Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
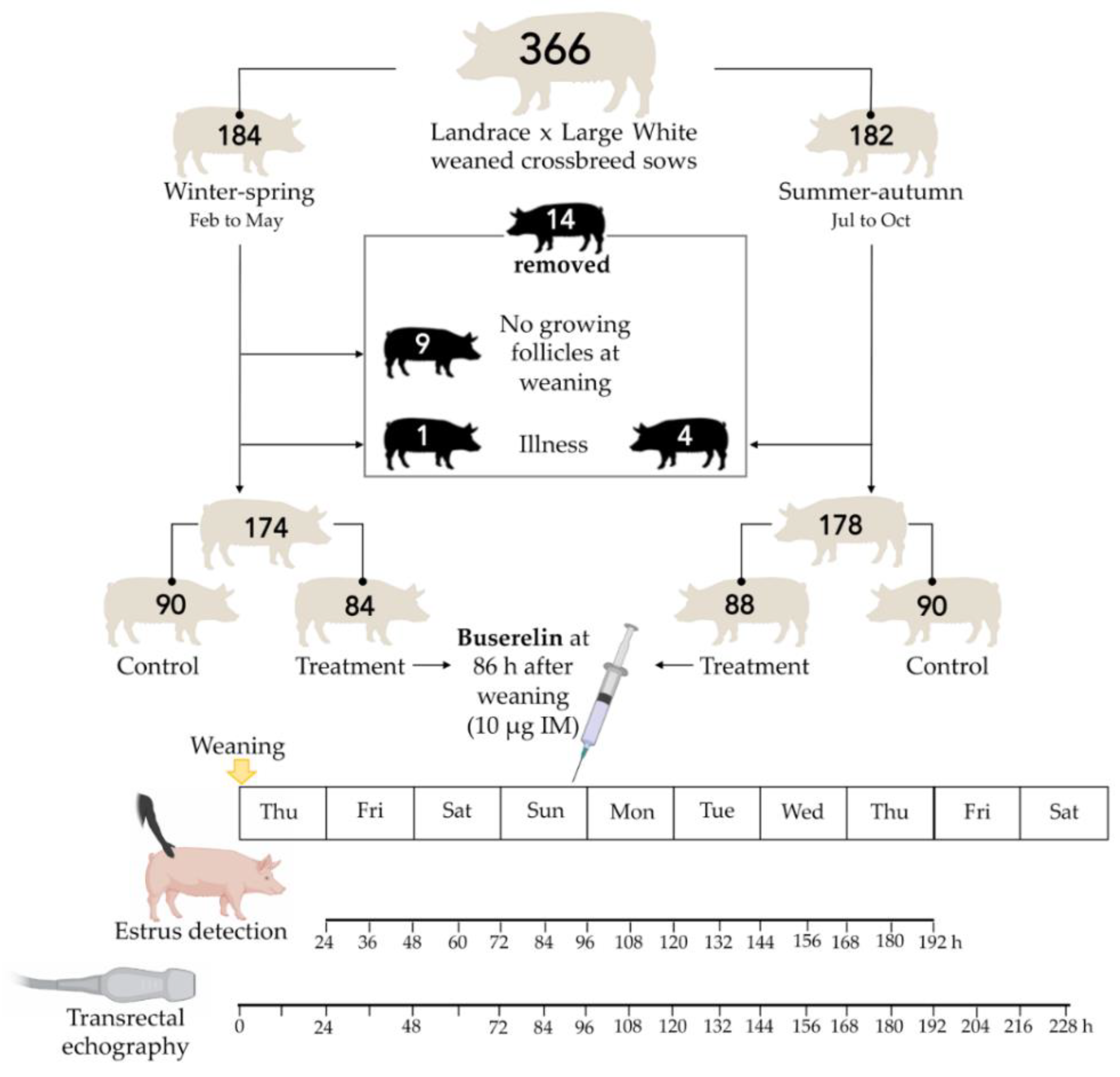
2.1. Farm, Animals and Handling
2.2. Estrus Detection and Insemination
2.3. Transrectal Ovarian Ultrasonography
2.4. Experimental Design
2.5. Statistical Analysis
3. Results
3.1. Reproductive Performance of GnRH Agonist-Treated Sows vs. Untreated Controls
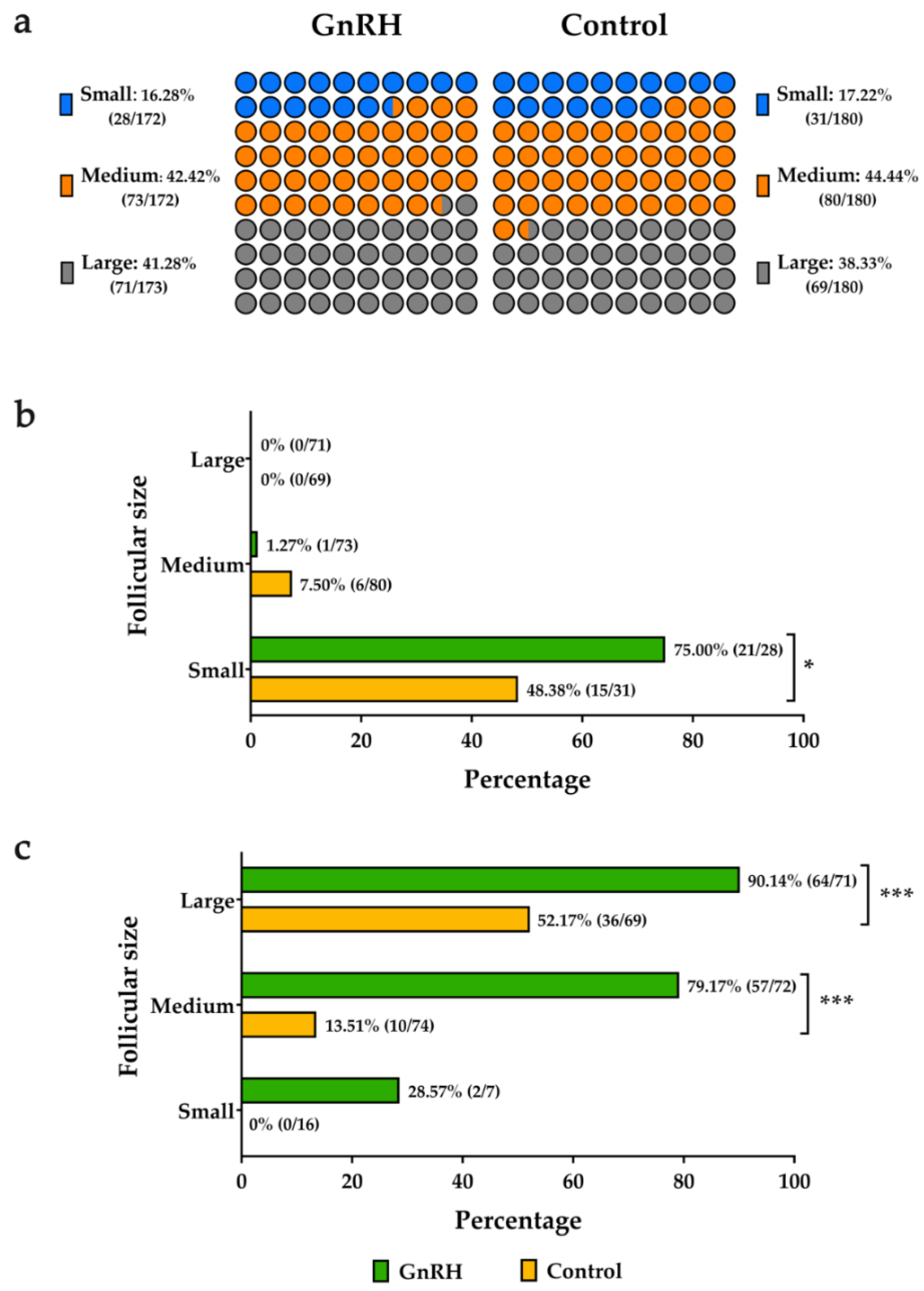
3.2. Influence of Follicular Size on the Response to GnRH Agonist Treatment
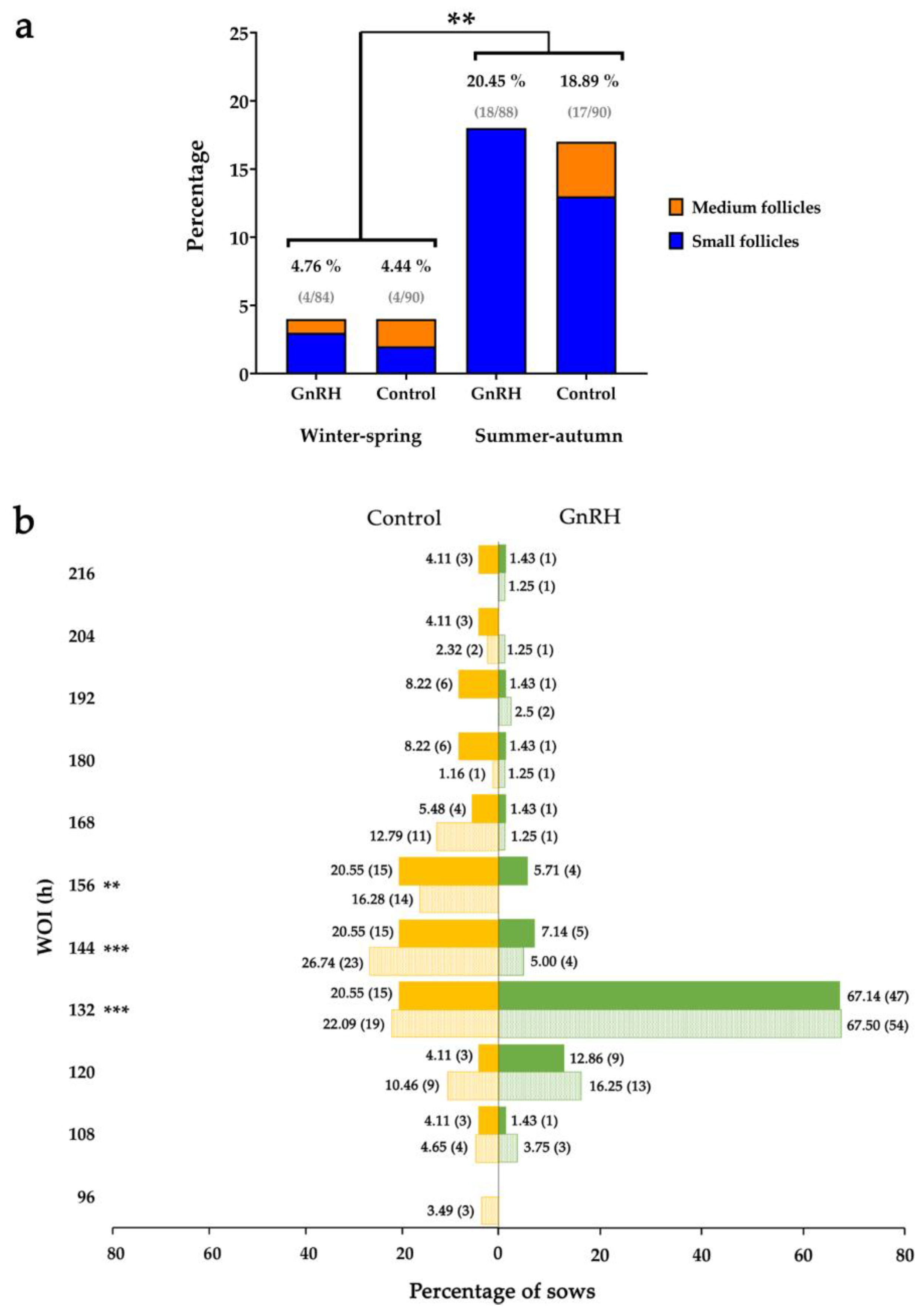
3.3. Influence of the Period of Weaning on the Response of Sows to GnRH Agonist Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bortolozzo, F.P.; Menegat, M.B.; Mellagi, A.P.G.; Bernardi, M.L.; Wentz, I. New Artificial Insemination Technologies for Swine. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 80–84. [Google Scholar] [CrossRef]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, O.; Björkman, S.; Oropeza-Moe, M.; Oliviero, C. Developments of reproductive management and biotechnology in the pig. Anim. Reprod. 2019, 16, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Driancourt, M.A. Fixed time artificial insemination in gilts and sows. Tools, schedules and efficacy. Soc. Reprod. Fertil. 2013, 68, 89–99. [Google Scholar]
- Kirkwood, R.N.; Kauffold, J. Advances in Breeding Management and Use of Ovulation Induction for Fixed-time AI. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 85–89. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef]
- Knox, R.V.; Esparza-Harris, K.C.; Johnston, M.E.; Webel, S.K. Effect of numbers of sperm and timing of a single, post-cervical insemination on the fertility of weaned sows treated with OvuGel®. Theriogenology 2017, 92, 197–203. [Google Scholar] [CrossRef]
- De Rensis, F.; Kirkwood, R.N. Control of estrus and ovulation: Fertility to timed insemination of gilts and sows. Theriogenology 2016, 86, 1460–1466. [Google Scholar] [CrossRef]
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]
- Brüssow, K.P.; Schneider, F.; Kanitz, W.; Rátky, J.; Kauffold, J.; Wähner, M. Studies on fixed-time ovulation induction in the pig. Soc. Reprod. Fertil. Suppl. 2009, 66, 187–195. [Google Scholar] [CrossRef]
- Nissen, A.K.; Lehn-Jensen, H.; Hyttel, P.; Greve, T. Follicular development and ovulation in sows: Effect of hCG and GnRH treatment. Acta Veter Scand. 1995, 36, 123–133. [Google Scholar]
- Cassar, G.; Kirkwood, R.N.; Poljak, Z.; Friendship, R. Effect of estrogen formulation and site of deposition on fertility of artificially inseminated sows treated with human chorionic gonadotrophin to induce ovulation. J. Swine Health Prod. 2004, 12, 285–287. [Google Scholar]
- Bolarín, A.; Hernández, M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Martinez, E.A.; Roca, J. Use of frozen-thawed semen aggravates the summer-autumn infertility of artificially inseminated weaned sows in the Mediterranean region. J. Anim. Sci. 2009, 87, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.L.; Ulguim, R.R.; Sbardella, P.E.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Fixed-time post-cervical artificial insemination in sows receiving porcine luteinising hormone at oestrus onset. Anim. Reprod. Sci. 2014, 144, 109–114. [Google Scholar] [CrossRef]
- McBride, M.; Amezcua, R.; Cassar, G.; O’Sullivan, T.; Friendship, R. Combining Fixed-Time Insemination and Improved Catheter Design in an Effort to Improve Swine Reproduction Efficiency. Animals 2019, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Martinat-Botté, F.; Venturi, E.; Guillouet, P.; Driancourt, M.A.; Terqui, M. Induction and synchronization of ovulations of nulliparous and multiparous sows with an injection of gonadotropin-releasing hormone agonist (Receptal). Theriogenology 2010, 73, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Driancourt, M.A.; Cox, P.; Rubion, S.; Harnois-Milon, G.; Kemp, B.; Soede, N.M. Induction of an LH surge and ovulation by buserelin (as Receptal) allows breeding of weaned sows with a single fixed-time insemination. Theriogenology 2013, 80, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Pearodwong, P.; Tretipskul, C.; Soede, N.M.; Tummaruk, P. Factors affecting estrus and ovulation time in weaned sows with induced ovulation by GnRH administration in different seasons. J. Veter Med. Sci. 2019, 81, 1567–1574. [Google Scholar] [CrossRef]
- Fries, H.C.C.; Souza, L.P.; Faccin, J.E.G.; Reckziegel, M.V.; Hernig, L.; Marimon, B.T.; Bernardi, M.L. Induction and synchronization of ovulation in sows using a Gonadotropin-releasing Hormone Analog (Lecirelin). Anim. Reprod. 2010, 55, 362–366. [Google Scholar]
- Knox, R.V.; Willenburg, K.L.; Rodriguez-Zas, S.L.; Greger, D.L.; Hafs, H.D.; Swanson, M.E. Synchronization of ovulation and fertility in weaned sows treated with intravaginal triptorelin is influenced by timing of administration and follicle size. Theriogenology 2011, 75, 308–319. [Google Scholar] [CrossRef]
- Dillard, D.S.; Flowers, W.L. Reproductive performance of sows associated with single, fixed-time insemination programs in commercial farms based on either average herd weaning-to-estrus intervals or postweaning estrous activity. Appl. Anim. Sci. 2020, 36, 100–107. [Google Scholar] [CrossRef]
- Knox, R.V. Factors influencing follicle development in gilts and sows and management strategies used to regulate growth for control of estrus and ovulation. J. Anim. Sci. 2019, 97, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.P.; Padilla, L.; Bolarin, A.; Rodriguez-Martinez, H.; Roca, J. Ovarian Follicle Growth during Lactation Determines the Reproductive Performance of Weaned Sows. Animals 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- De Rensis, F.; Ziecik, A.J.; Kirkwood, R.N. Seasonal infertility in gilts and sows: Aetiology, clinical implications and treatments. Theriogenology 2017, 96, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.P.; Sanchez-Osorio, J.; Bolarin, A.; Martinez, E.A.; Roca, J. Relevance of ovarian follicular development to the seasonal impairment of fertility in weaned sows. Vet. J. 2014, 199, 382–386. [Google Scholar] [CrossRef]
- Knox, R.V.; Probst-Miller, S. Evaluation of transrectal real-time ultrasound for use in identifying sources of reproductive failure in weaned sows. J. Swine Health Prod. 2004, 12, 71–74. [Google Scholar]
- Kauffold, J.; Peltoniemi, O.; Wehrend, A.; Althouse, G.C. Principles and Clinical Uses of Real-Time Ultrasonography in Female Swine Reproduction. Animals 2019, 9, 950. [Google Scholar]
- Bolarin, A.; Vazquez, J.M.; Parrilla, I.; Vazquez, J.L.; Martinez, E.A.; Roca, J. Validation of trans-rectal ultrasonography for counting preovulatory follicles in weaned sows. Anim. Reprod. Sci. 2009, 113, 137–142. [Google Scholar] [CrossRef]
- van de Wiel, D.F.; Booman, P. Post-weaning anoestrus in primiparous sows: LH patterns and effects of gonadotropin injection and boar exposure. Vet. Q. 1993, 15, 162–166. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.S.; Wang, X.Y.; Peng, J.L.; Huang, X.Q.; Tian, H.; Wei, Q.H.; Wang, L.Q. Effects of fixed-time artificial insemination using triptorelin on the reproductive performance of pigs: A meta-analysis. Animal 2020, 14, 1481–1492. [Google Scholar] [CrossRef]
- Ulguim, R.R.; Bortolozzo, F.P.; Wentz, I.; Johnston, M.; Webel, S.K.; Arend, L.; Knox, R. V Ovulation and fertility responses for sows receiving once daily boar exposure after weaning and OvuGel® followed by a single fixed time post cervical artificial insemination. Theriogenology 2018, 105, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bracken, C.J.; Radcliff, R.P.; McCormack, B.L.; Keisler, D.H.; Lucy, M.C. Decreased follicular size during late lactation caused by treatment with charcoal-treated follicular fluid delays onset of estrus and ovulation after weaning in sows. J. Anim. Sci. 2006, 84, 2110–2117. [Google Scholar] [CrossRef]
- Schuberth, H.J.; Taylor, U.; Zerbe, H.; Waberski, D.; Hunter, R.; Rath, D. Immunological responses to semen in the female genital tract. Theriogenology 2008, 70, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.G.; Robinson, R.S.; Mann, G.E.; Webb, R. Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim. Reprod. Sci. 2004, 82–83, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Holyoake, P.K.; Evans, G.; Grupen, C.G. Seasonal variation in the ovarian function of sows. Reprod. Fertil. Dev. 2012, 24, 822–834. [Google Scholar] [CrossRef]

| Characteristics | Weaned Sows | Probability | |
|---|---|---|---|
| GnRH | Control | ||
| Body condition 1 | 3.04 ± 0.02 | 2.99 ± 0.02 | Ns 2 |
| Lactation period (d) | 22.88 ± 0.12 | 22.87 ± 0.13 | ns |
| Parities | |||
| 1 | 29 (16.86%) | 33 (18.33%) | ns |
| 2 | 44 (25.58%) | 35 (19.44%) | |
| 3 | 23 (13.37%) | 28 (15.56%) | |
| 4 | 30 (17.44%) | 35 (19.44%) | |
| 5 | 21 (12.21%) | 28 (15.56%) | |
| 6 | 25 (14.53%) | 21 (11.67%) | |
| Ovary follicles 3 | |||
| Diameter (cm) | 0.37 ± 0.01 | 0.37 ± 0.01 | ns |
| Number 4 | 18.15 ± 0.32 | 18.23 ± 0.31 | ns |
| Variable | Farrowing Rate (%) | Litter Size | ||
|---|---|---|---|---|
| GnRH | Control | GnRH | Control | |
| All sows | 128/150 (85.33%) | 139/159 (87.42%) | 13.15 ± 0.28 | 13.15 ± 0.28 |
| Sows ovulating in time window * | 105/123 (85.37%) | 40/46 (86.96%) | 13.25 ± 0.29 | 13.98 ± 0.36 |
| Ovarian follicular diameter | ||||
| Small (<0.5 cm) | 6/28 a (21.43%) | 13/31 a (41.93%) | 11.17 ± 1.14 x | 12.00 ± 0.89 x |
| Medium (0.5–0.64 cm) | 62/73 b (84.93%) | 65/80 b (81.25%) | 12.68 ± 0.41 z | 12.91 ± 0.33 z |
| Large (>0.64 cm) | 60/71 b (84.51%) | 61/69 b (88.41%) | 13.70 ± 0.34 z | 13.75 ± 0.33 z |
| Period of weaning | ||||
| Winter–spring | 71/128 m (84.52%) | 77/139 m (85.56%) | 13.47 ± 0.30 x | 13.62 ± 0.29 x |
| Summer–autumn | 57/128 n (64.77%) | 62/128 n (68.89%) | 12.63 ± 0.46 z | 12.66 ± 0.37 z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, T.P.; Padilla, L.; Bolarin, A.; Rodriguez-Martinez, H.; Roca, J. Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin. Animals 2020, 10, 1979. https://doi.org/10.3390/ani10111979
Lopes TP, Padilla L, Bolarin A, Rodriguez-Martinez H, Roca J. Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin. Animals. 2020; 10(11):1979. https://doi.org/10.3390/ani10111979
Chicago/Turabian StyleLopes, Tania P., Lorena Padilla, Alfonso Bolarin, Heriberto Rodriguez-Martinez, and Jordi Roca. 2020. "Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin" Animals 10, no. 11: 1979. https://doi.org/10.3390/ani10111979
APA StyleLopes, T. P., Padilla, L., Bolarin, A., Rodriguez-Martinez, H., & Roca, J. (2020). Weaned Sows with Small Ovarian Follicles Respond Poorly to the GnRH Agonist Buserelin. Animals, 10(11), 1979. https://doi.org/10.3390/ani10111979









