Ecological and Behavioral Drivers of Supplemental Feeding Use by Roe Deer Capreolus capreolus in a Peri-Urban Context
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Monitoring of FS Use by Roe Deer
2.3. Processing of Camera Trapping Data
2.4. Statistical Analyses
2.4.1. Diel Pattern of Feeding Sites Use
2.4.2. Daily Number of Visits to Feeding Sites
2.4.3. Duration of Visits to Feeding Sites
3. Results
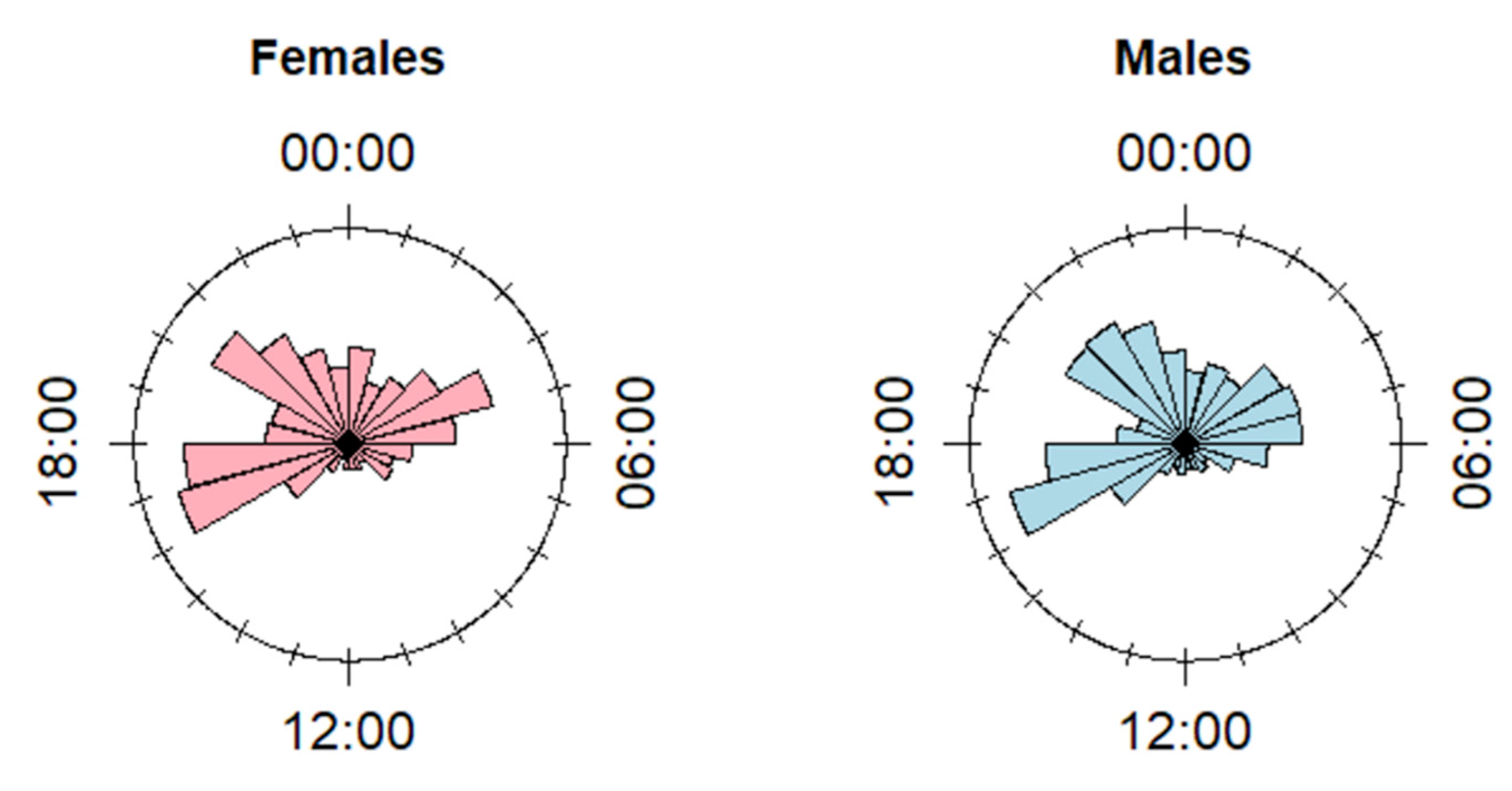
3.1. Diel Pattern of Feeding Sites Use
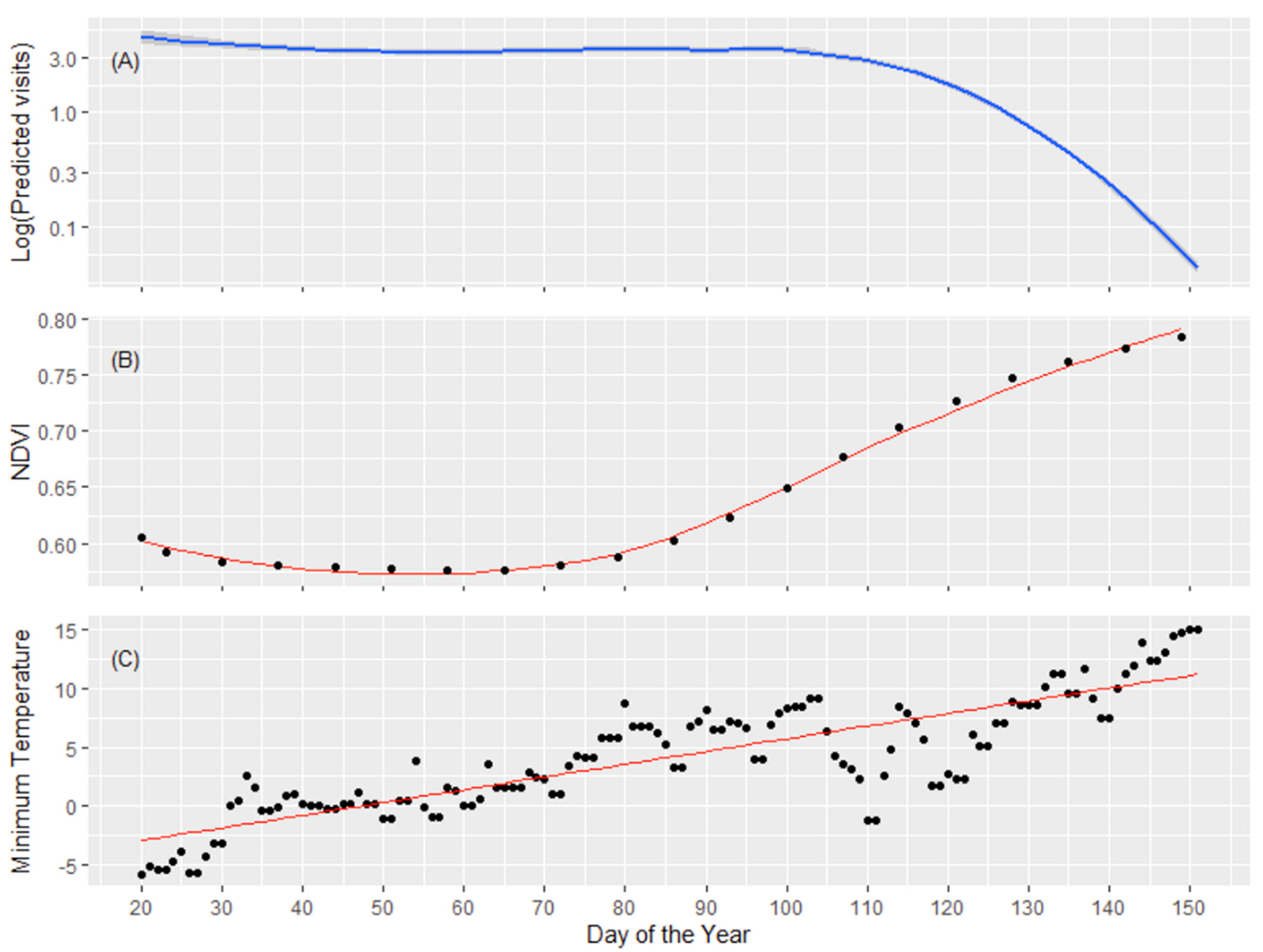
3.2. Daily Number of Visits to Feeding Sites
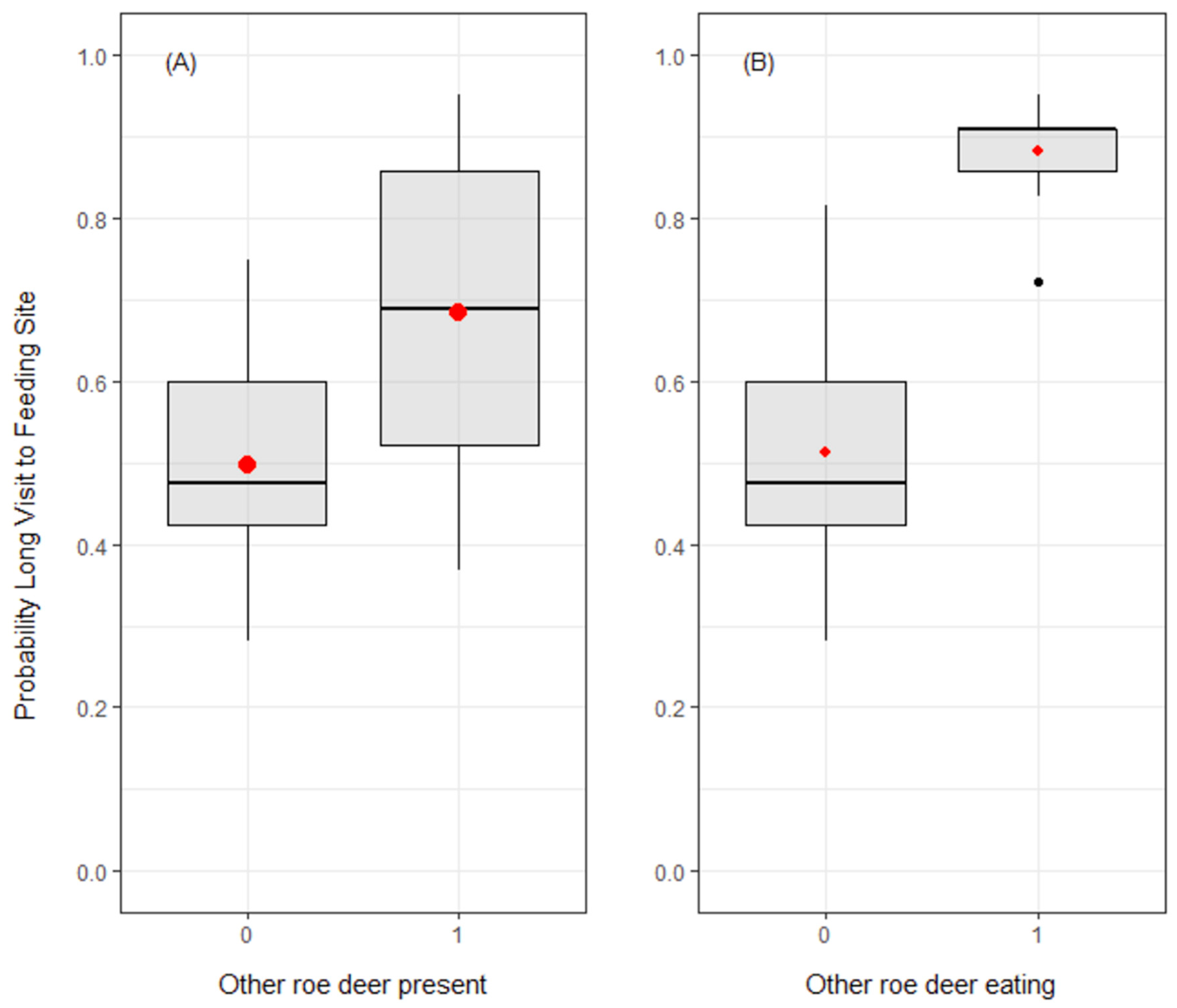
3.3. Duration of Visits to Feeding Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macgregor-fors, I. How to Measure the Urban-Wildland Ecotone: Redefining ‘Peri-Urban’ Areas. Ecol. Res. 2010, 25, 883–887. [Google Scholar] [CrossRef]
- Putman, R.J.; Staines, B.W. Supplementary Winter Feeding of Wild Red Deer Cervus Elaphus in Europe and North America: Justifications, Feeding Practice and Effectiveness. Mammal Rev. 2004, 34, 285–306. [Google Scholar] [CrossRef]
- Fležar, U.; Costa, B.; Bordjan, D.; Jerina, K.; Krofel, M. Free Food for Everyone: Artificial Feeding of Brown Bears Provides Food for Many Non-Target Species. Eur. J. Wildl. Res. 2019, 65, 1. [Google Scholar] [CrossRef]
- Reed, J.H.; Bonter, D.N. Supplementing Non-Target Taxa: Bird Feeding Alters the Local Distribution of Mammals. Ecol. Appl. 2018, 28, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.M.; Van Beest, F.M.; Schmidt, K.T.; Brook, R.K.; Storaas, T. To Feed or Not to Feed? Evidence of the Intended and Unintended Effects of Feeding Wild Ungulates. J. Wildl. Manag. 2014, 78, 1322–1334. [Google Scholar] [CrossRef]
- Arnold, J.M.; Gerhardt, P.; Steyaert, S.M.J.G.; Hochbichler, E.; Hackländer, K. Diversionary Feeding Can Reduce Red Deer Habitat Selection Pressure on Vulnerable Forest Stands, but Is Not a Panacea for Red Deer Damage. For. Ecol. Manag. 2018, 407, 166–173. [Google Scholar] [CrossRef]
- Pascual-Rico, R.; Pérez-García, J.M.; Sebastián-González, E.; Botella, F.; Giménez, A.; Eguía, S.; Sánchez-Zapata, J.A. Is Diversionary Feeding a Useful Tool to Avoid Human-Ungulate Conflicts? A Case Study with the Aoudad. Eur. J. Wildl. Res. 2018, 64, 67. [Google Scholar] [CrossRef]
- Oro, D.; Genovart, M.; Tavecchia, G.; Fowler, M.S.; Martínez-Abraín, A. Ecological and Evolutionary Implications of Food Subsidies from Humans. Ecol. Lett. 2013, 16, 1501–1514. [Google Scholar] [CrossRef]
- Becker, D.J.; Streicker, D.G.; Altizer, S. Linking Anthropogenic Resources to Wildlife-Pathogen Dynamics: A Review and Meta-Analysis. Ecol. Lett. 2015, 18, 483–495. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Cromsigt, J.P.G.M.; Edenius, L.; Malmsten, J.; Wam, H.K. Interactions between Ungulates, Forests, and Supplementary Feeding: The Role of Nutritional Balancing in Determining Outcomes. Mammal Res. 2017, 62. [Google Scholar] [CrossRef]
- Ricci, S.; Sandfort, R.; Pinior, B.; Mann, E.; Wetzels, S.U.; Stalder, G. Impact of Supplemental Winter Feeding on Ruminal Microbiota of Roe Deer Capreolus Capreolus. Wildl. Biol. 2019, 2019. [Google Scholar] [CrossRef]
- van Beest, F.M.; Loe, L.E.; Mysterud, A.; Milner, J.M. Comparative Space Use and Habitat Selection of Moose Around Feeding Stations. J. Wildl. Manag. 2010, 74, 219–227. [Google Scholar] [CrossRef]
- Jones, J.D.; Kauffman, M.J.; Monteith, K.L.; Scurlock, B.M.; Albeke, S.E.; Cross, P.C. Supplemental Feeding Alters Migration of a Temperate Ungulate. Ecol. Appl. 2014, 24, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Ossi, F.; Gaillard, J.M.; Hebblewhite, M.; Morellet, N.; Ranc, N.; Sandfort, R.; Kroeschel, M.; Kjellander, P.; Mysterud, A.; Linnell, J.D.C.; et al. Plastic Response by a Small Cervid to Supplemental Feeding in Winter across a Wide Environmental Gradient. Ecosphere 2017, 8. [Google Scholar] [CrossRef]
- Ranc, N.; Moorcroft, P.R.; Hansen, K.W.; Ossi, F.; Sforna, T.; Ferraro, E.; Brugnoli, A.; Cagnacci, F. Preference and Familiarity Mediate Spatial Responses of a Large Herbivore to Experimental Manipulation of Resource Availability. Sci. Rep. 2020, 10, 11946. [Google Scholar] [CrossRef] [PubMed]
- Ranc, N.; Moorcroft, P.R.; Ossi, F.; Cagnacci, F. Experimental Evidence of Memory-Based Foraging Decisions in a Large Wild Mammal. bioRxiv 2020. [Google Scholar] [CrossRef]
- Payne, A.; Philipon, S.; Hars, J.; Dufour, B.; Gilot-Fromont, E. Wildlife Interactions on Baited Places and Waterholes in a French Area Infected by Bovine Tuberculosis. Front. Vet. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Grenier, D.; Barrette, C.; Crête, M. Food Access by White-Tailed Deer (Odocoileus Virginianus) at Winter Feeding Sites in Eastern Quebec. Appl. Anim. Behav. Sci. 1999, 63, 323–337. [Google Scholar] [CrossRef]
- Côté, S.D. Determining Social Rank in Ungulates: A Comparison of Aggressive Interactions Recorded at a Bait Site and under Natural Conditions. Ethology 2000, 106, 945–955. [Google Scholar] [CrossRef]
- Espmark, Y. Social Behaviour of Roe Deer at Winter Feeding Stations. Appl. Anim. Ethol. 1974, 1, 35–47. [Google Scholar] [CrossRef]
- Gortázar, C.; Acevedo, P. Disease Risks and Overabundance of Game Species. Eur. J. Wildl. Res. 2006, 52, 81–87. [Google Scholar] [CrossRef]
- Sorensen, A.; van Beest, F.M.; Brook, R.K. Impacts of Wildlife Baiting and Supplemental Feeding on Infectious Disease Transmission Risk: A Synthesis of Knowledge. Prev. Vet. Med. 2014, 113, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, G.G.; Cross, P.C.; Cole, E.K.; Fuda, R.K.; Rogerson, J.D.; Scurlock, B.M.; Du Toit, J.T. Winter Feeding of Elk in the Greater Yellowstone Ecosystem and Its Effects on Disease Dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Forristal, V.E.; Creel, S.; Taper, M.L.; Scurlock, B.M.; Cross, P.C. Effects of Supplemental Feeding and Aggregation on Fecal Glucocorticoid Metabolite Concentrations in Elk. J. Wildl. Manag. 2012, 76, 694–702. [Google Scholar] [CrossRef]
- Tolhurst, B.A.; Delahay, R.J.; Walker, N.J.; Ward, A.I.; Roper, T.J. Behaviour of Badgers (Meles Mele) in Farm Buildings: Opportunities for the Transmission of Mycobacterium Bovis to Cattle? Appl. Anim. Behav. Sci. 2009, 117, 103–113. [Google Scholar] [CrossRef]
- Popova, E.D.; Zlatanova, D.P.; Todev, V. Diversity and Temporal Relationships between Mammals at Feeding Stations in Western Rhodope Mountains, Bulgaria. Acta Zool. Bulg. 2017, 69, 529–540. [Google Scholar] [CrossRef]
- Pagon, N.; Grignolio, S.; Pipia, A.; Bongi, P.; Bertolucci, C.; Apollonio, M. Seasonal Variation of Activity Patterns in Roe Deer in a Temperate Forested Area. Chronobiol. Int. 2013, 30, 772–785. [Google Scholar] [CrossRef]
- Bonnot, N.C.; Couriot, O.; Berger, A.; Cagnacci, F.; Ciuti, S.; De Groeve, J.E.; Gehr, B.; Heurich, M.; Kjellander, P.; Kröschel, M.; et al. Fear of the Dark? Contrasting Impacts of Humans versus Lynx on Diel Activity of Roe Deer across Europe. J. Anim. Ecol. 2020, 89, 132–145. [Google Scholar] [CrossRef]
- Mancinelli, S.; Peters, W.; Boitani, L.; Hebblewhite, M.; Cagnacci, F. Roe Deer Summer Habitat Selection at Multiple Spatio-Temporal Scales in an Alpine Environment. Hystrix 2015, 26, 132–140. [Google Scholar] [CrossRef]
- Mysterud, A.; Pérez-Barbería, F.J.; Gordon, I.J. The Effect of Season, Sex and Feeding Style on Home Range Area versus Body Mass Scaling in Temperate Ruminants. Oecologia 2001, 127, 30–39. [Google Scholar] [CrossRef]
- Meteotrentino. Available online: https://www.meteotrentino.it/ (accessed on 31 August 2020).
- Wildlife Office of the Autonomous Province of Trento. Analisi Delle Consistenze e Dei Prelievi Di Ungulati, Tetraonidi e Coturnice. Stagione Venatoria 2018; Wildlife Office of the Autonomous Province of Trento: Trento, Italy, 2018. [Google Scholar]
- Stealth Cam by GSM Outdoors|Home|HD Trail Cameras. Available online: https://www.stealthcam.com/ (accessed on 1 September 2020).
- Krishnappa, Y.S.; Turner, W.C. Software for Minimalistic Data Management in Large Camera Trap Studies. Ecol. Inform. 2014, 24, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the Satellite-Derived NDVI to Assess Ecological Responses to Environmental Change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Landler, L.; Ruxton, G.D.; Malkemper, E.P. The Hermans-Rasson Test as a Powerful Alternative to the Rayleigh Test for Circular Statistics in Biology. BMC Ecol. 2019, 19, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T. Rao’s Statistic for Homogeneity of Multiple Parameter. J. Stat. Plan. Inference 2001, 97, 101–111. [Google Scholar] [CrossRef]
- Zar, J.H. Watson’s Nonparametric Two-Sample Test. Behav. Res. Methods Instrum. 1976, 8, 513. [Google Scholar] [CrossRef]
- Skalski, J.R.; Hoffman, A.; Smith, S. Testing the Significance of Individual- and Cohort-Level Covariates in Animal Survival Studies. In Marked Individuals in the Study of Bird Population; North, P.M., Lebreton, J.-D., Eds.; Birkhauser-Verlag: Boston, MA, USA, 1993; pp. 9–28. [Google Scholar]
- Wood, S. Generalized Additive Models: An Introduction with R, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 1st ed.; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Walker, D.A.; Smith, T.J. Nine Pseudo R2 Indices for Binary Logistic Regression Models. J. Mod. Appl. Stat. Methods 2016, 15, 848–854. [Google Scholar] [CrossRef]
- James, D.; Hornik, K. Chron: Chronological Objects Which Can Handle Dates and Times 2020. R Package Version 2.3-56. Available online: https://cran.r-project.org/web/packages/chron/chron.pdf (accessed on 12 October 2020).
- Agostinelli, C.; Lund, U. R Package ‘Circular’: Circular Statistics (Version 0.4-93). 2013. Available online: https://r-forge.r-project.org/projects/circular/ (accessed on 12 October 2020).
- Agostinelli, C.; Agostinelli, M. S-plus original by Ulric Lund and R port by Claudio Agostinelli (2018). CircStats: Circular Statistics, from “Topics in Circular Statistics” (2001) 2018. R Package Version 0.2-6. Available online: https://cran.r-project.org/web/packages/CircStats/CircStats.pdf (accessed on 12 October 2020).
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. arXiv 2015, arXiv:1406.5823. [Google Scholar]
- Nathan, R.; Getz, M.W.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, E.P. A Movement Ecology Paradigm for Unifying Organismal Movement Research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Mori, E.; Bagnato, S.; Serroni, P.; Sangiuliano, A.; Rotondaro, F.; Marchianò, V.; Cascini, V.; Poerio, L.; Ferretti, F. Spatiotemporal Mechanisms of Coexistence in an European Mammal Community in a Protected Area of Southern Italy. J. Zool. 2020, 310, 232–245. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H. Habitat Use under Predation Risk: Hunting, Roads and Human Dwellings Influence the Spatial Behaviour of Roe Deer. Eur. J. Wildl. Res. 2013, 185–193. [Google Scholar] [CrossRef]
- Lone, K.; Loe, L.E.; Gobakken, T.; Linnell, J.D.C.; Odden, J.; Remmen, J.; Mysterud, A. Living and Dying in a Multi-Predator Landscape of Fear: Roe Deer Are Squeezed by Contrasting Pattern of Predation Risk Imposed by Lynx and Humans. Oikos 2014, 123, 641–651. [Google Scholar] [CrossRef]
- Paton, D.; Ciuti, S.; Quinn, M.; Boyce, M. Hunting Exacerbates the Response to Human Disturbance in Large Herbivores While Migrating through a Road Network. Ecosphere 2017, 8, e01841. [Google Scholar] [CrossRef]
- Picardi, S.; Basille, M.; Peters, W.; Ponciano, J.M.; Boitani, L.; Cagnacci, F. Movement Responses of Roe Deer to Hunting Risk. J. Wildl. Manag. 2019, 83, 43–51. [Google Scholar] [CrossRef]
- Cederlund, G. Activity Patterns in Moose and Roe Deer in a North Boreal Forest. Ecography 1989, 12, 39–45. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary Steps of Ecophysiological Adaptation and Diversification of Ruminants: A Comparative View of Their Digestive System. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef]
- De Groeve, J.; Van de Veghe, N.; Ranc, N.; Neutens, T.; Ometto, L.; Rota-Stabelli, O.; Cagnacci, F. Extracting spatio-temporal patterns in animal trajectories: An ecological application of sequence analysis methods. Methods Ecol. Evol. 2016, 7, 369–379. [Google Scholar] [CrossRef]
- De Groeve, J.; Cagnacci, F.; Ranc, N.; Nadège, C.; Gehr, B.; Heurich, M.; Hewison, A.J.M.; Linnell, J.D.C.; Morellet, N.; Mysterud, A.; et al. Individual Movement—Sequence Analysis Method (IM-SAM): Characterizing Spatio-Temporal Patterns of Animal Habitat Use across Landscapes. Int. J. Geogr. Inf. Sci. 2020, 34, 1530–1551. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Bar-David, S. Recursive Movement Patterns: Review and Synthesis across Species. Ecosphere 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Riotte-Lambert, L.; Benhamou, S.; Chamaillé-Jammes, S. Periodicity Analysis of Movement Recursions. J. Theor. Biol. 2013, 317, 238–243. [Google Scholar] [CrossRef]
- Maublanc, M.L.; Daubord, L.; Bideau, É.; Gerard, J.F. Experimental Evidence of Socio-Spatial Intolerance between Female Roe Deer. Ethol. Ecol. Evol. 2018, 30, 461–476. [Google Scholar] [CrossRef]
- Wolf Alpine Group. Wolf Population Status in the Alps: Pack Distribution and Trend up to 2016, with Focus on Year 2015–2016. 2018. Available online: http://www.lcie.org (accessed on 16 October 2020).
- Gehr, B.; Bonnot, N.; Heurich, M.; Cagnacci, F.; Ciuti, S.; Hewison, A.J.M.; Gaillard, J.-M.; Ranc, N.; Premier, J.; Vogt, K.; et al. Stay Home, Stay Safe—Site Familiarity Reduces Predation Risk in a Large Herbivore in Two Contrasting Study Sites. J. Anim. Ecol. 2020, 89, 1329–1339. [Google Scholar] [CrossRef]
- Robino, P.; Nebbia, P.; Tramuta, C.; Martinet, M.; Ferroglio, E.; De Meneghi, D. Identification of Mycobacterium Avium Subsp. Paratuberculosis in Wild Cervids (Cervus Elaphus Hippelaphus and Capreolus Capreolus) from Northwestern Italy. Eur. J. Wildl. Res. 2008, 54, 357–360. [Google Scholar] [CrossRef]
- Boadella, M.; Carta, T.; Oleaga, Á.; Pajares, G.; Muñoz, M.; Gortázar, C. Serosurvey for Selected Pathogens in Iberian Roe Deer. BMC Vet. Res. 2010, 6. [Google Scholar] [CrossRef]
- Perkins, S.E.; Cagnacci, F.; Stradiotto, A.; Arnoldi, D.; Hudson, P.J. Comparison of Social Networks Derived from Ecological Data: Implications for Inferring Infectious Disease Dynamics. J. Anim. Ecol. 2009, 78, 1015–1022. [Google Scholar] [CrossRef]
- Peters, W.; Hebblewhite, M.; Mysterud, A.; Eacker, D.; Hewison, A.J.M.; Linnell, J.D.C.; Focardi, S.; Urbano, F.; De Groeve, J.; Gehr, B.; et al. Large Herbivore Migration Plasticity along Environmental Gradients in Europe: Life-History Traits Modulate Forage Effects. Oikos 2018, 128. [Google Scholar] [CrossRef]
- Merkle, J.A.; Fortin, D.; Morales, J.M. A Memory-Based Forging Tactic Reveals an Adaptive Mechanism for Restricted Space-Use. Ecol. Lett. 2014, 17, 924–931. [Google Scholar] [CrossRef]
- Ranc, N.; Cagnacci, F.; Moorcroft, P.R. Memory Drives the Formation of Animal Home Ranges: Evidence from a Reintroduction. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kelt, D.A.; Heske, E.J.; Lambin, X.; Oli, M.K.; Orrock, J.L.; Ozgul, A.; Pauli, J.N.; Prugh, L.R.; Sollmann, R.; Sommer, S. Advances in Population Ecology and Species Interactions in Mammals. J. Mammal. 2020, 100, 965–1007. [Google Scholar] [CrossRef]

| Individual | Sex | DM | NV | MD (Sec) | ANOD | MNOD |
|---|---|---|---|---|---|---|
| 1 * | F | 132 | 672 | 94 | 1.23 | 3 |
| 2 | F | 132 | 219 | 382 | 1.59 | 3 |
| 3 * | F | 132 | 628 | 238 | 1.36 | 4 |
| 4 * | M | 132 | 485 | 130 | 1.41 | 3 |
| 5 | F | 128 | 1 | 210 | 1 | 0 |
| 6 * | F | 126 | 173 | 97 | 1.49 | 2 |
| 7 * | F | 114 | 180 | 49 | 1.51 | 2 |
| B ± SE | Pr (>|z|) | |
| Activation (O) | 0.82 ± 0.26 | 0.001 ** |
| EDF | p-Value | |
| S (Day of year) | 6.09 | <0.001 *** |
| S (Individual) | 4.59 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossi, F.; Ranc, N.; Moorcroft, P.; Bonanni, P.; Cagnacci, F. Ecological and Behavioral Drivers of Supplemental Feeding Use by Roe Deer Capreolus capreolus in a Peri-Urban Context. Animals 2020, 10, 2088. https://doi.org/10.3390/ani10112088
Ossi F, Ranc N, Moorcroft P, Bonanni P, Cagnacci F. Ecological and Behavioral Drivers of Supplemental Feeding Use by Roe Deer Capreolus capreolus in a Peri-Urban Context. Animals. 2020; 10(11):2088. https://doi.org/10.3390/ani10112088
Chicago/Turabian StyleOssi, Federico, Nathan Ranc, Paul Moorcroft, Priscilla Bonanni, and Francesca Cagnacci. 2020. "Ecological and Behavioral Drivers of Supplemental Feeding Use by Roe Deer Capreolus capreolus in a Peri-Urban Context" Animals 10, no. 11: 2088. https://doi.org/10.3390/ani10112088
APA StyleOssi, F., Ranc, N., Moorcroft, P., Bonanni, P., & Cagnacci, F. (2020). Ecological and Behavioral Drivers of Supplemental Feeding Use by Roe Deer Capreolus capreolus in a Peri-Urban Context. Animals, 10(11), 2088. https://doi.org/10.3390/ani10112088





