Phylogenetic Relationships of Turkish Indigenous Donkey Populations Determined by Mitochondrial DNA D-loop Region
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Determination, Sample Collection, and DNA Isolation
2.2. Amplification of mtDNA Control Regions (D-Loop) and Sequencing
2.3. Data Analyses
3. Results
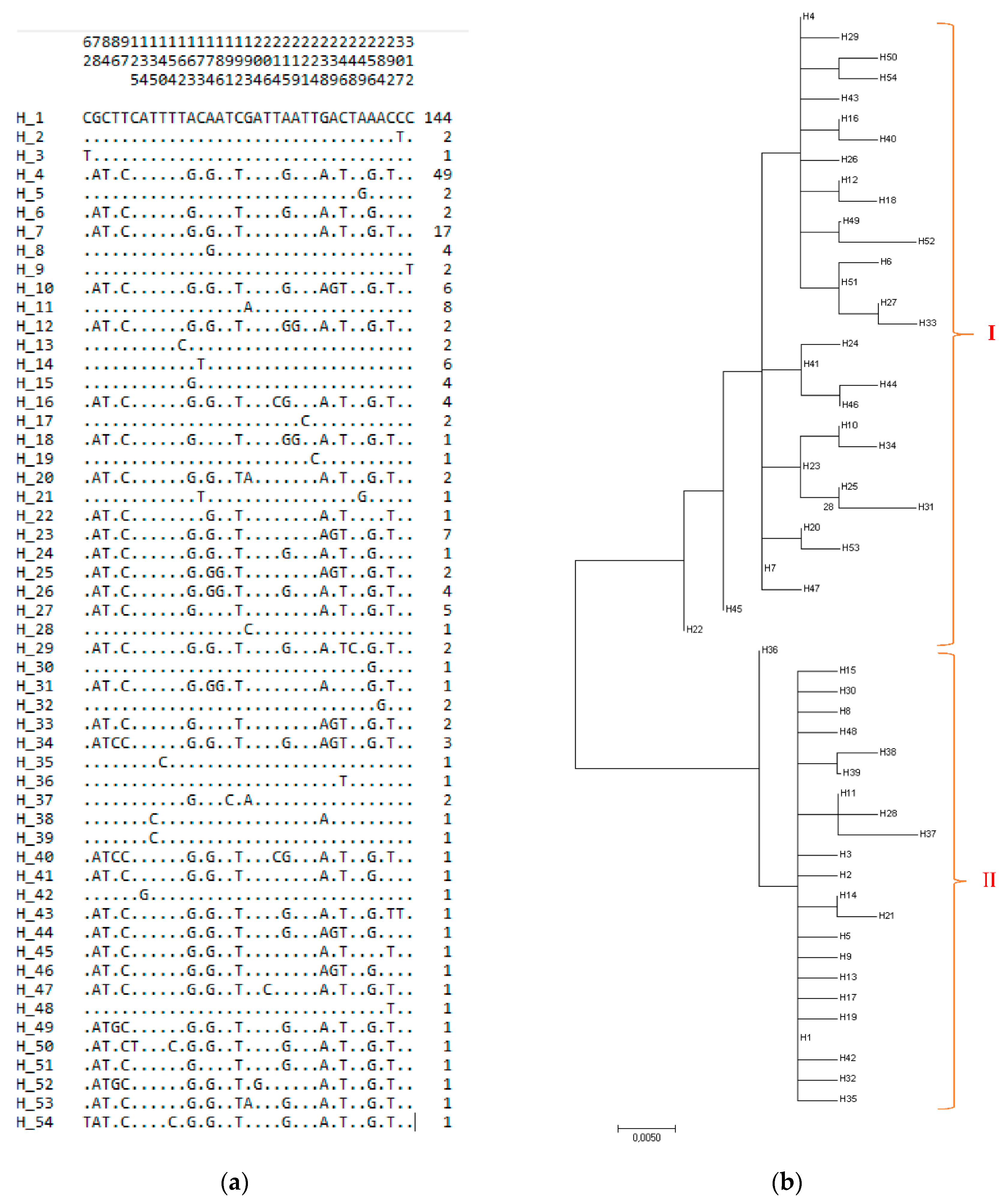
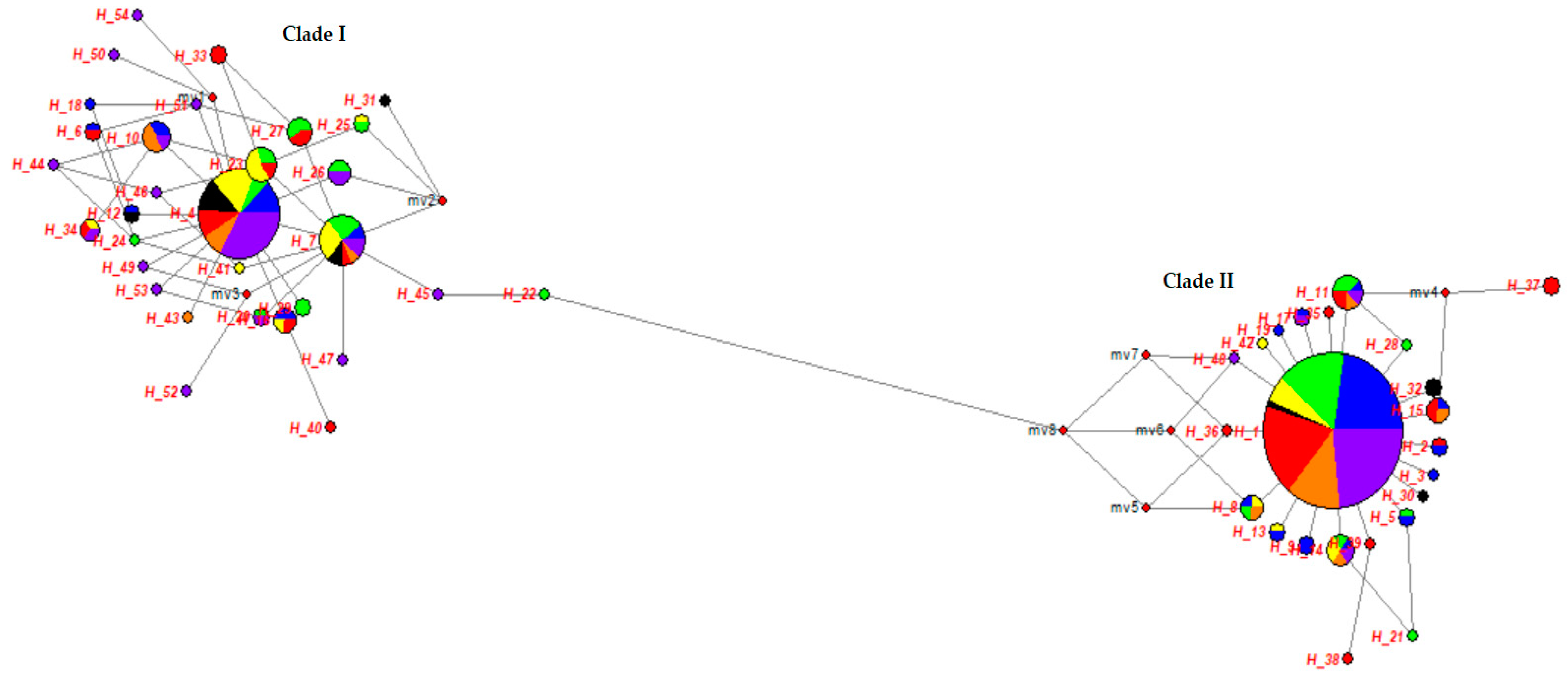
3.1. Genetic Diversity of the mtDNA D-Loop Analyses of Turkish Indigenous Donkeys
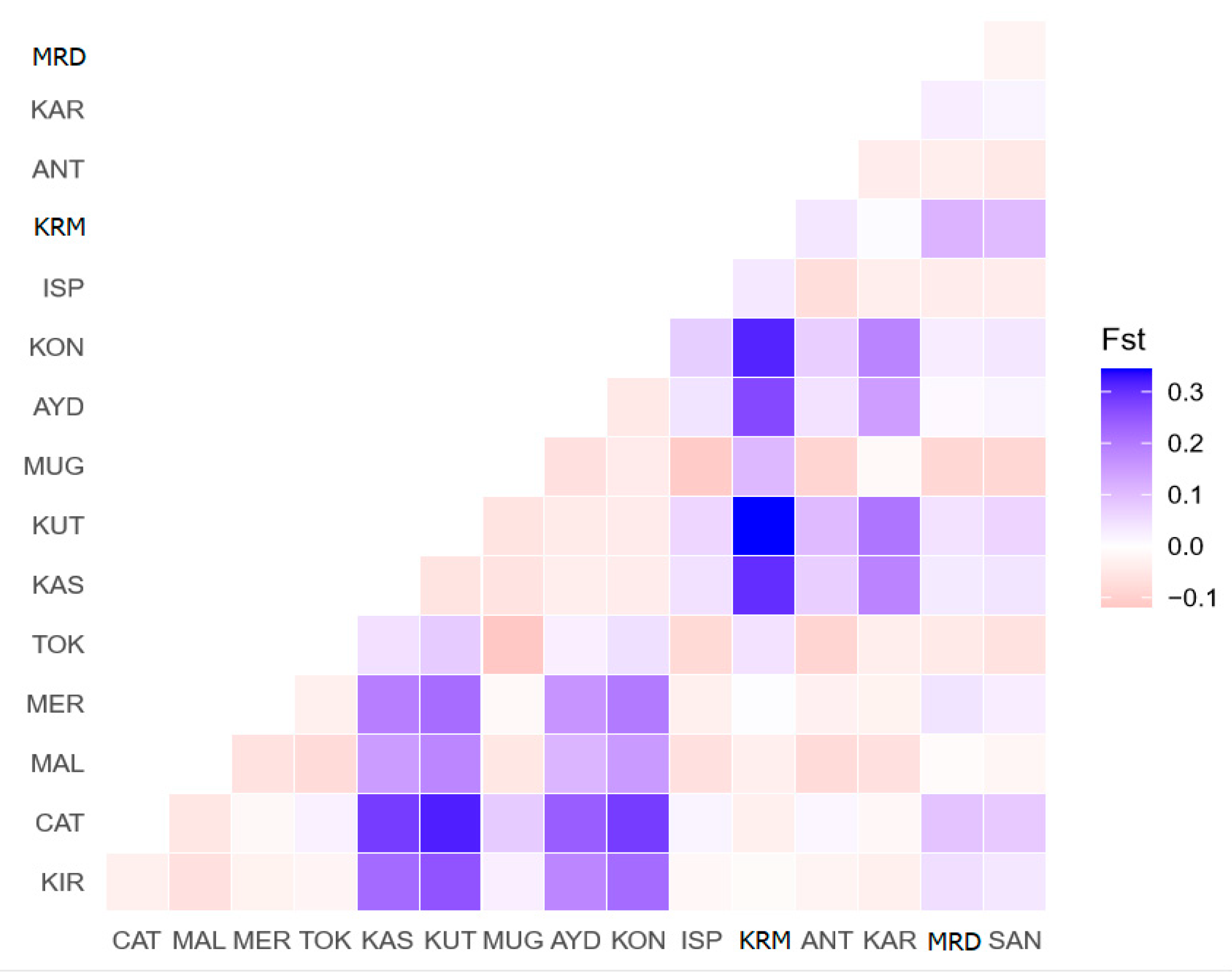
3.2. Turkish Donkey Populations Comparing with Other Donkey Populations
4. Discussion
4.1. Phylogenetic Relationships of Turkish Indigenous Donkey Populations
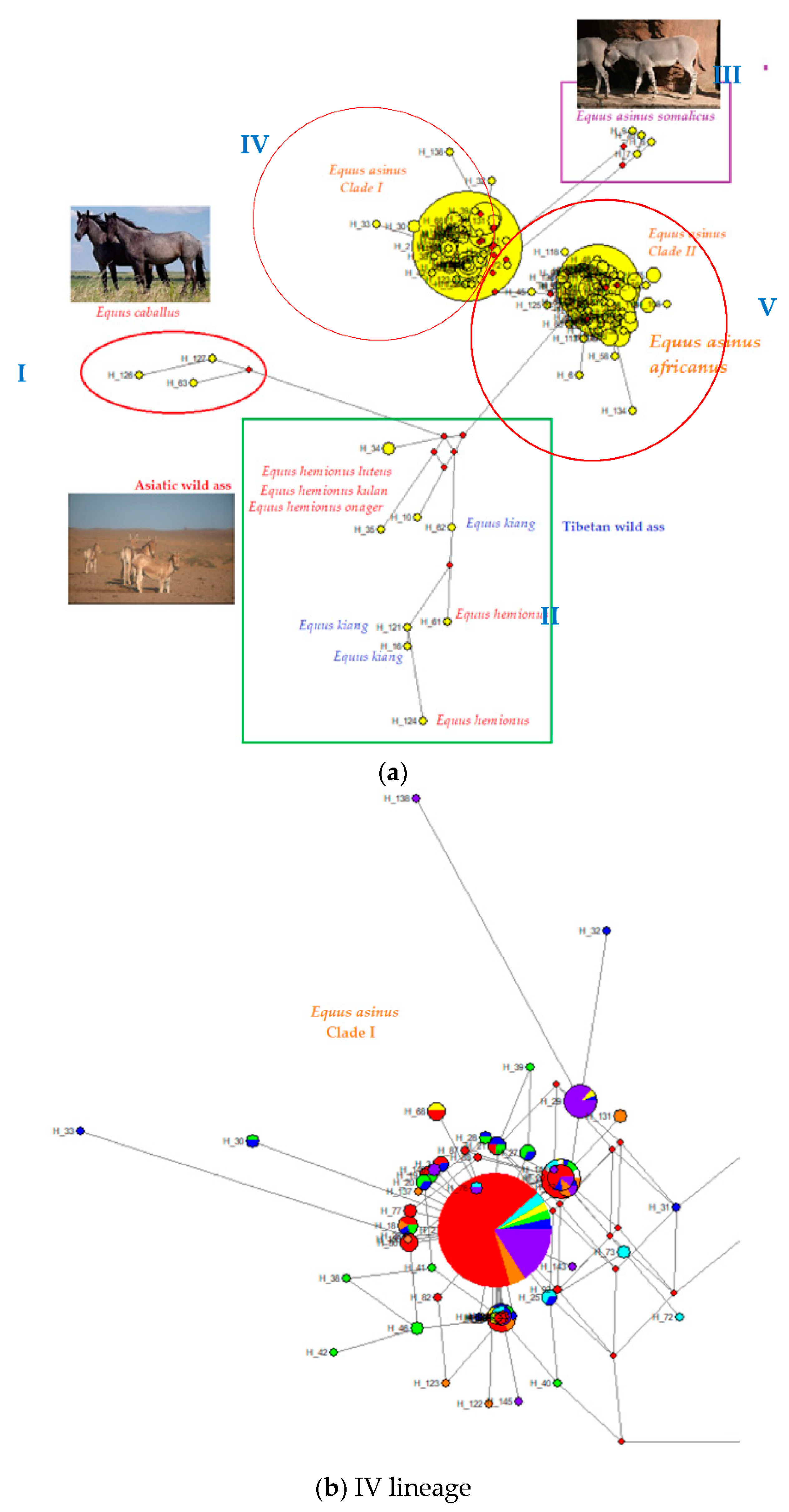
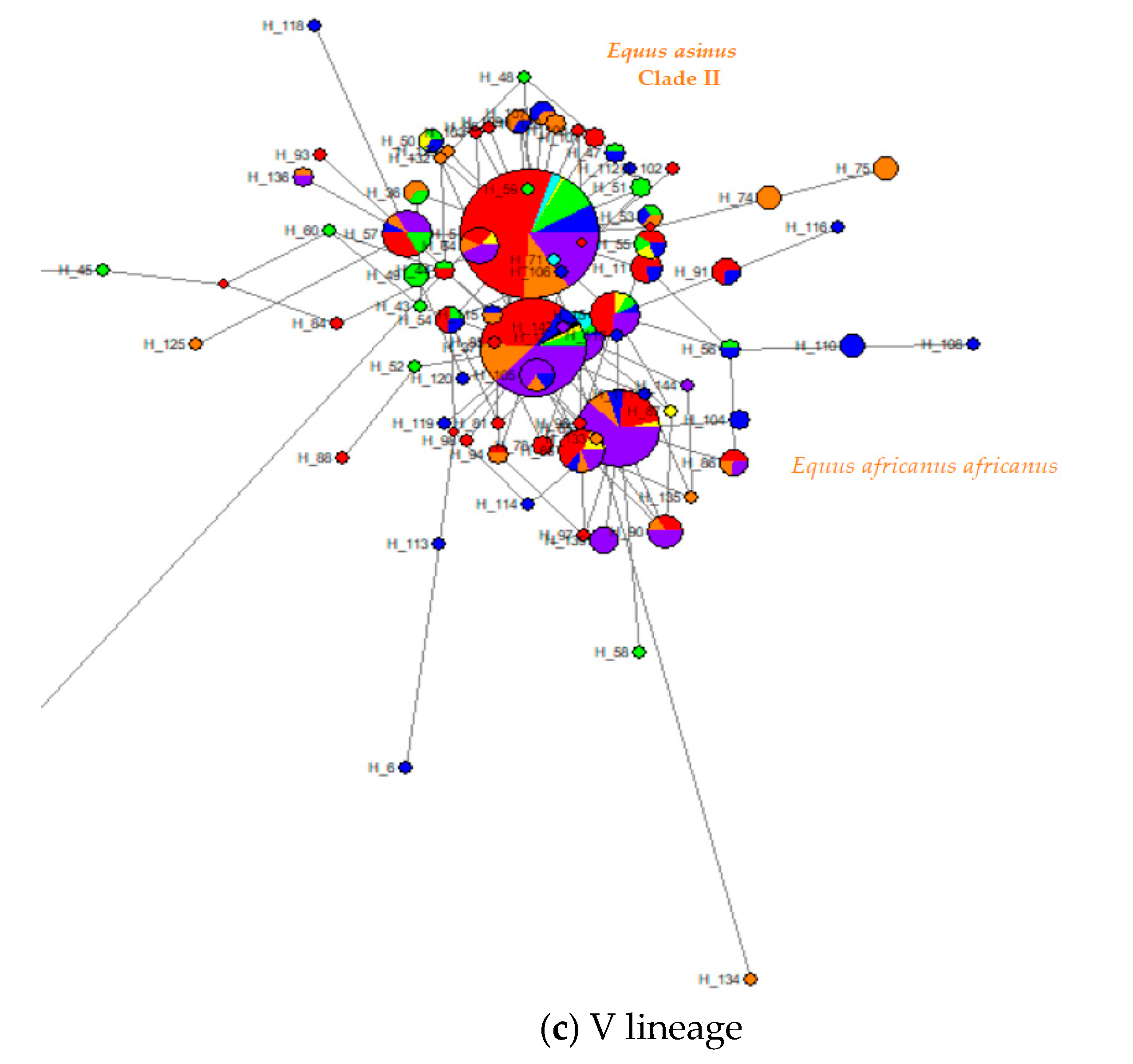
4.2. Phylogenetic Relationships between Turkish Native Donkeys and Other Donkeys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anthony, D.W.; Bogucki, P.; Comşa, E.; Gimbutas, M.; Jovanović, B.; Mallory, J.P.; Milisaukas, S. The “Kurgan Culture,” Indo-European Origins, and the Domestication of the Horse: A Reconsideration [and Comments and Replies]. Curr. Anthropol. 1986, 27, 291–313. [Google Scholar] [CrossRef]
- Levine, M.; Rassamakin, Y.; Kislenko, A.; Tatarintseva, N. Late prehistoric exploitation of the Eurasian steppe. Camb. Archeol. J. 1999, 9, b1–b6. [Google Scholar]
- Meadows, J.R.S.; Hanotte, O.; Drögemüller, C.; Calvo, J.; Godfrey, R.; Coltman, D.; Maddox, J.F.; Marzanov, N.; Kantanen, J.; Kijas, J.W. Globally dispersed Y chromosomal haplotypes in wild and domestic sheep. Anim. Genet. 2006, 37, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Aranguren-Mendez, J.; Beja-Pereira, A.; Avellanet, R.; Dzama, K.; Jordana, J. Mitochondrial DNA variation and genetic relationships in Spanish donkey breeds (Equus asinus). J. Anim. Breed. Genet. 2004, 121, 319–330. [Google Scholar] [CrossRef]
- Han, L.; Yu, H.X.; Cai, D.W.; Shi, H.L.; Zhu, H.; Zhou, H. Mitochondrial DNA analysis provides new insights into the origin of the Chinese domestic goat. Small Rumin. Res. 2010, 90, 41–46. [Google Scholar] [CrossRef]
- Cai, D.; Tang, Z.; Yu, H.; Han, L.; Ren, X.; Zhao, X.; Zhu, H.; Zhou, H. Early history of Chinese domestic sheep indicated by ancient DNA analysis of Bronze Age individuals. J. Archaeol. Sci. 2011, 38, 896–902. [Google Scholar] [CrossRef]
- Bruford, M.W.; Bradley, D.G.; Luikart, G. DNA markers reveal the complexity of livestock domestication. Nat. Rev. Genet. 2003, 4, 900–910. [Google Scholar] [CrossRef]
- Wilson, A.C.; Cann, R.L.; Carr, S.M.; George, M.; Gyllensten, U.B.; Helm-Bychowski, K.M.; Higuchi, R.G.; Palumbi, S.R.; Prager, E.M.; Sage, R.D.; et al. Mitochondrial DNA and two perspectives on evolutionary genetics. Biol. J. Linn. Soc. 1985, 26, 375–400. [Google Scholar] [CrossRef]
- Gissi, C.; Reyes, A.; Pesole, G.; Saccone, C. Lineage-specific evolutionary rate in mammalian mtDNA. Mol. Biol. Evol. 2000, 17, 1022–1031. [Google Scholar] [CrossRef]
- Wolf, C.; Rentsch, J.; Hübner, P. PCR-RFLP analysis of mitochondrial DNA: A reliable method for species identification. J. Agric. Food Chem. 1999, 47, 1350–1355. [Google Scholar] [CrossRef]
- Xu, X.; Gullberg, A.; Arnason, U. The complete mitochondrial DNA (mtDNA) of the donkey and mtDNA comparisons among four closely related mammalian species-pairs. J. Mol. Evol. 1996, 43, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, A.; Kavar, T.; Caput, P.; Mioc, B.; Pavic, V.; Dovc, P. Genetic diversity of three donkey populations in the Croatian coastal region. Anim. Genet. 2002, 33, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lopez Lopez, C.; Alonso, R.; De Aluja, A.S. Study of the genetic origin of the Mexican Creole donkey (Equus asinus) by means of the analysis of the D-loop region of mitochondrial DNA. Trop. Anim. Health Prod. 2005, 37, 173–188. [Google Scholar] [CrossRef]
- Rossel, S.; Marshall, F.; Peters, J.; Pilgram, T.; Adams, M.D.; O’Connor, D. Domestication of the donkey: Timing, processes, and indicators. Proc. Natl. Acad. Sci. USA 2008, 105, 3715–3720. [Google Scholar] [CrossRef]
- Stanisic, L.J.; Aleksic, J.M.; Dimitrijevic, V.; Simeunovic, P.; Glavinic, U.; Stevanovic, J.; Stanimirovic, Z. New insights into the origin and the genetic status of the Balkan donkey from Serbia. Anim. Genet. 2017, 48, 580–590. [Google Scholar] [CrossRef]
- Beja-Pereira, A.; England, P.R.; Ferrand, N.; Jordan, S.; Bakhiet, A.O.; Abdalla, M.A.; Mashkour, M.; Jordana, J.; Taberlet, P.; Luikart, G. African origins of the domestic donkey. Science 2004, 304, 1781. [Google Scholar] [CrossRef]
- Chen, S.Y.; Zhou, F.; Xiao, H.; Sha, T.; Wu, S.F.; Zhang, Y.P. Mitochondrial DNA diversity and population structure of four Chinese donkey breeds. Anim. Genet. 2006, 37, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Kimura, B.; Marshall, F.B.; Chen, S.; Rosenbom, S.; Moehlman, P.D.; Tuross, N.; Sabin, R.C.; Peters, J.; Barich, B.; Yohannes, H.; et al. Ancient DNA from Nubian and Somali wild ass provides insights into donkey ancestry and domestication. Proc. R. Soc. B Biol. Sci. 2011, 278, 50–57. [Google Scholar] [CrossRef]
- Kefena, E.; Dessie, T.; Tegegne, A.; Beja-Pereira, A.; Yusuf Kurtu, M.; Rosenbom, S.; Han, J.L. Genetic diversity and matrilineal genetic signature of native Ethiopian donkeys (Equus asinus) inferred from mitochondrial DNA sequence polymorphism. Livest. Sci. 2014, 167, 73–79. [Google Scholar] [CrossRef]
- Yılmaz, O.; Wilson, T.R. The domestic livestock resources of Turkey: Notes on donkeys. J. Anim. Plant Sci. 2013, 23, 651–656. [Google Scholar]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic diversity in farm animals—A review. Anim. Genet. 2010, 41, 6–31. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pansu, J.; Pompanon, F. Conservation genetics of cattle, sheep, and goats. Comptes Rendus Biol. 2011, 334, 247–254. [Google Scholar] [CrossRef]
- Arat, S. In vitro conservation and preliminary molecular identification of some Turkish domestic animal genetic resources (TURKHAYGEN-I). In Proceedings of the RBI 8th Global Conference on the Conservation of Animal Genetic Resources, Tekirdag, Turkey, 4–8 October 2011; pp. 51–58. [Google Scholar]
- Lei, C.Z.; Ge, Q.L.; Zhang, H.C.; Liu, R.Y.; Zhang, W.; Jiang, Y.Q.; Dang, R.H.; Zheng, H.L.; Hou, W.T.; Chen, H. African maternal origin and genetic diversity of Chinese domestic donkeys. Asian-Australas. J. Anim. Sci. 2007, 20, 645–652. [Google Scholar] [CrossRef]
- Gan, Q.F.; Lin, S.X.; Liang, X.W.; Meng, H.L.; Qiao, H.; Du, Y.F.; Ke, F.R.; Zheng, H.K. African maternal origin and genetic diversity of five chinese domestic donkeys breeds. J. Anim. Vet. Adv. 2011, 10, 3090–3094. [Google Scholar] [CrossRef]
- Pérez-Pardal, L.; Grizelj, J.; Traoré, A.; Cubric-Curik, V.; Arsenos, G.; Dovenski, T.; Marković, B.; Fernández, I.; Cuervo, M.; Álvarez, I.; et al. Lack of mitochondrial DNA structure in Balkan donkey is consistent with a quick spread of the species after domestication. Anim. Genet. 2014. [Google Scholar] [CrossRef]
- Cinar Kul, B.; Bilgen, N.; Akyuz, B.; Ertugrul, O. Molecular phylogeny of Anatolian and Cypriot donkey populations based on mitochondrial DNA and Y-chromosomal STRs. Ankara Univ. Vet. Fak. Derg. 2016, 63, 143–149. [Google Scholar] [CrossRef]
- Cozzi, M.C.; Valiati, P.; Cherchi, R.; Gorla, E.; Prinsen, R.T.M.M.; Longeri, M.; Bagnato, A.; Strillacci, M.G. Mitochondrial DNA genetic diversity in six Italian donkey breeds (Equus asinus). Mitochondrial DNA Part A 2018, 29, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual (3-Volume Set); Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; ISBN 978-087969577-4. [Google Scholar]
- Ma, X.Y.; Ning, T.; Adeola, A.C.; Li, J.; Esmailizadeh, A.; Lichoti, J.K.; Agwanda, B.R.; Isakova, J.; Aldashev, A.A.; Wu, S.F.; et al. Potential dual expansion of domesticated donkeys revealed by worldwide analysis on mitochondrial sequences. Zool. Res. 2020, 41, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 6. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Vilstrup, J.T.; Seguin-Orlando, A.; Stiller, M.; Ginolhac, A.; Raghavan, M.; Nielsen, S.C.A.; Weinstock, J.; Froese, D.; Vasiliev, S.K.; Ovodov, N.D.; et al. Mitochondrial phylogenomics of modern and ancient equids. PLoS ONE 2013, 8, e55950. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, Q.; Yang, C.; Wang, X.; Tian, F.; Wang, Y.; Ma, Y.; Ju, Z.; Huang, J.; Zhou, X.; et al. Characterization of complete mitochondrial genome of Dezhou donkey (Equus asinus) and evolutionary analysis. Curr. Genet. 2016, 62, 383–390. [Google Scholar] [CrossRef]
- Schubert, M.; Mashkour, M.; Gaunitz, C.; Fages, A.; Seguin-Orlando, A.; Sheikhi, S.; Alfarhan, A.H.; Alquraishi, S.A.; Al-Rasheid, K.A.S.; Chuang, R.; et al. Zonkey: A simple, accurate and sensitive pipeline to genetically identify equine F1-hybrids in archaeological assemblages. J. Archaeol. Sci. 2017, 78, 147–157. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Y.; Liu, F.; Jiang, C.; Gao, Y. Mitochondrial genome sequence of the Tibetan wild ass (Equus kiang). Mitochondrial DNA 2011, 22, 6–8. [Google Scholar] [CrossRef]
- Jónsson, H.; Schubert, M.; Seguin-Orlando, A.; Ginolhac, A.; Petersen, L.; Fumagalli, M.; Albrechtsen, A.; Petersen, B.; Korneliussen, T.S.; Vilstrup, J.T.; et al. Speciation with gene flow in equids despite extensive chromosomal plasticity. Proc. Natl. Acad. Sci. USA 2014, 111, 18655–18660. [Google Scholar] [CrossRef]
- Achilli, A.; Olivieri, A.; Soares, P.; Lancioni, H.; Kashani, B.H.; Perego, U.A.; Nergadze, S.G.; Carossa, V.; Santagostino, M.; Capomaccio, S.; et al. Mitochondrial genomes from modern horses reveal the major haplogroups that underwent domestication. Proc. Natl. Acad. Sci. USA 2012, 109, 2449–2454. [Google Scholar] [CrossRef]
- Xia, X.; Yu, J.; Zhao, X.; Yao, Y.; Zeng, L.; Ahmed, Z.; Shen, S.; Dang, R.; Lei, C. Genetic diversity and maternal origin of Northeast African and South American donkey populations. Anim. Genet. 2019, 50, 266–270. [Google Scholar] [CrossRef]
- Han, L.; Zhu, S.; Ning, C.; Cai, D.; Wang, K.; Chen, Q.; Hu, S.; Yang, J.; Shao, J.; Zhu, H.; et al. Ancient DNA provides new insight into the maternal lineages and domestication of Chinese donkeys. BMC Evol. Biol. 2014, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Ibiş, O. Mitogenome characterization of Turkish Anatolian Donkey (Equus asinus) and Its phylogenetic relationships. Türkiye Tarımsal Araştırmalar Derg. 2019, 6, 257–267. [Google Scholar] [CrossRef]
- Yalçın, E. Kırklareli Ili Eşek Çiftliği Populasyonlarının Çeşitli Vücut Ölçülerine Göre Morfometrik Karakterizasyonu ve mtDNA Polimorfizmi Yoluyla Genetik Çeşitliliğinin İncelenmesi. Master’s Thesis, Tekirdağ Namık Kemal Unıversity, Tekirdağ, Turkey, 2016. [Google Scholar]
- Tajima, F. Evolutionary relationship of DNA sequences in finite populations. Genetics 1983, 105, 437–460. [Google Scholar] [PubMed]
- Nei, M. Phylogenetic analysis in molecular evolutionary genetics. Annu. Rev. Genet. 1996, 30, 371–403. [Google Scholar] [CrossRef]
- Walsh, P.D. Sample size for the diagnosis of conservation units. Conserv. Biol. 2000, 14, 1533–1537. [Google Scholar] [CrossRef]
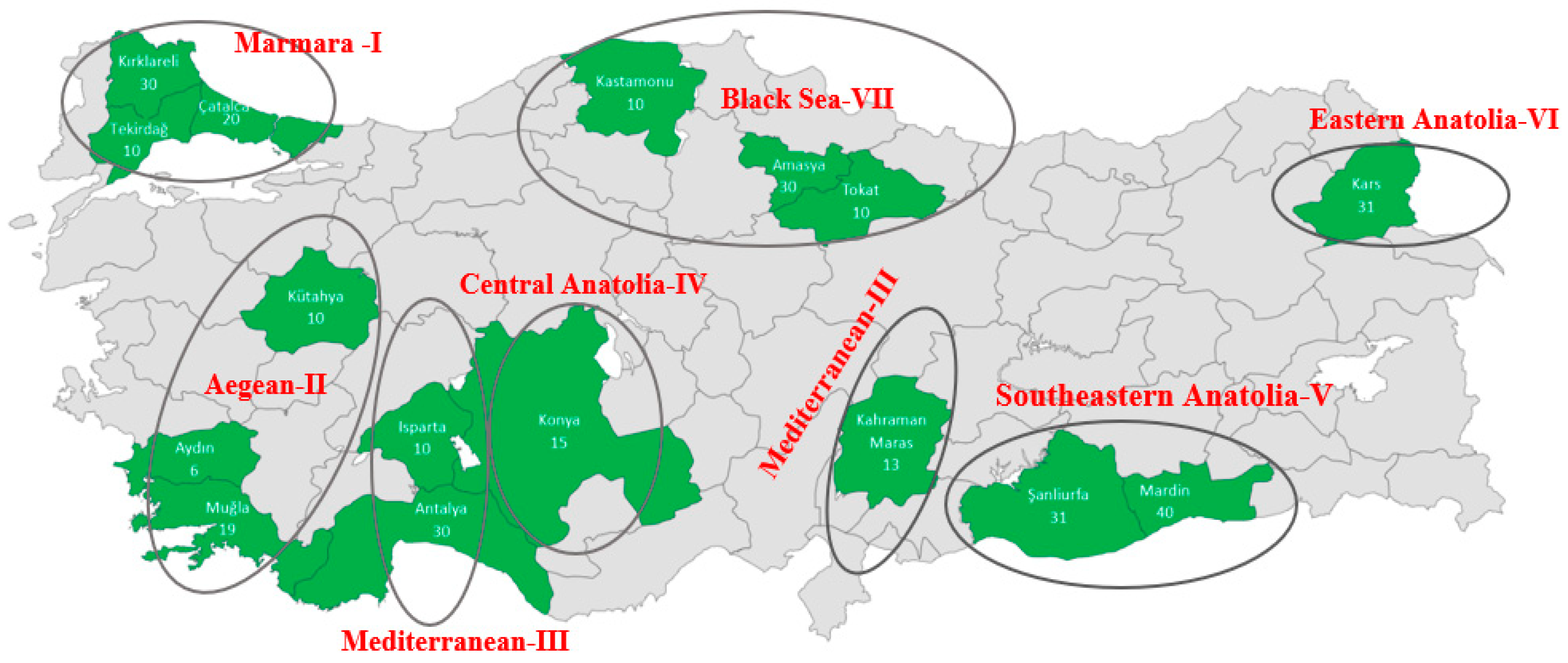


| Sampled Regions | Provinces | Geographical Coordinate | Number of Individuals |
|---|---|---|---|
| (I)-Marmara (MRM) | KIR | 41°51′ N 27°19′ E | 30 |
| CAT | 41°06′ N 28°30′ E | 20 | |
| MAL | 40°52′ N 26°57′ E | 10 | |
| (VII)-Black Sea (BSR) | MER | 40°53′ N 35°32′ E | 30 |
| TOK | 40°12′ N 36°27′ E | 10 | |
| KAS | 41°50′ N 32°54′ E | 10 | |
| (II)-Aegean (AER) | KUT | 39°21′ N 30°01′ E | 10 |
| MUG | 36°37′ N 29°26′ E | 19 | |
| AYD | 37°44′ N 28°01′ E | 6 | |
| (IV)-Central Anatolia (CAR) | KON | 37°38′ N 32°26′ E | 15 |
| (III)-Mediterranean (MDR) | ISP | 37°49′ N 30°44′ E | 10 |
| KRM | 37°30′ N 36°57′ E | 13 | |
| ANT | 36°50′ N 30°13′ E | 30 | |
| (VI)-Eastern Anatolia (EAR) | KAR | 40°36′ N 43°07′ E | 31 |
| (V)-South East Anatolia (SAR) | MRD | 37°18′ N 40°44′ E | 40 |
| SAN | 37°10′ N 38°50′ E | 31 | |
| Total | 315 | ||
| Provinces | n | NH | HD ± SD | S | SPI | SS | PH | SH | πD ± SD | k | Region |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KIR | 30 | 12 | 0.710 ± 0.007 | 18 | 11 | 7 | 1 | 11 | 0.01393 ± 0.0025 | 4.848 | MRM |
| CAT | 20 | 11 | 0.763 ± 0.0106 | 19 | 11 | 8 | 2 | 9 | 0.01288 ± 0.0036 | 4.495 | |
| MAL | 10 | 4 | 0.533 ± 0.180 | 12 | 11 | 1 | - | 4 | 0.01496 ± 0.0044 | 5.222 | |
| MER | 30 | 13 | 0.720 ± 0.091 | 16 | 15 | 1 | 3 | 10 | 0.01446 ± 0.0023 | 5.048 | BSR |
| TOK | 10 | 5 | 0.756 ± 0.130 | 13 | 12 | 1 | - | 5 | 0.01821 ± 0.0032 | 6.356 | |
| KAS | 10 | 7 | 0.911 ± 0.077 | 14 | 12 | 2 | 2 | 5 | 0.01731 ± 0.0036 | 6.022 | |
| KUT | 10 | 4 | 0.778 ± 0.091 | 13 | 12 | 1 | - | 4 | 0.01681 ± 0.0038 | 5.867 | AER |
| MUG | 19 | 9 | 0.871 ± 0.048 | 16 | 11 | 5 | 2 | 7 | 0.01696 ± 0.0020 | 5.918 | |
| AYD | 6 | 5 | 0.933 ± 0.122 | 14 | 10 | 4 | - | 5 | 0.02101 ± 0.0041 | 7.333 | |
| KRM | 13 | 4 | 0.654 ± 0.106 | 12 | 11 | 1 | - | 4 | 0.01646 ± 0.0026 | 5.744 | MDR |
| ISP | 10 | 7 | 0.867 ± 0.107 | 15 | 12 | 3 | 1 | 6 | 0.01917 ± 0.0033 | 6.689 | |
| ANT | 30 | 12 | 0.717 ± 0.090 | 18 | 15 | 3 | 5 | 7 | 0.01196 ± 0.0026 | 4.172 | |
| KON | 15 | 7 | 0.829 ± 0.082 | 14 | 12 | 2 | 3 | 4 | 0.01659 ± 0.0027 | 5.791 | CAR |
| KAR | 31 | 9 | 0.652 ± 0.091 | 15 | 12 | 3 | 1 | 8 | 0.01455 ± 0.0023 | 5.079 | EAR |
| MRD | 40 | 15 | 0.758 ± 0.060 | 18 | 12 | 6 | 8 | 7 | 0.01753 ± 0.0008 | 6.118 | SAR |
| SAN | 31 | 9 | 0.695 ± 0.076 | 17 | 13 | 4 | 3 | 6 | 0.01764 ± 0.0012 | 6.155 | |
| 315 | 54 | 0.763 ± 0.023 | 35 | 29 | 6 | 0.01680 ± 0.00046 | 5.745 |
| Region | NH | Haplotypes |
|---|---|---|
| MRM | 19 | Hap 1-19 |
| BSR | 17 | Hap 1, Hap 4, Hap 5, Hap 7, Hap 8 Hap 11, Hap 14, Hap 20 -29 |
| AER | 12 | Hap 1, Hap 4, Hap 7, Hap 8, Hap 13, Hap 14, Hap 16, Hap 23, Hap 25, Hap 34, Hap 41, Hap 42 |
| MDR | 18 | Hap 1, Hap 2, Hap 4, Hap 6, Hap 7, Hap 11, Hap 15, Hap 16, Hap 23, Hap 27, Hap 33-40 |
| CAR | 7 | Hap 1, Hap 4, Hap 7, Hap 12, Hap 30-32 |
| EAR | 9 | Hap 1, Hap 4, Hap 7, Hap 8, Hap 10, Hap 11, Hap 14, Hap 15, Hap 43 |
| SAR | 21 | Hap 1, Hap 4, Hap 7, Hap 10, Hap 11, Hap 14, Hap 17, Hap 20, Hap 26, Hap 34, Hap 44-54. |
| Sampling Regions | MRM | BSR | AER | MDR | CAR | EAR | SAR |
|---|---|---|---|---|---|---|---|
| MRM | - | 0.00036 | 0.00391 | −0.00014 | 0.00465 | −0.00024 | 0.00115 |
| BSR | 0.0242 | - | 0.00139 | 0.00011 | 0.00190 | −0.00007 | 0.00001 |
| AER | 0.2106 ** | 0.0765 * | - | 0.00323 | −0.00049 | 0.00273 | 0.00052 |
| MDR | −0.0099 | 0.0068 | 0.1734 ** | - | 0.00399 | −0.00026 | 0.00082 |
| CAR | 0.2523 ** | 0.1027 * | −0.0302 | 0.2130 ** | - | 0.00340 | 0.00069 |
| EAR | −0.0171 | −0.0052 | 0.01464 * | −0.0184 | 0.1843 ** | - | 0.00047 |
| SAR | 0.0685 * | 0.0004 | 0.0291 | 0.04799 * | 0.0376 | 0.0263 | - |
| Population | n | HD ± SD | πD ± SD | References |
|---|---|---|---|---|
| Turkish native donkeys | 315 | 0.763 ± 0.023 | 0.01680± 0.00046 | This study |
| Different countries’ donkeys | 10 | 0.956 ± 0.059 | 0.02349 ± 0.00598 | [11,36,37,38,39,40] |
| African origin domestic donkeys | 85 | 0.983 ± 0.007 | 0.02843 ± 0.00195 | [16] |
| Chinese donkeys | 146 | 0.890 ± 0.013 | 0.01759± 0.00053 | [17] |
| Balkan donkeys | 62 | 0.973 ± 0.011 | 0.01764 ± 0.00067 | [26] |
| Serbian donkeys | 23 | 0.949 ± 0.033 | 0.01922 ± 0.00136 | [15] |
| Nigerian, Iranian, Tadzhikistan, Chinese, Kenya, and Kyrgyzstan donkeys | 73 | 0.965 ± 0.010 | 0.02613 ± 0.00374 | [30] |
| Italian donkeys | 28 | 0.913 ± 0.033 | 0.01568 ± 0.00133 | [28] |
| Population | Pairwise Distance ± SD | πD ± SD |
|---|---|---|
| Clade I lineage | 1.4228 ± 0.8715 | 0.004136 ± 0.002802 |
| Clade II lineage | 2.1506 ± 1.1992 | 0.00525 ± 0.003857 |
| Source of Variation | Variance Component | Variance (%) | Fixation Index a | p-Value b |
|---|---|---|---|---|
| Hypothesis 1: 5 different clusters | ||||
| Among groups | 0.31406 | 7.95 | ΦCT: 0.07945 | 0.08211 ns |
| Among populations within groups | 0.06789 | 1.72 | ΦSC: 0.01866 | 0.0303 * |
| Within populations | 3.57081 | 90.34 | ΦST: 0.09663 | 0.000 *** |
| Hypothesis 2: Turkish native donkey populations; genetic distances tree | ||||
| Among groups | 0.21621 | 7.31 | ΦCT: 0.07314 | 0.0303 * |
| Among populations within groups | 0.01157 | 0.39 | ΦSC: 0.00422 | 0.3304 ns |
| Within populations | 2.72850 | 92.29 | ΦST: 0.07705 | 0.000 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkan Ünal, E.; Özdil, F.; Kaplan, S.; Gürcan, E.K.; Genç, S.; Arat, S.; Soysal, M.İ. Phylogenetic Relationships of Turkish Indigenous Donkey Populations Determined by Mitochondrial DNA D-loop Region. Animals 2020, 10, 1970. https://doi.org/10.3390/ani10111970
Özkan Ünal E, Özdil F, Kaplan S, Gürcan EK, Genç S, Arat S, Soysal Mİ. Phylogenetic Relationships of Turkish Indigenous Donkey Populations Determined by Mitochondrial DNA D-loop Region. Animals. 2020; 10(11):1970. https://doi.org/10.3390/ani10111970
Chicago/Turabian StyleÖzkan Ünal, Emel, Fulya Özdil, Selçuk Kaplan, Eser Kemal Gürcan, Serdar Genç, Sezen Arat, and Mehmet İhsan Soysal. 2020. "Phylogenetic Relationships of Turkish Indigenous Donkey Populations Determined by Mitochondrial DNA D-loop Region" Animals 10, no. 11: 1970. https://doi.org/10.3390/ani10111970
APA StyleÖzkan Ünal, E., Özdil, F., Kaplan, S., Gürcan, E. K., Genç, S., Arat, S., & Soysal, M. İ. (2020). Phylogenetic Relationships of Turkish Indigenous Donkey Populations Determined by Mitochondrial DNA D-loop Region. Animals, 10(11), 1970. https://doi.org/10.3390/ani10111970







