Feasibility of on/at Line Methods to Determine Boar Taint and Boar Taint Compounds: An Overview
Abstract
:Simple Summary
Abstract
1. Introduction
2. Description of on Line/at Line Available Methods
2.1. Sensory Evaluation (also Referred to as Human Nose Scoring)
2.1.1. General Aspects
2.1.2. Boar Taint Evaluation
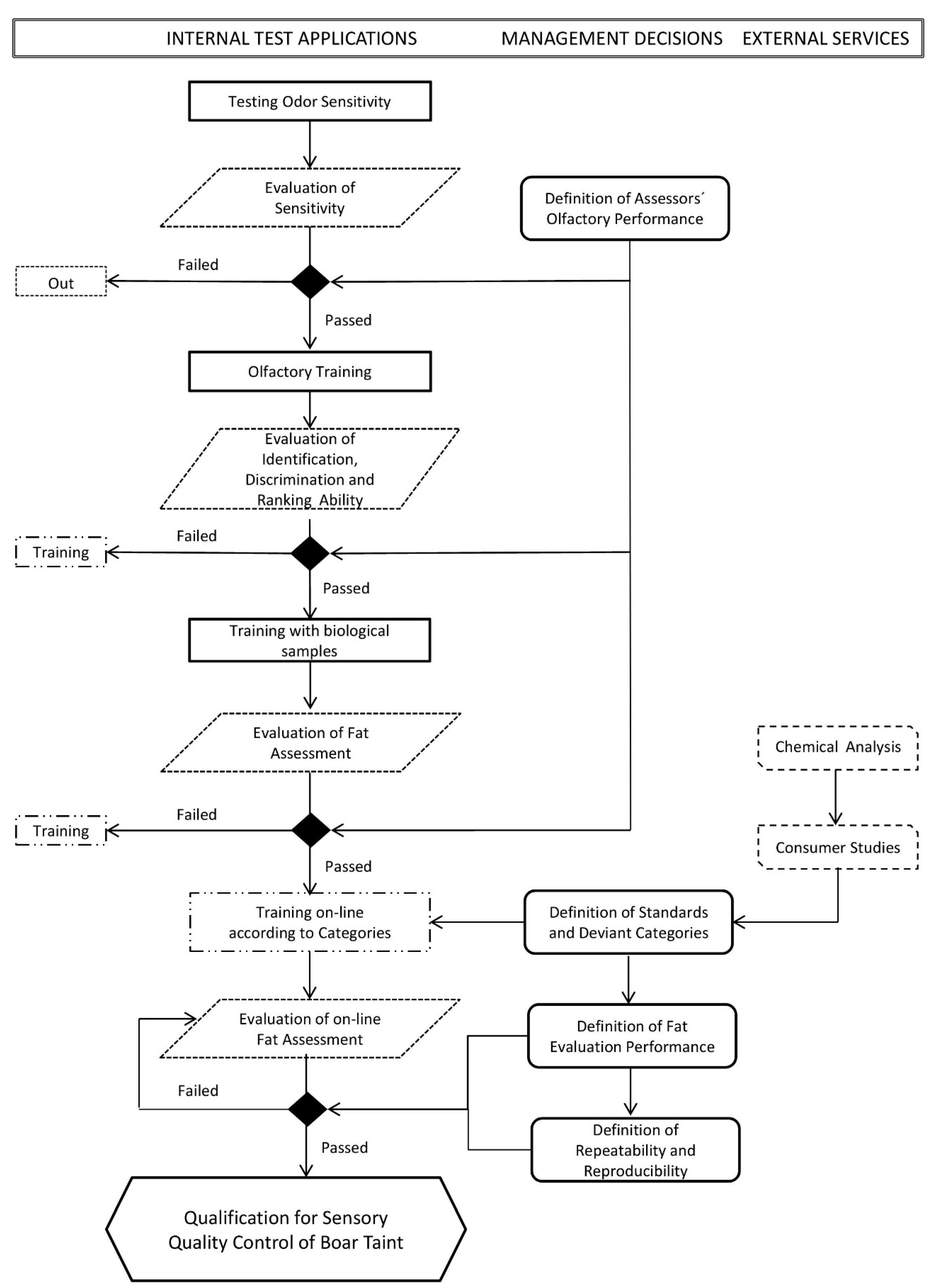
Selection and Training of Assessors
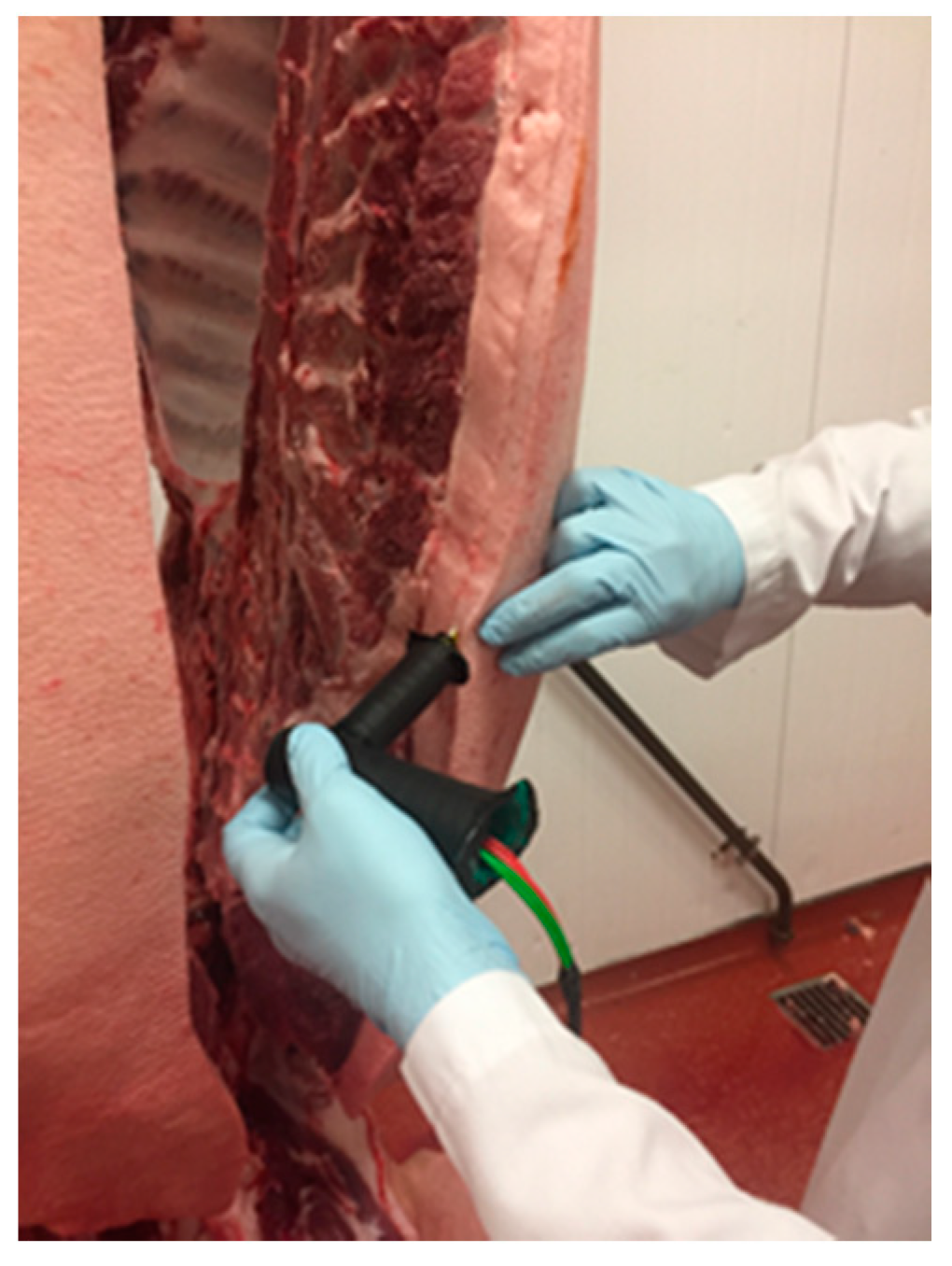
Sample Preparation
Performance of the Analysis
Implementation Costs
2.2. Colorimetric Method
2.2.1. General Aspects and Boar Taint Determination
2.2.2. Sample Preparation and Analysis
2.2.3. Performance of the Analysis and Speed Capacity
2.2.4. Implementation Costs
2.3. Laser Diode Thermal Desorption Ion Source Tandem Mass Spectrometry
2.3.1. General Aspects
2.3.2. Sample Preparation and Analysis
2.3.3. Performance of Analysis and Speed Capacity
2.3.4. Implementation Costs
2.4. Rapid Evaporative Ionization Mass Spectroscopy
2.4.1. Performance Analysis
2.4.2. Implementation Costs
2.5. Raman Spectroscopy
2.5.1. General Aspects and Applications
2.5.2. Boar Taint Evaluation
Sample Preparation
Performance of Analysis and Speed Capacity
Implementation Costs
2.6. Electrochemical Biosensors
2.6.1. General Aspects and Applications
2.6.2. Boar Taint Determination
Sample Preparation
Performance of Analysis
Implementation Costs
3. General Discussion and SWOT Analysis
3.1. Technology Readiness Level
3.2. Type of Measurement
3.3. Traceability and Sample Pre-Treatment
3.4. Speed and Capacity
3.5. Performance
3.6. Investment Costs
3.7. Other Considerations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pomar, C.; Marcoux, M.; Gispert, M.; Font i Furnols, M.; Daumas, G. Determining the Lean Meat Content of Pork Carcasses. In Improving the Sensory and Nutritional Quality of Fresh Meat; Kerry, J.P., Ledward, D., Eds.; Woodhead Publishing CRC Press: Cambridge, UK, 2008; pp. 493–518. [Google Scholar]
- Font i Furnols, M.; Gispert, M. Comparison of different devices for predicting the lean meat percentage of pig carcasses. Meat Sci. 2009, 83, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, B.; Lambooij, E.; Buist, W.G.; Vereijken, P. Lean meat prediction with HGP, CGM and CSB-Image-Meater, with prediction accuracy evaluated for different proportions of gilts, boars and castrated boars in the pig population. Meat Sci. 2012, 90, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.M.; Busk, H.; Oksama, M.; Seynaeve, M.; Gispert, M.; Walstra, P.; Hansson, I.; Olsen, E. The estimated accuracy of the EU reference dissection method for pig carcass classification. Meat Sci. 2006, 73, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, G.; Carreras, R.; Font-i-Furnols, M.; Gispert, M.; Serra, M.; Marti-Puig, P. Automatic ham classification method based on support vector machine model increases accuracy and benefits compared to manual classification. Meat Sci. 2019, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.M.; Petersen, H.; Engelsen, S.B. An On-Line Near-Infrared (NIR) Transmission Method for Determining Depth Profiles of Fatty Acid Composition and Iodine Value in Porcine Adipose Fat Tissue. Appl. Spectrosc. 2012, 66, 218–226. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Fulladosa, E.; Prevolnik Povše, M.; Čandek-Potokar, M. Future Trends in Non-Invasive Technologies Suitable for Quality Determinations. In A Handbook of Reference Methods for Meat Quality Assessment; Font-i-Furnols, M., Potokar, M.Č., Maltin, C., Povše, M.P., Eds.; FAIM, European Cooperation in Science and Technology (COST): Edinburg, UK, 2015; pp. 90–103. [Google Scholar]
- Beganović, A.; Hawthorne, L.M.; Bach, K.; Huck, C.W. Critical Review on the Utilization of Handheld and Portable Raman Spectrometry in Meat Science. Foods 2019, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Scheeder, M.; Müller Richli, M. Neues Bewertungssystem für die Fettqualität bei Mastschweinen. ETH-Schr. Tierernährung 2014, 37, 148–151. [Google Scholar]
- Sørensen, K.M.; Christensen, M.; Engelsen, S.B. Three-dimensionsl images of porcine carcass fat quality using spatially resolved near infrared spectroscopy. NIR News 2013, 24, 9–11. [Google Scholar] [CrossRef]
- Regulation (EC) No 854/2004 of the European Parliament and on the Council Laying down Specific Rules for the Organisation of Official Control on Products of Animal Origin Intended for Human Consumption Communities; L139; Official Journal of the European Union: Brussels, Belgium, 2004; p. 139/206.
- Panella-Riera, N.; Blanch, M.; Kallas, Z.; Chevillon, P.; Garavaldi, A.; Gil, M.; Gil, J.M.; Font-i-Furnols, M.; Oliver, M.A. Consumers’ segmentation based on the acceptability of meat from entire male pigs with different boar taint levels in four European countries: France, Italy, Spain and United Kingdom. Meat Sci. 2016, 114, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Claus, R.; Hoffman, B.; Karg, H. Determination of 5 -androst-16-en-3-one, a boar taint steroid in pigs, with reference to relationships to testosterone. J. Anim. Sci. 1971, 33, 1293–1297. [Google Scholar] [CrossRef]
- Patterson, R.L.S. 5α-androst-16-ene-3-one: Compound responsible for taint in boar fat. J. Sci. Food Agric. 1969, 19, 31–38. [Google Scholar] [CrossRef]
- Vold, E. Fleischproduktionseigenschaften bei Ebern und Kastraten IV: Organoleptische und gaschromatographische Untersuchungen wasserdampf-flüchtiger Stoffe des Rückenspeckes von Ebern. Meldinger fra NorgesLandbrukshøgskole 1970, 49, 1–25. [Google Scholar]
- Walstra, P.; Maarse, H. Investigation into Sex Odour of Entire Male Pigs; IVO Rapport C-147 (September); IVO: Lelystad, The Netherlands, 1970; pp. 1–30. [Google Scholar]
- Brennan, J.J.; Shand, P.J.; Fenton, M.; Nicholls, L.L.; Aherne, F.X. Androstenone, androstenol and odor intensity in backfat of 100-and 130-kg boars and gilts. Can. J. Anim. Sci. 1986, 66, 615–624. [Google Scholar] [CrossRef]
- García-Regueiro, J.A.; Diaz, I. Evaluation of the contribution of skatole, indole, androstenone and androstenols to boar-taint in back fat of pigs by HPLC and capillary gas chromatography (CGC). Meat Sci. 1989, 25, 307–316. [Google Scholar] [CrossRef]
- Rius Solé, M.A.; García Regueiro, J.A. Role of 4-phenyl-3-buten-2-one in boar taint: Identification of new compounds related to sensorial descriptors in pig fat. J. Agric. food Chem. 2001, 49, 5303–5309. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Squires, E.J. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 2009, 3, 1508–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, J.; Gerlach, C.; Meier-Dinkel, L.; Elsinghorst, P.W.; Boeker, P.; Schmarr, H.-G.; Wüst, M. 2-Aminoacetophenone—A hepatic skatole metabolite as a potential contributor to boar taint. Food Res. Int. 2014, 62, 35–42. [Google Scholar] [CrossRef]
- Font-i-Furnols, M. Consumer studies on sensory acceptability of boar taint: A review. Meat Sci. 2012, 92, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Meier-Dinkel, L.; Strack, M.; Höinghaus, K.; Mörlein, D. Consumers dislike boar taint related off-flavours in pork chops regardless of a meal context. Meat Sci. 2016, 122, 119–124. [Google Scholar] [CrossRef]
- Prusa, K.; Nederveld, H.; Runnels, P.L.; Li, R.; King, V.L.; Crane, J.P. Prevalence and relationships of sensory taint, 5α-androstenone and skatole in fat and lean tissue from the loin (Longissimus dorsi) of barrows, gilts, sows, and boars from selected abattoirs in the United States. Meat Sci. 2011, 88, 96–101. [Google Scholar] [CrossRef]
- Borrisser-Pairó, F.; Panella-Riera, N.; Zammerini, D.; Olivares, A.; Garrido, M.D.; Martínez, B.; Gil, M.; García-Regueiro, J.A.; Oliver, M.A. Prevalence of boar taint in commercial pigs from Spanish farms. Meat Sci. 2016, 111, 177–182. [Google Scholar] [CrossRef]
- Mathur, P.K.; ten Napel, J.; Bloemhof, S.; Heres, L.; Knol, E.F.; Mulder, H.A. A human nose scoring system for boar taint and its relationship with androstenone and skatole. Meat Sci. 2012, 91, 414–422. [Google Scholar] [CrossRef]
- Aluwé, M.; Tuyttens, F.A.; Millet, S. Field experience with surgical castration with anaesthesia, analgesia, immunocastration and production of entire male pigs: Performance, carcass traits and boar taint prevalence. Animal 2015, 9, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyrman, E.; Millet, S.; Tuyttens, F.A.M.; Ampe, B.; Janssens, S.; Buys, N.; Wauters, J.; Vanhaecke, L.; Aluwé, M. On farm intervention studies on reduction of boar taint prevalence: Feeding strategies, presence of gilts and time in lairage. Res. Vet. Sci. 2018, 118, 508–516. [Google Scholar] [CrossRef]
- Backus, G.; Higuera, M.; Juul, N.; Nalon, E.; de Bryne, N. Second Progress Report 2015–2017 on the European Declaration on Alternatives to Surgical Castration of Pigs. Available online: https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf (accessed on 1 July 2020).
- Fuchs, G. The correlation between the 5α-androst-16-ene-3-one content and the sex odour intensity in boar fat. Swed. J. Agric. Res. 1971, 1, 233–237. [Google Scholar]
- Borggaard, C.; Birkler, R.; Meinert, L.; Støier, S. At-Line Rapid Instrumental Method for Measuring the Boar Taint Components Androstenone and Skatole in Pork Fat. Available online: https://www.dti.dk/specialists/analytical-method-for-meat-from-entire-male-pigs/39301?cms.query=LDTD (accessed on 4 May 2020).
- Hemeryck, L.Y.; Stead, S.L.; Decloedt, A.; Huysman, S.; Balog, J.; DeSpiegeleer, M.; Pringle, S.D.; Boland, A.; Vanhaecke, L. First Implementation of Rapid Evaporative Ionisation Mass Spectrometry (REIMS) for the at-Line Screening of Boar Carcasses in the Slaughterhouse. In Proceedings of the 67th Conference of Mass Spectrometry and Allied Topics (ASMS), Atlanta, GA, USA, 2–6 June 2019. [Google Scholar]
- Ampuero Kragten, S.; Verkuylen, B.; Dahlmans, H.; Hortos, M.; Garcia-Regueiro, J.A.; Dahl, E.; Andresen, O.; Feitsma, H.; Mathur, P.K.; Harlizius, B. Inter-laboratory comparison of methods to measure androstenone in pork fat. Animal 2011, 5, 1634–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, J.E.; Brunius, C.; Zamaratskaia, G. Review of analytical methods to measure boar taint compounds in porcine adipose tissue: The need for harmonised methods. Meat Sci. 2012, 90, 9–19. [Google Scholar] [CrossRef]
- Buttinger, G.; Karasek, L.; Verlinde, P.; Wenzl, T. In House Validation of a Reference Method for the Determination of Boar Taint Compounds by LC-MSMS; Publications Office of the European Union: Geel, Belgium, 2014. [Google Scholar]
- Buttinger, G.; Wenzl, T. Development of Reference Methods for the Detection and the Measurement of the Main Compounds Responsible for Boar Taint; Publications Office of the European Union: Geel, Belgium, 2014. [Google Scholar]
- Haugen, J.E.; van Wagenberg, C.; Backus, G.; Nielsen, B.E.; Borgaard, C.; Bonneau, M.; Panella-Riera, N.; Aluwé, M. BoarCheck: A Study of Rapid Methods for Boar Taint Use or Being Developed at Slaughter Plants in the European Union. BoarCheck Project Final Report. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_research_boarcheck_20140901.pdf (accessed on 4 May 2020).
- Verplanken, K.; Wauters, J.; Van Durme, J.; Claus, D.; Vercammen, J.; De Saeger, S.; Vanhaecke, L. Rapid method for the simultaneous detection of boar taint compounds by means of solid phase microextraction coupled to gas chromatography/mass spectrometry. J. Chromatogr. A 2016, 1462, 124–133. [Google Scholar] [CrossRef]
- Squires, E.J.; Bone, C.; Cameron, J. Pork production with entire males: Directions for control of boar taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef]
- Rius, M.A.; García-Regueiro, J.A. Skatole and indole concentrations in Longissimus dorsi and fat samples of pigs. Meat Sci. 2001, 59, 285–291. [Google Scholar] [CrossRef]
- Wauters, J.; Vercruysse, V.; Aluwé, M.; Verplanken, K.; Vanhaecke, L. Boar taint compound levels in back fat versus meat products: Do they correlate? Food Chem. 2016, 206, 30–36. [Google Scholar] [CrossRef]
- Coker, M.D.; West, R.L.; Brendemuhl, J.H.; Johnson, D.D.; Stelzleni, A.M. Effects of live weight and processing on the sensory traits, androstenedione concentration and 5-alpha-androst-16-en-3-one (androstenone) concentration in boar meat. Meat Sci. 2009, 82, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the Characteristics of Olive Oil and Olive-Residue Oil and on the Relevant Methods of Analysis; L248; Official Journal of the European Communities: Brussels, Belgium, 1991; pp. 1–83.
- Rogers, L. Sensory Panel Management: A Practical Handbook for Recruitment, Training and Performance; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Trautmann, J. Sensory Quality Control of Boar Taint. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2016. [Google Scholar]
- Muñoz, A.M. Sensory evaluation in quality control: An overview, new developments and future opportunities. Food Qual. Prefer. 2002, 13, 329–339. [Google Scholar] [CrossRef]
- Trautmann, J.; Gertheiss, J.; Wicke, M.; Mörlein, D. How olfactory acuity affects the sensory assessment of boar fat: A proposal for quantification. Meat Sci. 2014, 98, 255–262. [Google Scholar] [CrossRef]
- Font i Furnols, M.; Guerrero, L.; Serra, X.; Rius, M.A.; Oliver, M.A. Sensory characterization of boar taint in entire male pigs. J. Sens. Stud. 2000, 15, 393–410. [Google Scholar] [CrossRef]
- Garrido, M.D.; Egea, M.; Linares, M.B.; Martínez, B.; Viera, C.; Rubio, B.; Borrisser-Pairó, F. A procedure for sensory detection of androstenone in meat and meat products from entire male pigs: Development of a panel training. Meat Sci. 2016, 122, 60–67. [Google Scholar] [CrossRef]
- Mörlein, D.; Meier-Dinkel, L.; Moritz, J.; Sharifi, A.R.; Knorr, C. Learning to smell: Repeated exposure increases sensitivity to androstenone, a major component of boar taint. Meat Sci. 2013, 94, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lunde, K.; Skuterud, E.; Egelandsdal, B.; Font i Furnols, M.; Nute, G.R.; Bejerholm, C.; Nilsen, A.; Stenstrøm, Y.H.; Hersleth, M. The importance of the recruitment method for androstenone sensitivity with respect to accurate sensory evaluation of androstenone tainted meat. Food Qual. Prefer. 2010, 21, 648–654. [Google Scholar] [CrossRef]
- Mörlein, D.; Christensen, R.H.B.; Gertheiss, J. Validation of boar taint detection by sensory quality control: Relationship between sample size and uncertainty of performance indicators. Meat Sci. 2015, 100, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, J.; Meier-Dinkel, L.; Gertheiss, J.; Mörlein, D. Boar taint detection: A comparison of three sensory protocols. Meat Sci. 2016, 111, 92–100. [Google Scholar] [CrossRef]
- Whittington, F.M.; Zammerini, D.; Nute, G.R.; Baker, A.; Hughes, S.I.; Wood, J.D. Comparison of heating methods and the use of different tissues for sensory assessment of abnormal odours (boar taint) in pig meat. Meat Sci. 2011, 88, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, K.M.; Aluwé, M.; Vanhaecke, L.; Heres, L.; Duchateau, L.; Vandendriessche, F.; Tuyttens, F.A.M. Evaluation of different heating methods for the detection of boar taint by means of the human nose. Meat Sci. 2013, 94, 125–132. [Google Scholar] [CrossRef] [PubMed]
- van Wagenberg, C. Sensory Detection System for Boar Taint. In Boars on the Way. Research Program ‘Stopping the Castration of Piglets’; LEI Wageningen UR: Wageningen, The Netherlands, 2013; p. 33. [Google Scholar]
- Mortensen, A.B. A Method for Detecting Obnoxious Taint such as Boar Taint in Individual Animal Bodies, Preferably Carcasses or Parts Thereof. U.S. Patent No. 4,563,428, 7 January 1986. [Google Scholar]
- Mortensen, A.B.; Sørensen, S.E. Relationship between boar taint and skatole determined with a new analysis method. In Proceedings of the 30th European Meeting of Meat Research Workers, Bristol, UK, 9–14 September 1984; pp. 394–396. [Google Scholar]
- Hansen-Møller, J. Analytical methods for determination of boar taint compounds. In Skatole and Boar Taint; Jensen, W.K., Ed.; Danish Meat Research Institute: Roskilde, Denmark, 1998; pp. 21–40. [Google Scholar]
- Auger, S.; Larcoursière, J.; Pierrre, P. High-throughput Analysis of Indole, Skatole and Androstenone in pork fat by LDTD-MS/MS. Available online: https://phytronix.com/wp-content/uploads/2018/09/AN-1804-High-throughput-analysis-of-Indole-Skatole-and-Androstenone-in-pork-fat-by-LDTD-MSMS.pdf (accessed on 4 May 2020).
- Støier, S. A New Instrumental Boar Taint Detection Method. Available online: https://www.boarsontheway.com/wp-content/uploads/2019/11/Boar-taint-detection-02102019-aangepast.pdf (accessed on 4 May 2020).
- Lund, B.W.; Borggaard, C. Simultaneously Detection of Off-Note or Boar Taint Related Compounds in Animal Tissue. U.S. Patent Application No. 15/555,421, 15 February 2018. [Google Scholar]
- Schäfer, K.C.; Dénes, J.; Albrecht, K.; Szaniszló, T.; Balog, J.; Skoumal, R.; Katona, M.; Tóth, M.; Balogh, L.; Takáts, Z. In vivo, in situ tissue analysis using rapid evaporative ionization mass spectrometry. Angew. Chem. Int. Ed. 2009, 48, 8240–8242. [Google Scholar] [CrossRef]
- Verplanken, K.; Stead, S.; Jandova, R.; Poucke, C.V.; Claereboudt, J.; Bussche, J.V.; Saeger, S.; Takats, Z.; Wauters, J.; Vanhaecke, L. Rapid evaporative ionization mass spectrometry for high-throughput screening in food analysis: The case of boar taint. Talanta 2017, 169, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, S.; Bee, G. Prevention of boar taint in pig production. Abstracts of the 19th Symposium of the Nordic Committee for Veterinary Scientific Cooperation, Gardermoen, Norway, 21–22 November 2005. Acta Vet. Scand. 2006, 48 (Suppl. 1), S6. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H.; Stratoudaki, T.; Doulgeridis, M.; Anglos, D. Pigment Identification in Painted Artworks: A Dual Analytical Approach Employing Laser-Induced Breakdown Spectroscopy and Raman Microscopy. Appl. Spectrosc. 2000, 54, 463–469. [Google Scholar] [CrossRef]
- Deidda, R.; Sacre, P.-Y.; Clavaud, M.; Coïc, L.; Avohou, H.; Hubert, P.; Ziemons, E. Vibrational spectroscopy in analysis of pharmaceuticals: Critical review of innovative portable and handheld NIR and Raman spectrophotometers. TrAC Trends Anal. Chem. 2019, 114, 251–259. [Google Scholar] [CrossRef]
- Baker, M.J.; Byrne, H.J.; Chalmers, J.; Gardner, P.; Goodacre, R.; Henderson, A.; Kazarian, S.G.; Martin, F.L.; Moger, J.; Stone, N.; et al. Clinical applications of infrared and Raman spectroscopy: State of play and future challenges. Analyst 2018, 143, 1735–1757. [Google Scholar] [CrossRef] [PubMed]
- Chisanga, M.; Muhamadali, H.; Ellis, D.I.; Goodacre, R. Enhancing Disease Diagnosis: Biomedical Applications of Surface-Enhanced Raman Scattering. Appl. Sci. 2019, 9, 1163. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Ying, Y. Applications of Raman Spectroscopy in Agricultural Products and Food Analysis: A Review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Wang, Q. Raman Spectroscopic Characterization and Analysis of Agricultural and Biological Systems. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Liu, X.; Schmidt, H.; Morlein, D. Feasibility of boar taint classification using a portable Raman device. Meat Sci. 2016, 116, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Damez, J.L.; Clerjon, S. Quantifying and predicting meat and meat products quality attributes using electromagnetic waves: An overview. Meat Sci. 2013, 95, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Sowoidnich, K.; Kronfeldt, H.D. A prototype hand-held Raman sensor for the in situ characterization of meat quality. Appl. Spectrosc. 2010, 64, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, K.M.; Westley, C.; Goodacre, R.; Engelsen, S.B. Simultaneous quantification of the boar-taint compounds skatole and androstenone by surface-enhanced Raman scattering (SERS) and multivariate data analysis. Anal. Bioanal. Chem. 2015, 407, 7787–7795. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Westmacott, K.; Honeychurch, K.C.; Crew, A.; Pemberton, R.M.; Hart, J.P. Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses. Biosensors 2016, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Honeychurch, K.C. Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors 2019, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Batra, B.; Narwal, V.; Kalra, V.; Sharma, M.; Rana, J.S. Folic acid biosensors: A review. Process Biochem. 2020, 92, 343–354. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.C.; Paulsen, I.T.; Williams, T.C. Blueprints for Biosensors: Design, Limitations, and Applications. Genes 2018, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Westmacott, K.L.; Crew, A.P.; Doran, O.; Hart, J.P. Novel, rapid, low-cost screen-printed (bio)sensors for the direct analysis of boar taint compounds androstenone and skatole in porcine adipose tissue: Comparison with a high-resolution gas chromatographic method. Biosens. Bioelectron. 2020, 150, 111837. [Google Scholar] [CrossRef]
- Hart, J.; Crew, A.; McGuire, N.; Doran, O. Sensor and method for detecting androstenone or skatole in Boar Taint. European Patent Application No. EP2,966,441A1, 13 January 2016. [Google Scholar]
- Font-i-Furnols, M.; Čandek-Potokar, M.; Panella-Riera, N.; Haugen, J.E.; Bahelka, I. Quality control in entire male pig production with particular emphasis on boar taint detection. In Proceedings of the 69th European Association of Animal Production (EAAP) Annual Meeting, Dubrovnik, Croatia, 27–30 August 2018. [Google Scholar]
- Christensen, R.H.; Nielsen, D.B.; Aaslyng, M.D. Estimating the risk of dislike: An industry tool for setting sorting limits for boar taint compounds. Food Qual. Prefer. 2019, 71, 209–216. [Google Scholar] [CrossRef]
- Aluwé, M.; Aaslyng, M.; Backus, G.; Bonneau, M.; Chevillon, P.; Haugen, J.E.; Meier-Dinkel, L.; Morlein, D.; Oliver, M.A.; Snoek, H.M.; et al. Consumer acceptance of minced meat patties from boars in four European countries. Meat Sci. 2018, 137, 235–243. [Google Scholar] [CrossRef]
- Horizon 2020. Work Programme 2014–2015. Annex G. Available online: https://ec.europa.eu/research/participants/data/ref/h2020/wp/2014_2015/annexes/h2020-wp1415-annex-g-trl_en.pdf (accessed on 10 August 2020).
- Trautmann, J.; Meier-Dinkel, L.; Gertheiss, J.; Mörlein, D. Noise and accustomation: A pilot study of trained assessors’ olfactory performance. PLoS ONE 2017, 12, e0174697. [Google Scholar] [CrossRef]
- Bonneau, M.; Kempster, A.J.; Claus, R.; Claudi-Magnussen, C.; Diestre, A.; Tornberg, E.; Walstra, P.; Chevillon, P.; Weiler, U.; Cook, G.L. An international study on the importance of androstenone and skatole for boar taint: I. Presentation of the programme and measurement of boar taint compounds with different analytical procedures. Meat Sci. 2000, 54, 251–259. [Google Scholar] [CrossRef]
- Blanch, M.; Panella-Riera, N.; Chevillon, P.; Furnols, M.F.; Gil, M.; Gil, J.M.; Kallas, Z.; Oliver, M.A. Impact of consumer’s sensitivity to androstenone on acceptability of meat from entire male pigs in three European countries: France, Spain and United Kingdom. Meat Sci. 2012, 90, 572–578. [Google Scholar] [CrossRef]
- Bonneau, M.; Walstra, P.; Claudi-Magnussen, C.; Kempster, A.J.; Tornberg, E.; Fischer, K.; Diestre, A.; Siret, F.; Chevillon, P.; Claus, R.; et al. An international study on the importance of androstenone and skatole for boar taint: IV. Simulation studies on consumer dissatisfaction with entire male pork and the effect of sorting carcasses on the slaughter line, main conclusions and recommendations. Meat Sci. 2000, 54, 285–295. [Google Scholar] [CrossRef]
- Matthews, K.R.; Homer, D.B.; Punter, P.; Béague, M.P.; Gispert, M.; Kempster, A.J.; Agerhem, H.; Claudi-Magnussen, C.; Fischer, K.; Siret, F.; et al. An international study on the importance of androstenone and skatole for boar taint: III. Consumer survey in seven European countries. Meat Sci. 2000, 54, 271–283. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Aaslyng, M.D.; Backus, G.B.C.; Han, J.; Kuznetsova, T.G.; Panella-Riera, N.; Semenova, A.A.; Zhang, Y.; Oliver, M.A. Russian and Chinese consumers’ acceptability of boar meat patties depending on their sensitivity to androstenone and skatole. Meat Sci. 2016, 121, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Rius, M.A.; Hortós, M.; García-Regueiro, J.A. Influence of volatile compounds on the development of off-flavours in pig back fat samples classified with boar taint by a test panel. Meat Sci. 2005, 71, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Engesser, J. Alternatives for Boar Taint Reduction and Elimination Besides Surgical Castration and Destroying Testicular Tissue. Ph.D. Thesis, University of Leipzig, Leipzig, Germany, 2015. [Google Scholar]
- Weiler, U.; Font i Furnols, M.; Fischer, K.; Kemmer, H.; Oliver, M.A.; Gispert, M.; Dobrowolski, A.; Claus, R. Influence of differences in sensitivity of Spanish and German consumers to perceive androstenone on the acceptance of boar meat differing in skatole and androstenone concentrations. Meat Sci. 2000, 54, 297–304. [Google Scholar] [CrossRef]
- Dougherty, D.P.; Wright, G.A.; Yew, A.C. Computational model of the cAMP-mediated sensory response and calcium-dependent adaptation in vertebrate olfactory receptor neurons. Proc. Natl. Acad. Sci. USA 2005, 102, 10415–10420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Dinkel, L.; Gertheiss, J.; Müller, S.; Wesoly, R.; Mörlein, D. Evaluating the performance of sensory quality control: The case of boar taint. Meat Sci. 2015, 100, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Stefanski, V.; Weiler, U. Influence of sampling procedure, sampling location and skin contamination on skatole and indole concentrations in adipose tissue of pigs. Meat Sci. 2016, 111, 85–91. [Google Scholar] [CrossRef]
- Batorek Lukač, N.; Velikonja-Bolta, Š.; Škrlep, M.; Tomažin, U.; Čandek-Potokar, M. Effect of sampling location on concentration of boar taint compounds in fat tissue. Adv. Anim. Biosci. Altern. Piglet Castration 2018, 9 (Suppl. 1), S39. [Google Scholar]
- Haugen, J.E.; Flåtten, A.; Andresen, Ø. Intracarcass variation of androstenone, skatole and indole fat levels in entire male pigs. In Proceedings of the EAAP Working Group Entire Male Pig, Uppsala, Sweden, 8–9 June 2005. [Google Scholar]
- Claus, R. Messung des Ebergeruchstoffes im Fett von Schweinen Mittels Eines Radioimmunotests. 1. Mitteilung; Technical University of Munich: Munich, Germany, 1975. [Google Scholar]
- Malmfors, B.; Lundström, K.; Hansson, L.; Gahne, B. The effect of HCG and LH-RH on 5α-androstenone levels in plasma & adipose tissue of boars. Swed. J. Agric. Res. 1976, 6, 73–79. [Google Scholar]
- Andresen, Ø.; Bakke, H. 5α-androstenone in fat from boars selected for rate of gain and thickness of backfat, and from boars used in artificial insemination service. Acta Vet. Scand. 1975, 16, 492–502. [Google Scholar]


| Internal Factors | External Factors | |||
|---|---|---|---|---|
| Strengths | Weaknesses | Opportunities | Threats | |
| Human nose | R: Already applied in abattoirs, TRL-9 T: Online as well as at line, determine sensory perception of boar taint S: Does not require sampling if on line C: Immediate result P: it can easily identify carcasses with high levels of boar taint I: Low initial investment | T: Based on human performance, no quantification of AND and SKA, need of initial and periodic training, level of detection intrinsic to the assessors, need of turn-over of assessors due to fatigue S: Needs sampling plan to keep traceability if at line P: High variability in accuracy, high training efforts are needed to identify carcasses with low levels of boar taint, results from individual assessors cannot be linked to consumer acceptance in a scientific/objective way | C: Adaptable to an increase of productivity (more assessors) I: Easy to implement at slaughter line (no big infrastructures needed) O: Possibility to give feedback of boar taint content to genetic companies/breeding programs | C/I: High speed of the lines requires more assessors, so higher costs emerge P/I: Higher performance goals require multiple assessors O: It is a short-term strategy because probably it will be replaced by automatic methods when available. |
| Colorimetric method | R: Used in abattoirs for many years, TRL-9 T: Quantitative SKA equivalents, objective, direct measurement C: Result available after chilling before enter cooling room P: Robust | T: Only SKA equivalents determination (AND not measured) S: At line, need of sampling plan to keep traceability, need of sampling pre-treatment I: High initial investment O: Environmental contamination (chemical residues) | I: Relatively easy to implement at slaughter line (no big infrastructures needed) O: Possibility to give feedback of SKA equivalents content to genetic companies/breeding programs | |
| LDTD-MS/MS 1 | R: Already available at the market, TRL-8/9 T: Quantitative SKA and AND, objective, direct measurement C: Long intervals between maintenance, result available after chilling before entering to the cooling room, 360 samples/h (DTI 3) P: Robust I: Analytical cost 1€/sample (DTI 3) (including consumables and excluding personnel, maintenance, depreciation of investment) | S: At line, need of sampling plan to keep traceability, need sampling pre-treatment I: High initial investment (not only LDTD-MS/MS device) O: Environmental contamination (chemical residues), need of disposable items to minimize cleaning and cross-contamination | C: Possibility of automation O: Possibility to give feedback of AND and SKA content to genetic companies/breeding programs, equipment could be used for determination of other compounds (not related to boar taint) P: Relationship between AND/SKA levels and consumer acceptance partly known [87,88] and is currently being further investigated. | I: High speed lines would need adjustments of the device, personnel, maintenance and depreciation costs not included in the 1€ per sample. Probably highly variable according to slaughter plants and chains. |
| REIMS 2 | T: Objective S: On line does not require sampling or sample pre-treatment. I: Low operational cost | R: TRL-5 T: Indirect measurement, dependent on discriminant models’ training set, classification in yes-no taint C: Needs maintenance (cleaning) after low number of samples I: High initial investment O: Environmental contamination (chemical residues), need of disposable items to minimize cleaning and cross-contamination | I: Relatively easy to implement at slaughter line (no big infrastructures needed) O: Equipment can be used to determine other compounds (not related to boar taint) | P: Variability of carcass characteristics in different slaughter plants (could decrease robustness), actual performance in industrial conditions (relationships of results with AND/SKA levels or consumer acceptance) still unknown. I: High speed lines would need adjustments of the device |
| Raman spectroscopy | T: Objective I: Low analytical cost | R: TRL-4 T: Indirect measurement of boar taint or AND/SKA, dependent on discriminant models’ training set or calibration set S: At line needs sampling plan to keep traceability, need sampling pre-treatment C: High acquisition time | R: Portable device exists, and it could be implemented on slaughter line provided that measurement/data acquisition become vaster without affecting performance O: Equipment can be used to determine other compounds (other than boar taint), possibility to give feedback of boar taint classification (Raman) or AND and SKA content (SERS) to genetic companies/breeding programs | P: Variability in slaughter plant carcass characteristics (could decrease robustness), actual performance in industrial conditions (relationships of results with AND/SKA levels (SERS)/boar taint classification (Raman) or consumer acceptance) still unknown I: High speed lines would need adjustments of the device |
| Electrochemical biosensors | T: Quantitative SKA and AND, objective S: Does not require sampling or sample pre-treatment. C: Result available after chilling before entering the cooling room | R: TRL-6 S: Need to keep traceability due to time lapse I: Need of disposable items to minimize cleaning and cross-contamination O: Environmental contamination (if disposable) | C: Possibility of automation P: Relationship between AND/SKA levels and consumer acceptance partly known [87,88] and is currently being further investigated O: Equipment can be used to determine other compounds (other than boar taint), possibility to give feedback of AND and SKA content to genetic companies/breeding programs | I: High speed lines would need adjustments of the device, costs (disposable probes, personnel, maintenance, depreciation) unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font-i-Furnols, M.; Martín-Bernal, R.; Aluwé, M.; Bonneau, M.; Haugen, J.-E.; Mörlein, D.; Mörlein, J.; Panella-Riera, N.; Škrlep, M. Feasibility of on/at Line Methods to Determine Boar Taint and Boar Taint Compounds: An Overview. Animals 2020, 10, 1886. https://doi.org/10.3390/ani10101886
Font-i-Furnols M, Martín-Bernal R, Aluwé M, Bonneau M, Haugen J-E, Mörlein D, Mörlein J, Panella-Riera N, Škrlep M. Feasibility of on/at Line Methods to Determine Boar Taint and Boar Taint Compounds: An Overview. Animals. 2020; 10(10):1886. https://doi.org/10.3390/ani10101886
Chicago/Turabian StyleFont-i-Furnols, Maria, Raúl Martín-Bernal, Marijke Aluwé, Michel Bonneau, John-Erik Haugen, Daniel Mörlein, Johanna Mörlein, Núria Panella-Riera, and Martin Škrlep. 2020. "Feasibility of on/at Line Methods to Determine Boar Taint and Boar Taint Compounds: An Overview" Animals 10, no. 10: 1886. https://doi.org/10.3390/ani10101886
APA StyleFont-i-Furnols, M., Martín-Bernal, R., Aluwé, M., Bonneau, M., Haugen, J.-E., Mörlein, D., Mörlein, J., Panella-Riera, N., & Škrlep, M. (2020). Feasibility of on/at Line Methods to Determine Boar Taint and Boar Taint Compounds: An Overview. Animals, 10(10), 1886. https://doi.org/10.3390/ani10101886





