Animal Research beyond the Laboratory: Report from a Workshop on Places Other than Licensed Establishments (POLEs) in the UK
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
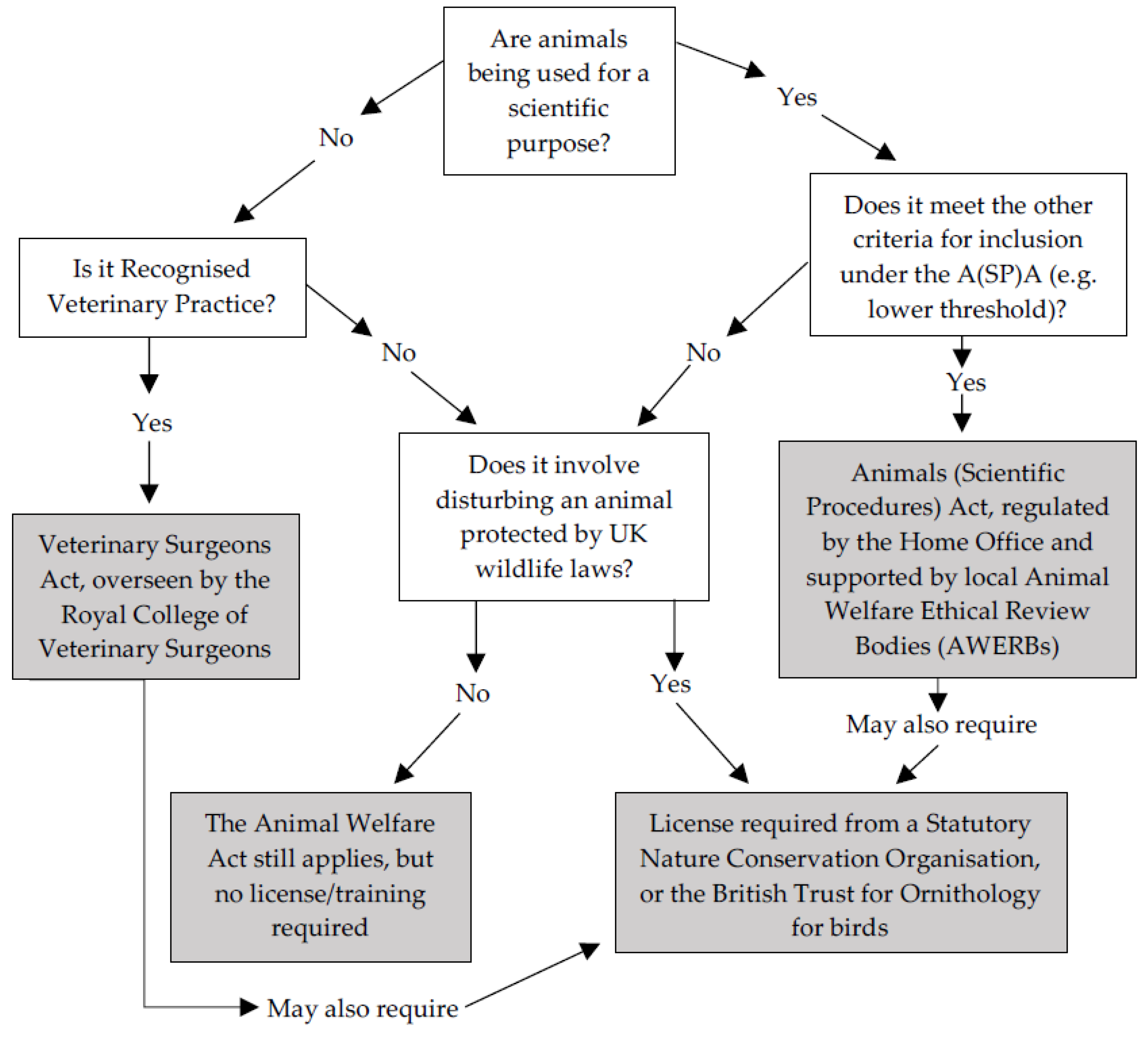
- How does the category of animal (e.g., pets, wild animals, those housed in zoos or farms) shape ethical obligations, veterinary treatment, and humane end-points? How does the A(SP)A manage these ethical obligations and influence decisions?
- How are boundaries drawn between work under and outside of the A(SP)A, and how do these boundaries shape research and animal welfare practice and regulation?
- How do the general public and other stakeholders engage with research at POLEs?
- How does taking scientific research with animals out of the laboratory shape the knowledge produced?
- How is research with animals outside of the laboratory best regulated?
3. Results and Discussion
3.1. Practical and Ethical Contrasts between Research at POLEs and in the Laboratory
3.2. Transparency and Profile of POLEs
3.3. Ethical Review
3.4. Training, Competency, and Support
3.5. Regulation and Cultures of Care
3.6. Defining Science
4. Conclusions
- Support and training. It was agreed that further support and training networks would be helpful in allowing those working at POLEs under the A(SP)A to share experiences and best practice. Such networks would also ideally connect researchers working under the A(SP)A with those undertaking subthreshold research, such as veterinarians, citizen scientists, and ecological consultants.
- Ethical review. Workshop contributors were supportive of the idea that if they do not do so already, AWERBs should be encouraged to learn about how research may be described, peer-reviewed, approved, and carried out differently at POLEs compared with in laboratories. As part of this discussion, it was proposed that AWERBs could be encouraged to oversee animal research not falling under the A(SP)A such as work that is subthreshold, based overseas, or involving animal taxa such as insects and crustaceans not currently classified as protected under the A(SP)A. Some AWERBs already extend their mandates in this manner, although the scale of this extension is unknown. This echoes the idea that AWERBs should be viewed as hubs for ethics and welfare discussion [49,50].
- Cultures of care. It was proposed that methods for encouraging and ensuring harm−benefit analysis and attention to animal welfare within nonregulated work, such as the marking and tagging of wild animals not protected under the WCA, should be explored. This may involve regulation, or nonregulatory means of encouraging cultures of care such as the distribution of a plainly written and widely accessible guide that prompts consideration of ethics and purpose, although implementation and funding of such activities remain unclear.
- Setting of boundaries. Specific regulatory grey areas identified by some workshop participants relate to the boundaries set between research and practice, such as RVP. It was proposed that ambiguity relating to RVP should be clarified by the RCVS.
- Statistics and transparency. Some workshop attendees felt that it would be helpful to have access to further published statistics on the numbers of research projects and animals worked on in nonlaboratory settings, both for research carried out under the A(SP)A and subthreshold research, such as the trapping and marking of wildlife performed by citizen scientists and ecological consultants. This might require not only action by the Home Office and SNCOs, but also the creation of a new database where work requiring no licenses could be registered. Although it remains unclear how such a project would be implemented, funded, and kept secure, there may be scope for enrolling established citizen science platforms such as Zooniverse (https://www.zooniverse.org/).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Home Office. Guidance on the Operation of the Animals (Scientific Procedures) Act 1986; Home Office: London, UK, 2014. [Google Scholar]
- ASRU. Animals (Scientific Procedures) Act 1986: Working with Animals Taken from the Wild; Animals in Science Regulation Unit, Home Office: London, UK, 2016. [Google Scholar]
- Fry, D. How different countries control animal experiments outside recognized establishments. Altex Proc 2012, 12, 309–313. [Google Scholar]
- Paul, E.; Sikes, R.S. Wildlife researchers running the permit maze. ILAR J. 2013, 54, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Wilson, A.D.M.; Elvidge, C.K.; Lennox, R.J.; Jepsen, N.; Colotelo, A.H.; Brown, R.S. Ten practical realities for institutional animal care and use committees when evaluating protocols dealing with fish in the field. Rev. Fish. Biol. Fish. 2016, 26, 123–133. [Google Scholar] [CrossRef]
- Curzer, H.J.; Wallace, M.; Perry, G. Environmental research ethics. Environ. Ethics 2013, 35, 95–114. [Google Scholar] [CrossRef]
- Russow, L.-M.; Theran, P. Ethical issues concerning animal research outside the laboratory. ILAR J. 2003, 44, 187–190. [Google Scholar] [CrossRef]
- Sikes, R.S.; Paul, E. Fundamental differences between wildlife and biomedical research. ILAR J. 2013, 54, 5–13. [Google Scholar] [CrossRef]
- Lane, J.M.; McDonald, R.A. Welfare and ‘best practice’ in field studies of wildlife. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals; Wiley-Blackwell: Chichester, UK, 2010; pp. 92–106. [Google Scholar]
- European Commission. Directive 2010/63/EU; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Geen, G.R.; Robinson, R.A.; Baillie, S.R. Effects of tracking devices on individual birds—A review of the evidence. J. Avian Biol. 2019, 50, e01823. [Google Scholar] [CrossRef]
- Vandenabeele, S.; Wilson, R.; Grogan, A. Tags on seabirds: How seriously are instrument-induced behaviours considered? Anim. Welf. 2011, 20, 559–571. [Google Scholar]
- Vandenabeele, S.P.; Shepard, E.L.; Grogan, A.; Wilson, R.P. When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar. Biol. 2012, 159, 1–14. [Google Scholar] [CrossRef]
- Bodey, T.W.; Cleasby, I.R.; Bell, F.; Parr, N.; Schultz, A.; Votier, S.C.; Bearhop, S. A phylogenetically controlled meta-analysis of biologging device effects on birds: Deleterious effects and a call for more standardized reporting of study data. Methods Ecol. Evol. 2018, 9, 946–955. [Google Scholar] [CrossRef]
- Norecopa. A consensus statement from the participants. In Harmonisation of the Care and Use of Wild and Domestic Mammals and Birds in Field Research: Gardermoen, 26–27 October; Norecopa: Oslo, Norway, 2017. [Google Scholar]
- Wilson, R. Tags on birds: How much are our guidelines flights of fancy? In Harmonisation of the Care and Use of Wild and Domestic Mammals and Birds in Field Research: Gardermoen, 26–27 October; Norecopa: Oslo, Norway, 2017. [Google Scholar]
- Lindsjö, J.; Cvek, K.; Spangenberg, E.M.F.; Olsson, J.N.G.; Stéen, M. The dividing line between wildlife research and management—implications for animal welfare. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.; Reynolds, S.J.; Lane, J.; Dickey, R.; Greenhough, B. Getting to grips with wildlife research by citizen scientists: What role for regulation? People Nat. 2020. [Google Scholar] [CrossRef]
- Anderson, J.; Jukes, H. Clinical trials in practice: What do you need to know? Practice 2007, 29, 546. [Google Scholar] [CrossRef]
- Braidwood, J. Veterinary clinical trials in the UK. Vet. Rec. 2013, 173, 225. [Google Scholar] [CrossRef] [PubMed]
- Hockey, G. Recognised veterinary practice and clinical trials. Vet. Rec. 2015, 176, 659. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N. How lack of clarity in regulation is stifling veterinary research. Vet. Rec. 2014, 174, 148. [Google Scholar] [CrossRef]
- Lucke, J. Recognised veterinary practice. Vet. Rec. 2007, 161, 795. [Google Scholar] [CrossRef] [PubMed]
- Moens, Y.; Brodbelt, D.; Seymour, C.; Brearley, J.; Clutton, E.; Taylor, P. Use of the medicines cascade. Vet. Rec. 2007, 161, 731. [Google Scholar] [CrossRef]
- Nanjiani, I.; Hume, D.; Fitzpatrick, J.; Murray, J. “Recognised veterinary practice” in the context of clinical field trials. Vet. Rec. 2015, 176, 552. [Google Scholar] [CrossRef]
- Sparkes, A.; Wray, J.; Leece, E.; de Risio, L.; Murphy, S.; Furneaux, R.; Sansom, J.; Coatesworth, J.; Dean, S.; Noakes, D.; et al. Recognised veterinary practice. Vet. Rec. 2007, 161, 827. [Google Scholar] [CrossRef]
- Fordyce, P.; Mullan, S. Nature and governance of veterinary clinical research conducted in the UK. Vet. Rec. 2017, 180, 69. [Google Scholar] [CrossRef] [PubMed]
- Clutton, E. Clinical studies, pain and ethics. J. Small Anim. Pract. 2009, 50, 59–60. [Google Scholar] [CrossRef]
- Clutton, E.; Bradbury, G.; Chennells, D.; Dennison, N.; Duncan, J.; Few, B.; Flecknell, P.; Golledge, H.; McKeegan, D.; Murphy, K.; et al. Pets in clinical trials. Vet. Rec. 2017, 181, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Fürdös, I.; Fazekas, J.; Singer, J.; Jensen-Jarolim, E. Translating clinical trials from human to veterinary oncology and back. J. Transl. Med. 2015, 13, 265. [Google Scholar] [CrossRef]
- Innes, J. A call for better regulation of veterinary medical devices. Vet. Rec. 2018, 183, 229. [Google Scholar] [CrossRef]
- Yeates, J.; Everitt, S.; Innes, J.F.; Day, M.J. Ethical and evidential considerations on the use of novel therapies in veterinary practice. J. Small Anim. Pract. 2013, 54, 119–123. [Google Scholar] [CrossRef]
- Yeates, J.W. Ethical principles for novel therapies in veterinary practice. J. Small Anim. Pract. 2016, 57, 67–73. [Google Scholar] [CrossRef]
- Gieryn, T.F. City as truth-spot: Laboratories and field-sites in urban studies. Soc. Stud. Sci. 2006, 36, 5–38. [Google Scholar] [CrossRef]
- Greenhough, B. Tales of an island-laboratory: Defining the field in geography and science studies. Trans. Inst. Br. Geogr. 2006, 31, 224–237. [Google Scholar] [CrossRef]
- Kohler, R.E. Landscapes and Labscapes: Exploring the Lab-Field Border in Biology; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Laber, K.; Kennedy, B.W.; Young, L. Field studies and the IACUC: Protocol review, oversight, and occupational health and safety considerations. Lab. Anim. 2007, 36, 27–33. [Google Scholar] [CrossRef]
- Mulcahy, D.M. Does the Animal Welfare Act apply to free-ranging animals? ILAR J. 2003, 44, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Gorman, R.; Greenhough, B.; Hobson-West, P.; Kirk, R.G.W.; Message, R.; Myelnikov, D.; Palmer, A.; Roe, E.; Ashall, V.; et al. Animal research nexus: A new approach to the connections between science, health and animal welfare. Med. Humanit. 2020. [Google Scholar] [CrossRef] [PubMed]
- Animal Research Nexus Summary Notes from POLEs Workshop, 30th Sept-1st Oct. Available online: https://animalresearchnexus.org/index.php/publications/summary-notes-poles-workshop-30th-sept-1st-oct-2019 (accessed on 19 November 2019).
- Animals in Science Committee. Review of Harm-Benefit Analysis in the Use of Animals in Research; Home Office: London, UK, 2017. [Google Scholar]
- Margulis, S. Zoos as venues for research: Changes in focus, changes in perception. In Increasing Legal Rights for Zoo Animals: Justice on the Ark; Donahue, J., Ed.; Lexington Books: Lanham, MD, USA, 2017; pp. 49–78. [Google Scholar]
- Grimm, D. Can clinical trials on dogs and cats help people? Science 2016, 11. [Google Scholar] [CrossRef]
- Lanyon, L. Evidence-based veterinary medicine: A clear and present challenge. Vet. Rec. 2014, 174, 173. [Google Scholar] [CrossRef] [PubMed]
- Pet projects need a helping hand: Clinical trials with cats and dogs offer great promise for animal and human medicine but risk being stifled by overzealous regulations. Nat. Ed. 2016, 540, 169.
- Snow, J. Man’s BestFriend: How Veterinary Research Could Save Human Lives; The Guardian: London, UK, 2015. [Google Scholar]
- ASRU. Animals (Scientific Procedures) Act 1986: Re-Homing and Setting Free of Animals; Animals in Science Regulation Unit, Home Office: London, UK, 2015. [Google Scholar]
- Robinson, R.A.; Leech, D.I.; Clark, J.A. The Online Demography Report: Bird Ringing and Nest Recording in Britain & Ireland in 2018; British Trust for Ornithology: Thetford, UK, 2019. [Google Scholar]
- Hawkins, P.; Hobson-West, P. Delivering Effective Ethical Review: The AWERB as a “Forum for Discussion”; RSPCA Research Animals Department: Horsham, UK, 2017. [Google Scholar]
- RSPCA; LASA. Guiding Principles on Good Practice for Animal Welfare and Ethical Review Bodies; Royal Society for the Prevention of Cruelty to Animals; Laboratory Animal Science Association: Horsham, UK; Hull, UK, 2015. [Google Scholar]
- Bee, M.; Bernal, X.; Calisi, R.; Carere, C.; Carter, T.; Fuertbauer, I.; Ha, J.C.; Hubrecht, R.; Jennings, D.; Metcalfe, N.; et al. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2020, 159, i–xi. [Google Scholar] [CrossRef]
- PLoS ONE Submission Guidelines. Available online: https://journals.plos.org/plosone/s/submission-guidelines (accessed on 12 October 2020).
- Eitzel, M.; Cappadonna, J.; Santos-Lang, C.; Duerr, R.; West, S.E.; Virapongse, A.; Kyba, C.; Bowser, A.; Cooper, C.; Sforzi, A.; et al. Citizen science terminology matters: Exploring key terms. Citiz. Sci. 2017, 2, 1–20. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Chapman, D.S.; Sheppard, L.J.; Roy, H.E. Choosing and Using Citizen Science: A Guide to When and How to Use Citizen Science to Monitor Biodiversity and the Environment; Centre for Ecology & Hydrology: Crowmarsh Gifford, UK, 2014. [Google Scholar]
- Natural England References and Experience Needed to Support Your Protected Species Application or Registration. Available online: https://www.gov.uk/government/publications/reference-to-support-a-protected-species-licence/protected-species-licences-guidance-on-getting-references-to-support-applications (accessed on 26 March 2020).
- Wilson, R.P.; Holton, M.; Wilson, V.L.; Gunner, R.; Tysse, B.; Wilson, G.I.; Quintana, F.; Duarte, C.; Scantlebury, D.M. Towards informed metrics for examining the role of human-induced animal responses in tag studies on wild animals. Integr. Zool. 2019, 14, 17–29. [Google Scholar] [CrossRef]
- Shonfield, J.; Do, R.; Brooks, R.J.; McAdam, A.G. Reducing accidental shrew mortality associated with small-mammal livetrapping I: An inter- and intrastudy analysis. J. Mammal. 2013, 94, 745–753. [Google Scholar] [CrossRef]
- RCVS. Recognised veterinary practice. In Code of Professional Conduct for Veterinary Surgeons and Supporting Guidance; Royal College of Veterinary Surgeons: London, UK, 2018; pp. 153–158. [Google Scholar]
- Bishop, J.; Hartley, A.; Hosey, G.; Masters, N.; Melfi, V. Legal and ethical issues in zoo research. In Handbook of Zoo & Aquarium Research: Guidelines for Conducting Research in Zoos and Aquariums; Bishop, J., Hosey, G., Plowman, A., Eds.; British & Irish Association of Zoos & Aquariums: London, UK, 2013; pp. 15–21. [Google Scholar]
- Palmer, A.; Message, R.; Greenhough, B. Edge cases at the boundaries of animal research law: Constituting the regulatory borderlands of the UK’s Animals (Scientific Procedures) Act. In Preparation.
- Greenhough, B.; Roe, E. Exploring the role of Animal Technologists in implementing the 3Rs: An ethnographic investigation of the UK university sector. Sci. Technol. Hum. Values 2018, 43, 694–722. [Google Scholar] [CrossRef]
- Tidemann, S.C.; Gosler, A. Ethno-Ornithology: Birds, Indigenous Peoples, Culture and Society; Routledge: London, UK, 2010. [Google Scholar]
- Cooper, C.B.; Lewenstein, B.V. Two meanings of citizen science. In The Rightful Place of Science: Citizen Science; Cavalier, D., Kennedy, E.B., Eds.; Consortium for Science, Policy & Outcomes, Arizona State University: Tempe, AZ, USA, 2016; pp. 51–62. [Google Scholar]
- Resnik, D.B.; Elliott, K.C.; Miller, A.K. A framework for addressing ethical issues in citizen science. Environ. Sci. Policy 2015, 54, 475–481. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, A.; Greenhough, B.; Hobson-West, P.; Message, R.; Aegerter, J.N.; Belshaw, Z.; Dennison, N.; Dickey, R.; Lane, J.; Lorimer, J.; et al. Animal Research beyond the Laboratory: Report from a Workshop on Places Other than Licensed Establishments (POLEs) in the UK. Animals 2020, 10, 1868. https://doi.org/10.3390/ani10101868
Palmer A, Greenhough B, Hobson-West P, Message R, Aegerter JN, Belshaw Z, Dennison N, Dickey R, Lane J, Lorimer J, et al. Animal Research beyond the Laboratory: Report from a Workshop on Places Other than Licensed Establishments (POLEs) in the UK. Animals. 2020; 10(10):1868. https://doi.org/10.3390/ani10101868
Chicago/Turabian StylePalmer, Alexandra, Beth Greenhough, Pru Hobson-West, Reuben Message, James N. Aegerter, Zoe Belshaw, Ngaire Dennison, Roger Dickey, Julie Lane, Jamie Lorimer, and et al. 2020. "Animal Research beyond the Laboratory: Report from a Workshop on Places Other than Licensed Establishments (POLEs) in the UK" Animals 10, no. 10: 1868. https://doi.org/10.3390/ani10101868
APA StylePalmer, A., Greenhough, B., Hobson-West, P., Message, R., Aegerter, J. N., Belshaw, Z., Dennison, N., Dickey, R., Lane, J., Lorimer, J., Millar, K., Newman, C., Pullen, K., Reynolds, S. J., Wells, D. J., Witt, M. J., & Wolfensohn, S. (2020). Animal Research beyond the Laboratory: Report from a Workshop on Places Other than Licensed Establishments (POLEs) in the UK. Animals, 10(10), 1868. https://doi.org/10.3390/ani10101868





