGAG | 20 | 60.12 | ||
| PPAR-α | Forward | GGGCCATAGTGTGAGTGTGA | 20 | 59.75 | 245 |
| Reverse | TGGGTGTCCACCATGTCTAC | 20 | 59.78 | ||
| ATGL | Forward | AAAACGTCCTGGTGACCCCAGT | 21 | 59.98 | 104 |
| Reverse | TAGGAGGAATGATGCCACAGTACA | 24 | 60.03 | ||
| FAS | Forward | ACAGCTGCAGACCCAGAATC | 20 | 60.04 | 307 |
| Reverse | GTAGAAGGCAGAGGCTGCAA | 20 | 60.04 | ||
| DGAT2 | Forward | AGAGGAGCTGTAAGCTCGGA | 20 | 60.03 | 157 |
| Reverse | AGTGCCTTTGAGGAATCCCG | 20 | 60.04 | ||
| SREBP | Forward | ATGTCCCCATGTTCCCACTG | 20 | 59.67 | 137 |
| Reverse | GCTAACGCATATGCCTCCCA | 20 | 60.25 | ||
| β-actin | Forward | GGATTCACTCTGAGCGCCG | 19 | 58.43 | 203 |
| Reverse | CCGTCTCCTTACCTTTGGGTG | 21 | 59.12 |
| Diets | WG (%) | SR (%) | FCR | CF (%) | HSI (%) | VIS (%) |
|---|---|---|---|---|---|---|
| LC-0 | 722.45 ± 6.21 | 91.11 ± 1.11 | 1.13 ± 0.54 B | 2.96 ± 4.67 | 1.76 ± 7.05 | 10.84 ± 18.04 A |
| LC-400 | 739.35 ± 19.11 | 95.56 ± 2.94 | 1.09 ± 0.35 A | 3.06 ± 8.06 | 1.73 ± 10.35 | 12.98 ± 51.66 B |
| LC-1200 | 766.75 ± 48.54 | 92.22 ± 2.22 | 1.09 ± 1.56 A,B | 3.09 ± 6.52 | 1.61 ± 10.22 | 11.75 ± 39.55 A |
| HC-0 | 753.32 ± 42.11 a | 94.44 ± 2.94 | 1.10 ± 1.81 b | 2.96 ± 5.76 a | 2.08 ± 11.26 b,* | 11.07 ± 21.41 b |
| HC-400 | 867.73 ± 43.46 b | 88.33 ± 5.00 | 1.12 ± 3.09 b | 3.14 ± 6.01 b | 1.55 ± 10.10 a | 11.30 ± 25.83 b |
| HC-1200 | 777.85 ± 9.53 a | 97.78 ± 1.11 | 0.96 ± 0.42 a | 3.03 ± 6.40 a,b | 1.76 ±9.91 a | 9.92 ± 23.63 a |
| AN0VA (P) | ||||||
| MI | 0.140 | 0.486 | 0.120 | 0.073 | 0.012 | 0.002 |
| carbohydrates | 0.042 | 0.795 | 0.029 | 0.940 | 0.223 | 0.001 |
| MI × carbohydrates | 0.143 | 0.073 | 0.081 | 0.506 | 0.043 | 0.017 |
| Diets | Moisture (%) | Crude Lipid (%) | Crude Protein (%) |
|---|---|---|---|
| LC-0 | 74.06 ± 0.11 A | 13.87 ± 0.77 B | 45.72 ± 0.19 A |
| LC-400 | 76.70 ± 0.47 B | 11.90 ± 0.91 A | 54.77 ± 2.61 B,** |
| LC-1200 | 73.62 ± 0.56 A | 15.62 ± 0.53 A,B | 45.26 ± 0.69 A |
| HC-0 | 74.65 ± 0.21 | 15.80 ± 0.37 b | 43.77 ± 0.82 |
| HC-400 | 74.39 ± 0.37 | 15.00 ± 0.46 b | 44.07 ± 0.39 |
| HC-1200 | 74.65 ± 0.43 | 13.28 ± 0.18 a | 44.14 ± 1.02 |
| AN0VA (P) | |||
| MI | 0.003 | 0.109 | 0.013 |
| carbohydrates | 0.488 | 0.133 | 0.068 |
| MI × carbohydrates | 0.006 | 0.003 | 0.076 |
| Diets | Serum Glucose | Serum INS | Liver Glycogen | Muscle Glycogen |
|---|---|---|---|---|
| LC-0 | 4.23 ± 0.18 | 70.35 ± 1.56 A | 17.68 ± 0.92 B | 1.42 ± 0.16 |
| LC-400 | 4.69 ± 0.31 | 85.75 ± 2.61 C | 17.66 ± 2.48 B | 1.44 ± 0.27 |
| LC-1200 | 4.89 ± 0.22 | 80.01 ± 0.49 B | 14.07 ± 0.96 A | 1.46 ± 0.15 |
| HC-0 | 5.44 ± 0.43 | 73.53 ± 0.81 a | 16.90 ± 0.51 b | 1.56 ± 0.18 |
| HC-400 | 5.19 ± 0.32 | 83.87 ± 2.72 b | 14.57 ± 1.32 a | 1.67 ± 0.20 |
| HC-1200 | 5.68 ± 0.22 | 80.07 ± 1.68 b | 14.58 ± 0.87 a | 1.58 ± 0.12 |
| AN0VA (P) | ||||
| MI | 0.119 | 0.000 | 0.000 | 0.924 |
| carbohydrates | 0.000 | 0.767 | 0.154 | 0.507 |
| MI × carbohydrates | 0.418 | 0.405 | 0.077 | 0.650 |
| Diets | Serum TG | Serum HDL-C | Serum LDL-C | Serum T-CHO | Liver TG |
|---|---|---|---|---|---|
| LC-0 | 2.05 ± 0.26 B | 1.05 ± 0.08 | 2.72 ± 0.26 | 2.16 ± 0.96 | 0.31 ± 0.02 B |
| LC-400 | 1.77 ± 0.14 A,B | 0.96 ± 0.07 | 2.87 ± 0.19 | 2.18 ± 0.13 | 0.22 ± 0.33 A |
| LC-1200 | 0.35 ± 0.12 A | 1.15 ± 0.07 | 2.82 ± 0.10 | 2.19 ± 0.12 | 0.21 ± 0.03 A |
| HC-0 | 2.25 ± 0.09 b | 1.21 ± 0.04 a | 2.46 ± 0.12 | 1.90 ± 0.05 a | 0.29 ± 0.13 b |
| HC-400 | 1.98 ± 0.20 b | 1.58 ± 0.09 b,** | 2.83 ± 0.21 | 2.47 ± 0.14 b | 0.20 ± 0.02 a |
| HC-1200 | 1.31 ± 0.10 a | 2.02 ± 0.13 c,** | 2.93 ± 0.05 | 2.49 ± 0.11 b | 0.21 ± 0.02 a |
| AN0VA (P) | |||||
| MI | 0.000 | 0.000 | 0.202 | 0.052 | 0.000 |
| carbohydrates | 0.734 | 0.000 | 0.655 | 0.662 | 0.074 |
| MI × carbohydrates | 0.389 | 0.002 | 0.567 | 0.112 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Pan, J.; Wang, X.; Huang, Y.; Qin, C.; Qiao, F.; Qin, J.; Chen, L. Alleviation of the Adverse Effect of Dietary Carbohydrate by Supplementation of Myo-Inositol to the Diet of Nile Tilapia (Oreochromis niloticus). Animals 2020, 10, 2190. https://doi.org/10.3390/ani10112190
Zhu J, Pan J, Wang X, Huang Y, Qin C, Qiao F, Qin J, Chen L. Alleviation of the Adverse Effect of Dietary Carbohydrate by Supplementation of Myo-Inositol to the Diet of Nile Tilapia (Oreochromis niloticus). Animals. 2020; 10(11):2190. https://doi.org/10.3390/ani10112190
Chicago/Turabian StyleZhu, Jiahua, Jingyu Pan, Xiaodan Wang, Yuxing Huang, Chuanjie Qin, Fang Qiao, Jianguang Qin, and Liqiao Chen. 2020. "Alleviation of the Adverse Effect of Dietary Carbohydrate by Supplementation of Myo-Inositol to the Diet of Nile Tilapia (Oreochromis niloticus)" Animals 10, no. 11: 2190. https://doi.org/10.3390/ani10112190
APA StyleZhu, J., Pan, J., Wang, X., Huang, Y., Qin, C., Qiao, F., Qin, J., & Chen, L. (2020). Alleviation of the Adverse Effect of Dietary Carbohydrate by Supplementation of Myo-Inositol to the Diet of Nile Tilapia (Oreochromis niloticus). Animals, 10(11), 2190. https://doi.org/10.3390/ani10112190






