Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro uNDF Determination
2.2. Digestibility Determination with uNDF as the Internal Marker
(faecal nutrient content/diet nutrient content)]
2.3. Statistical Analysis
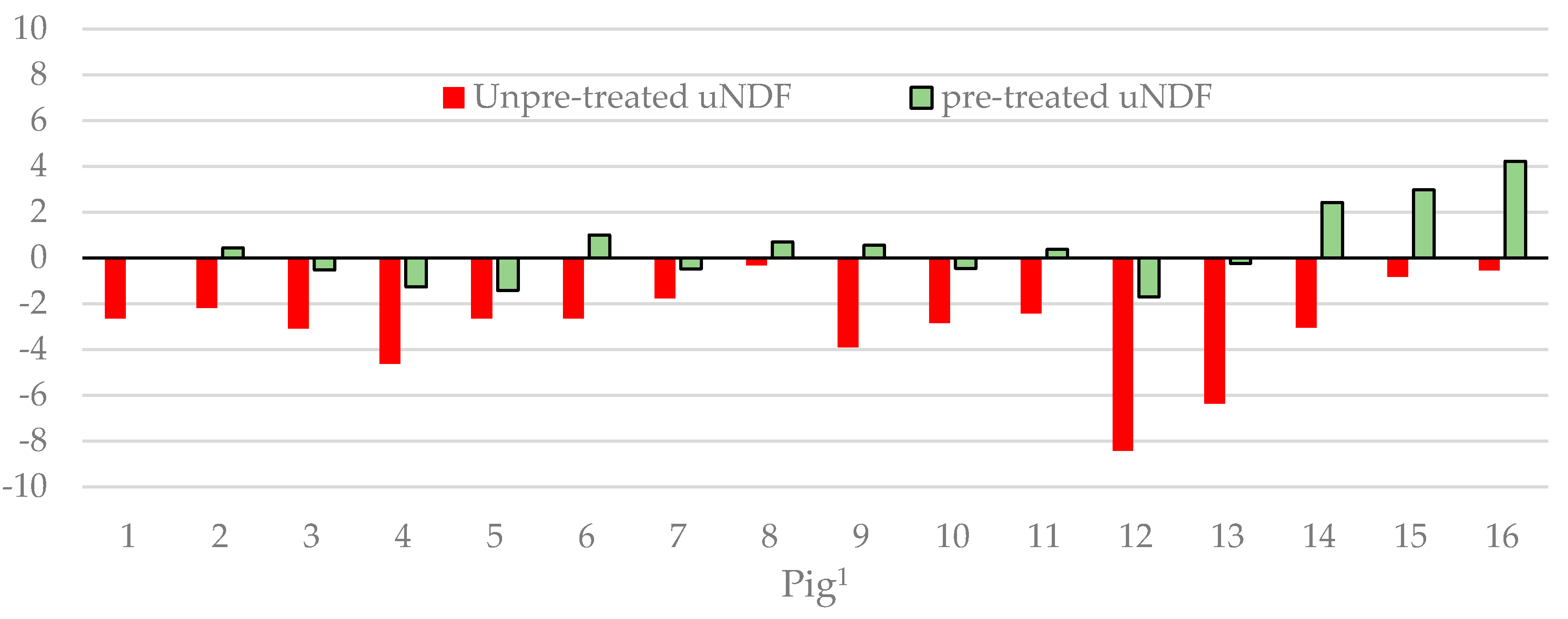
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niemi, J.K.; Sevón-Aimonen, M.-L.; Pietola, K.; Stalder, K.J. The value of precision feeding technologies for grow–finish swine. Livest. Sci. 2010, 129, 13–23. [Google Scholar] [CrossRef]
- Le Goff, G.; Noblet, J. Comparative total tract digestibility of dietary energy and nutrients in growing pigs and adult sows. J. Anim. Sci. 2001, 79, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Andretta, I.; Pomar, C.; Rivest, J.; Pomar, J.; Lovatto, P.A.; Neto, J.R. The impact of feeding growing–finishing pigs with daily tailored diets using precision feeding techniques on animal performance, nutrient utilization, and body and carcass composition. J. Anim. Sci. 2014, 92, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, D.; Bonnal, L.; Jaguelin-Peyraud, Y.; Noblet, J. Predicting feed digestibility from NIRS analysis of pig faeces. Animal 2014, 9, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S.; Lynch, P.; O’Mara, F.; Caffrey, P. A comparison of total collection and marker technique for the measurement of apparent digestibility of diets for growing pigs. Anim. Feed. Sci. Technol. 2001, 89, 49–58. [Google Scholar] [CrossRef]
- Jagger, S.; Wiseman, J.; Cole, D.J.A.; Craigon, J. Evaluation of inert markers for the determination of ileal and faecal apparent digestibility values in the pig. Br. J. Nutr. 1992, 68, 729–739. [Google Scholar] [CrossRef]
- Chiaravalli, M.; Rapetti, L.; Graziosi, A.R.; Galassi, G.; Crovetto, G.; Colombini, S. Comparison of Faecal versus Rumen Inocula for the Estimation of NDF Digestibility. Animals 2019, 9, 928. [Google Scholar] [CrossRef]
- Cotanch, K.W.; Grant, R.J.; Van Amburgh, M.E.; Zontini, A.; Fustini, M.; Palmonari, A.; Formigoni, A. Applications of uNDF in Ration Modeling and Formulation. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, Syracuse, NY, USA, 21–23 October 2014. [Google Scholar]
- Fustini, M.; Palmonari, A.; Canestrari, G.; Bonfante, E.; Mammi, L.; Pacchioli, M.; Sniffen, G.; Grant, R.; Cotanch, K.; Formigoni, A. Effect of undigested neutral detergent fiber content of alfalfa hay on lactating dairy cows: Feeding behavior, fiber digestibility, and lactation performance. J. Dairy Sci. 2017, 100, 4475–4483. [Google Scholar] [CrossRef]
- Righi, F.; Simoni, M.; Visentin, G.; Manuelian, C.L.; Currò, S.; Quarantelli, A.; De Marchi, M. The use of near infrared spectroscopy to predict faecal indigestible and digestible fibre fractions in lactating dairy cattle. Livest. Sci. 2017, 206, 105–108. [Google Scholar] [CrossRef]
- Adesogan, A. Effect of bag type on the apparent digestibility of feeds in ANKOM DaisyII incubators. Anim. Feed. Sci. Technol. 2005, 119, 333–344. [Google Scholar] [CrossRef]
- Damiran, D.; DelCurto, T.; Bohnert, D.; Findholt, S. Comparison of techniques and grinding size to estimate digestibility of forage based ruminant diets. Anim. Feed. Sci. Technol. 2008, 141, 15–35. [Google Scholar] [CrossRef]
- Earing, J.E.; Cassill, B.D.; Hayes, S.H.; VanZant, E.S.; Lawrence, L.M. Comparison of in vitro digestibility estimates using the DaisyII incubator with in vivo digestibility estimates in horses. J. Anim. Sci. 2010, 88, 3954–3963. [Google Scholar] [CrossRef]
- Tassone, S.; Renna, M.; Barbera, S.; Valle, E.; Fortina, R. In Vitro Digestibility Measurement of Feedstuffs in Donkeys Using the DaisyII Incubator. J. Equine Vet. Sci. 2019, 75, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tagliapietra, F.; Cattani, M.; Hindrichsen, I.K.; Hansen, H.H.; Colombini, S.; Bailoni, L.; Schiavon, S. True dry matter digestibility of feeds evaluated in situ with different bags and in vitro using rumen fluid collected from intact donor cows. Anim. Prod. Sci. 2012, 52, 338–346. [Google Scholar] [CrossRef]
- Seifried, N.; Steingaß, H.; Rodehutscord, M. In vitro and in situ evaluation of secondary starch particle losses from nylon bags during the incubation of different cereal grains. Anim. Feed. Sci. Technol. 2015, 210, 26–36. [Google Scholar] [CrossRef]
- Mabjeesh, S.; Cohen, M.; Arieli, A. In Vitro Methods for Measuring the Dry Matter Digestibility of Ruminant Feedstuffs: Comparison of Methods and Inoculum Source. J. Dairy Sci. 2000, 83, 2289–2294. [Google Scholar] [CrossRef]
- Galassi, G.; Malagutti, L.; Colombini, S.; Rapetti, L.; Gallo, L.; Schiavon, S.; Tagliapietra, F.; Crovetto, G.M. Nitrogen and Energy Partitioning in Two Genetic Groups of Pigs Fed Low-Protein Diets at 130 Kg Body Weight. Ital. J. Anim. Sci. 2015, 14, 293–298. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th rev. ed.; The National Academies Press: Washington, DC, USA, 2012.
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Ankom Technology. In Vitro True Digestibility Using the DAISY Incubator. 2005. Ankom Technology. 2020, p. 1. Available online: http:www.ankom.commediadocumentsIVDMD_0805_D200.pdf (accessed on 13 September 2010).
- Schalla, A.; Meyer, L.; Meyer, Z.; Onetti, S.; Schultz, A.; Goeser, J. Hot topic: Apparent total-tract nutrient digestibilities measured commercially using 120-hour in vitro indigestible neutral detergent fiber as a marker are related to commercial dairy cattle performance. J. Dairy Sci. 2012, 95, 5109–5114. [Google Scholar] [CrossRef]
- Tassone, S.; Fortina, R.; Peiretti, P.G. In Vitro Techniques Using the DaisyII Incubator for the Assessment of Digestibility: A Review. Animals 2020, 10, 775. [Google Scholar] [CrossRef]
- Lopes, J.; Shaver, R.; Hoffman, P.; Akins, M.; Bertics, S.; Gencoglu, H.; Coors, J. Type of corn endosperm influences nutrient digestibility in lactating dairy cows. J. Dairy Sci. 2009, 92, 4541–4548. [Google Scholar] [CrossRef]
- Lund, P.; Weisbjerg, M.R.; Hvelplund, T.; Knudsen, K.E.B. Determination of digestibility of different forages in dairy cows using indigestible NDF as marker. Acta Agric. Scand. Sect. A Anim. Sci. 2007, 57, 16–29. [Google Scholar] [CrossRef]
- Morris, D.; Rebelo, L.; Dieter, P.; Lee, C. Validating intrinsic markers and optimizing spot sampling frequency to estimate fecal outputs. J. Dairy Sci. 2018, 101, 7980–7989. [Google Scholar] [CrossRef]
- De Carvalho, G.G.P.; Garcia, R.; Pires, A.J.V.; Silva, R.R.; Detmann, E.; Oliveira, R.; Ribeiro, L.S.O. Long-term Bias of Internal Markers in Sheep and Goat Digestion Trials. Asian-Australas. J. Anim. Sci. 2013, 26, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-C.; Croom, J.; Chuang, S.-T.; Chiou, P.W.S.; Yu, B. Development of a Dynamic System Simulating Pig Gastric Digestion. Asian-Australas. J. Anim. Sci. 2008, 21, 1522–1528. [Google Scholar] [CrossRef]
| CONV 1 | LP 2 | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | Total Faecal Collection | Pre-treated uNDF | Unpre-treated uNDF | Total Faecal Collection | Pre-treated uNDF | Unpre-treated uNDF | D 3 | M 4 | D*M 5 | |
| DM 6 | 87.6 a | 87.5 a | 84.8 b | 86.9 a | 88.0 a | 82.8 c | 0.344 | 0.072 | <0.001 | 0.037 |
| OM 7 | 89.3 a | 89.1 a | 86.8 b | 89.5 a | 89.5 a | 85.0 c | 0.303 | 0.060 | <0.001 | 0.039 |
| NDF 8 | 57.7 a | 56.9 a b | 47.8 c | 54.8 a b | 52.9 b | 32.4 d | 1.174 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battelli, M.; Rapetti, L.; Rota Graziosi, A.; Colombini, S.; Crovetto, G.M.; Galassi, G. Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs. Animals 2020, 10, 2007. https://doi.org/10.3390/ani10112007
Battelli M, Rapetti L, Rota Graziosi A, Colombini S, Crovetto GM, Galassi G. Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs. Animals. 2020; 10(11):2007. https://doi.org/10.3390/ani10112007
Chicago/Turabian StyleBattelli, Marco, Luca Rapetti, Andrea Rota Graziosi, Stefania Colombini, Gianni Matteo Crovetto, and Gianluca Galassi. 2020. "Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs" Animals 10, no. 11: 2007. https://doi.org/10.3390/ani10112007
APA StyleBattelli, M., Rapetti, L., Rota Graziosi, A., Colombini, S., Crovetto, G. M., & Galassi, G. (2020). Use of Undigested NDF for Estimation of Diet Digestibility in Growing Pigs. Animals, 10(11), 2007. https://doi.org/10.3390/ani10112007







