Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
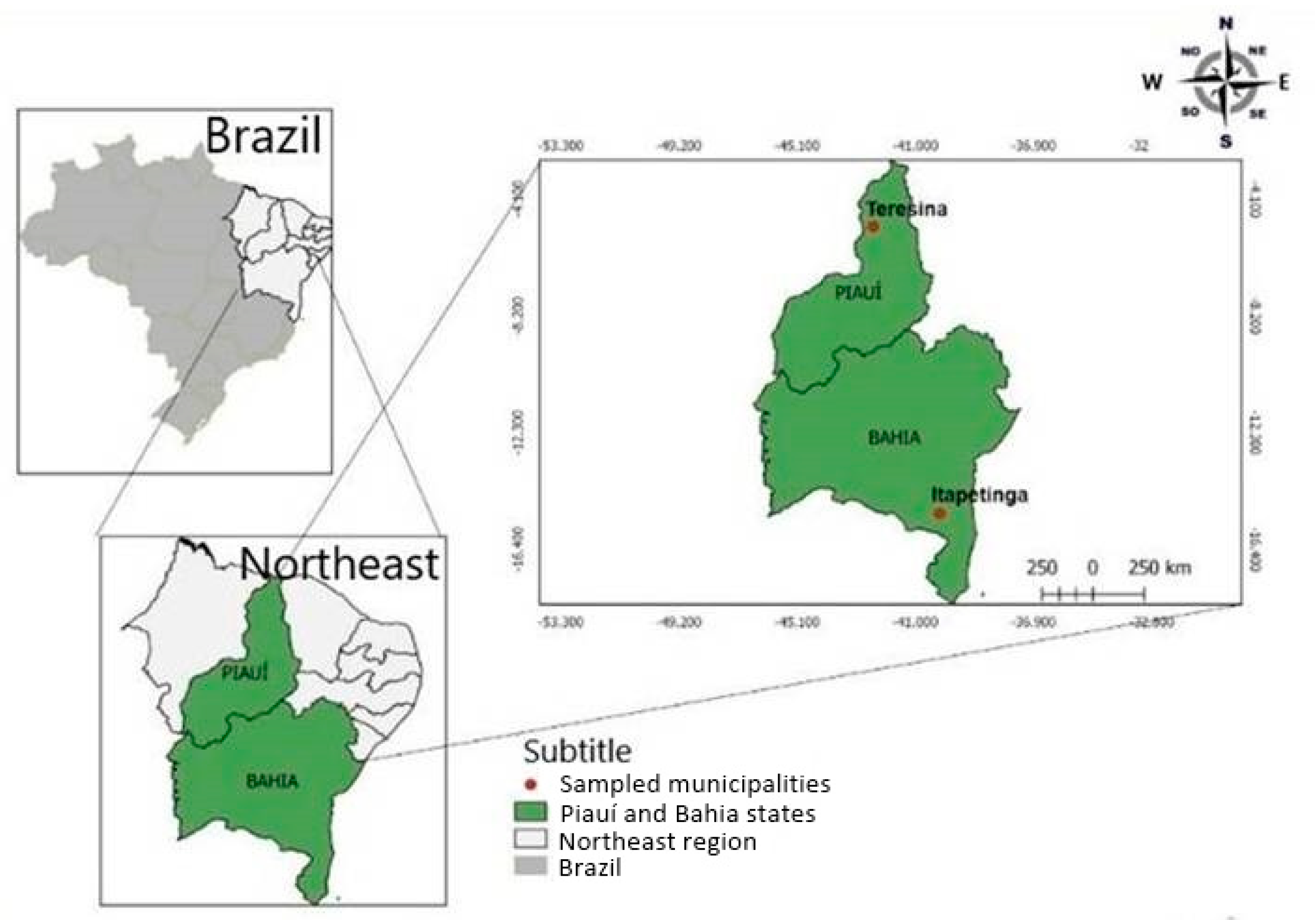
2.2. Sampling
2.3. DNA Amplification, Molecular Markers, and Genotyping
2.4. Statistical and Genetic Analyses
3. Results
3.1. Inter- and Intrabreed Genetic Diversity of the Three Populations of Chickens from the Brazilian Northeast
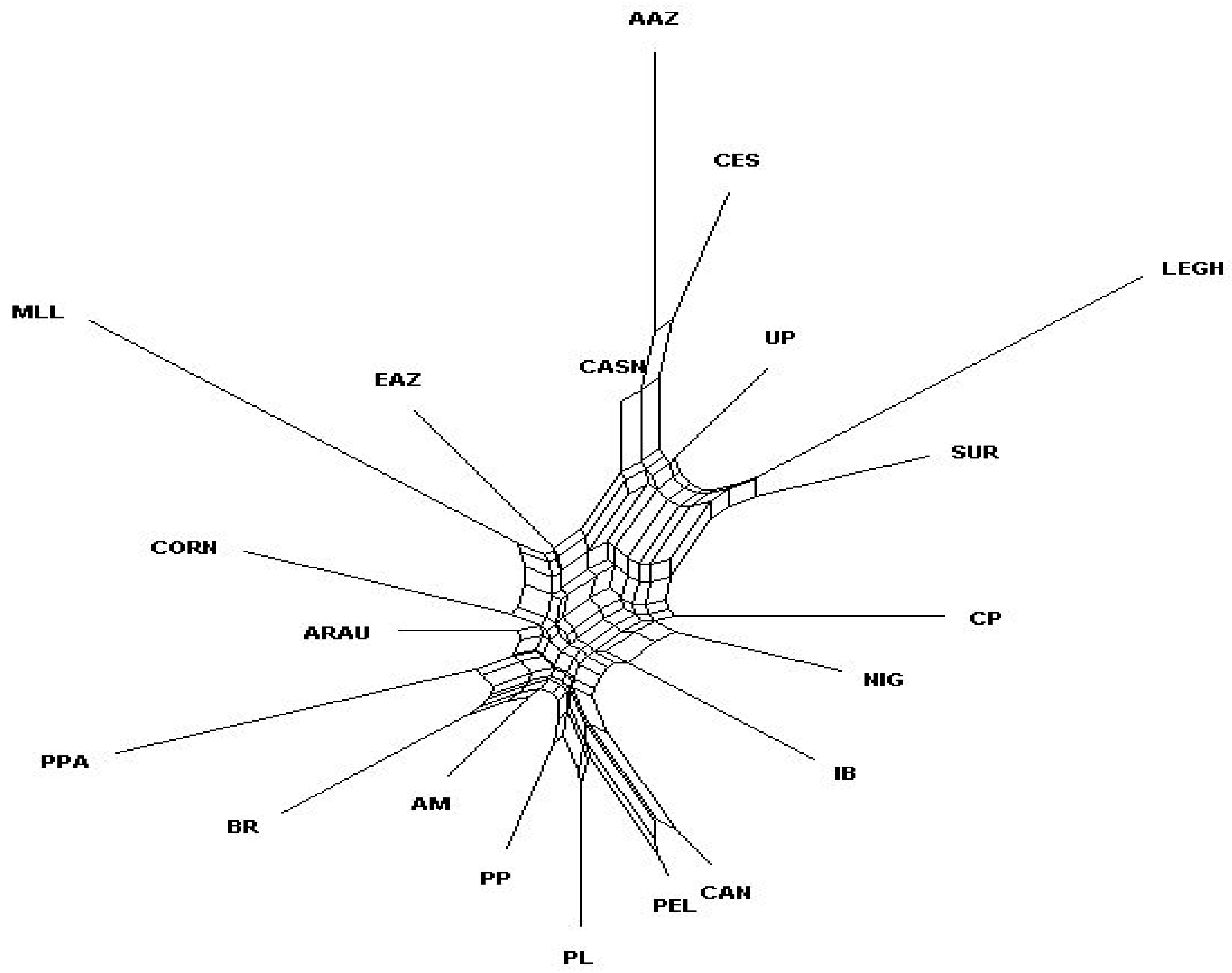
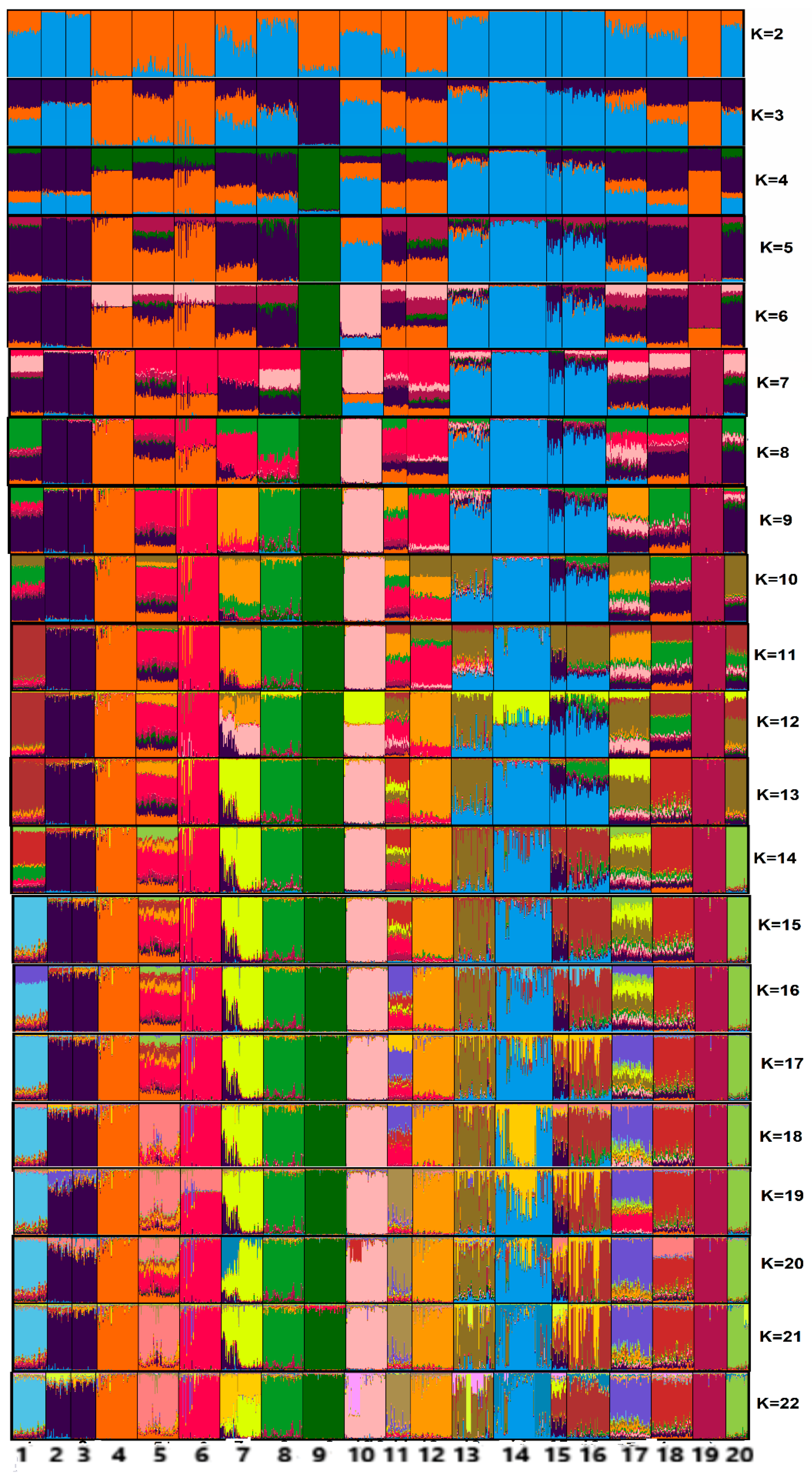
3.2. Genetic Relationships between Brazilian and Exotic Chicken Breeds
4. Discussion
4.1. Genetic Diversity within and among the Three Populations of Chickens from the Brazilian Northeast
4.2. Genetic Relationships between Brazilian and Exotic Chicken Breeds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Global plan of action for animal genetic resources and the Interlaken Declaration International Technical Conference on Animal Genetic Resources for Food and Agriculture. Rome, Italy. 2007. Available online: http://www.fao.org/3/a1404e/a1404e00.htm (accessed on 1 September 2020).
- Almeida, E.C.J.; Carneiro, P.L.S.; Nunes, L.A.; Pereira, A.H.R.; Farias Filho, R.V.; Malhado, C.H.M.; Bittencourt, T.C.B.S.C. Características físicas de ovos de galinhas nativas comparadas a linhagem de postura. Arch. Zootec. 2019, 68, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.A.; Bonafé, C.M.; Almeida, M.J.O.; Rodriguez-Rodriguez, M.P.; Sarmento, J.L.R.; Silva, M.A.; Oliveira, M.B.; Sousa, P.R.; Carvalho, A.A. Padrão racial fenotípico de galinhas brasileiras da raça Canela-Preta. Arch. Zootec. 2017, 66, 195–202. [Google Scholar]
- Ghernouti, N.; Bodinier, M.; Ranebi, D.; Maftah, A.; Petit, D.; Gaouar, S.B.S. Control region of mtDNA identifies three migration events of sheep breeds in Algeria. Small Rumin. Res. 2017, 155, 66–71. [Google Scholar] [CrossRef]
- Delgado Bermejo, J.V.; Barba Capote, C.J.; Aguirre Riofrío, E.L.; Cabezas Congo, R.; Cevallos Falquez, O.F.; Rizzo Zamora, L.G.; Vargas Burgos, J.C.; Navas González, F.J.; Álvarez Franco, L.Á.; Biobovis, C.; et al. Molecular inference in the colonization of cattle in Ecuador. Res. Vet. Sci. 2020, 132, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.A.; Bonafé, C.M.; Rodriguez-Rodriguez, M.D.P.; Almeida, M.J.O.; Sarmento, J.L.R.; Britto, F.B.; Silva, M.A. Caracterização genética e estrutura populacional de galinhas caipiras Canela-Preta. Pesq. Agropec. Bras. 2016, 51, 1899–1906. [Google Scholar] [CrossRef] [Green Version]
- Toalombo Vargas, P.A.; León, J.M.; Fiallos Ortega, L.R.; Martinez, A.; Villafuerte Gavilanes, A.A.; Delgado, J.V.; Landi, V. Deciphering the patterns of genetic admixture and diversity in the Ecuadorian Creole chicken. Animals 2019, 9, 670. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, D.A.; Bonafé, C.M.; Rodriguez-Rodriguez, M.D.P.; Almeida, M.J.O.; Sarmento, J.L.R.; Britto, F.B.; Silva, M.A. Variabilidade genética de 12 loci de microssatélites em galinhas crioulas Canela-Preta. Arq. Bras. Med. Vet. Zootec. 2018, 70, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Macri, M.; Martínez, A.M.; Landi, V.; Canales, A.; Arando, A.; Delgado, J.V.; Camacho, M.E. Diversidad genética de la raza Gallina Utrerana. Actas Iberoam. Conserv. Anim. 2019, 13, 52–59. [Google Scholar]
- Ceccobelli, S.; Di Lorenzo, P.; Lancioni, H.; Ibáñez, L.V.M.; Tejedor, M.T.; Castellini, C.; Landi, V.; Martínez, A.M.; Bermejo, J.V.D.; Pla, J.L.V. Genetic diversity and phylogeographic structure of sixteen Mediterranean chicken breeds assessed with microsatellites and mitochondrial DNA. Livest. Sci. 2015, 175, 27–36. [Google Scholar] [CrossRef]
- Walsh, P.S.; Varlaro, J.; Reynolds, R. A rapid chemiluminescent method for quantitation of human DNA. Nucleic Acids Res. 1992, 20, 5061–5065. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). Molecular Genetic Characterization of Animal Genetic Resources. FAO Animal Production and Health Guidelines. No. 9. Rome, 2011. Available online: http://www.fao.org/3/i2413e/i2413e00.htm (accessed on 1 September 2020).
- Ceccobelli, S.; Lorenzo, P.D.; Lancioni, H.; Castellini, C.; Ibáñez, L.V.M.; Sabbioni, A.; Sarti, F.M.; Weigend, S.; Lasagna, E. Phylogeny, genetic relationships and population structure of five Italian local chicken breeds. Ital. J. Anim. Sci. 2013, 12, e66. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar]
- Rousset, F. genepop’007: A complete re-implementation of the genepop software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2005, 23, 254–267. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; vonHoldt, B.M. Structure Harvester: A website and program for visualizing Structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnault, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [Green Version]
- Wimmers, K.; Ponsuksili, S.; Hardge, T.; Valle-Zarate, A.; Mathur, P.K.; Horst, P. Genetic distinctness of African, Asian and South American local chickens. Anim. Genet. 2000, 31, 159–165. [Google Scholar] [CrossRef]
- López-Zavala, R.; Cano-Camacho, H.; Chassin-Noria, O.; Oyama, K.; Vázquez-Marrufo, G.; Zavala-Páramo, M.G. Diversidad genética y estructura de poblaciones de pavos domésticos mexicanos. Rev. Mex. Cienc. Pecu. 2013, 4, 417–434. [Google Scholar]
- Abebe, A.S.; Mikko, S.; Johansson, A.M. Genetic diversity of five local Swedish chicken breeds detected by microsatellite markers. PLoS ONE 2015, 10, e0120580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementino, C.S. Caracterização genética de galinhas naturalizadas na região Meio-Norte do Brasil, com uso de microssatélites. Master’s Thesis, UFPI-Federal University of Piauí, Teresina, Piauí, Brazil, 24 June 2010. Available online: http://ainfo.cnptia.embrapa.br/digital/bitstream/item/123466/1/DissertacaoCintiaFabio.pdf (accessed on 10 September 2020). (In Portuguese).
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 1997; 519p. [Google Scholar]
- Dancause, K.N.; Vilar, M.G.; Steffy, R.; Lum, J.K. Characterizing genetic diversity of contemporary pacific chickens using mitochondrial DNA analyses. PLoS ONE 2011, 6, e16843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drescher, S. A abolição brasileira em perspectiva comparativa. Hist. Soc. 1995, 2, 115–162. [Google Scholar]


| Breed | Abbreviation | Country/Region | Sample Size |
|---|---|---|---|
| Canela-Preta | CP | Brazil | 40 |
| Caneluda do Catolé | CAN | Brazil | 30 |
| Peloco | PEL | Brazil | 30 |
| Andaluza Azul | AAZ | Spain | 50 |
| Castellana Negra | CASN | Spain | 50 |
| Combatiente Español | CES | Spain | 50 |
| Extremeña Azul | EAZ | Spain | 50 |
| Ibicenca | IB | Spain | 50 |
| Mallorquina | MLL | Spain | 50 |
| Pita Pinta | PPA | Spain | 50 |
| Sureña | SUR | Spain | 30 |
| Utrerana Perdiz | UP | Spain | 50 |
| Leghorn | LEGH | Europe | 40 |
| Cornish | CORN | Europe | 26 |
| Araucana | ARAU | Chile | 50 |
| Nigerian chickens | NIG | Nigeria | 50 |
| Amarela | AM | Portugal | 50 |
| Branca | BR | Portugal | 69 |
| Preta Lusitânica | PL | Portugal | 19 |
| Pedrês Portuguesa | PP | Portugal | 52 |
| Total | 886 |
| Genetic Group | N | Na | Ho | He | UHe | F | HWE (p-Value) | |
|---|---|---|---|---|---|---|---|---|
| CP | Mean | 39.8 | 4.960 | 0.617 | 0.618 | 0.626 | −0.001 | 0.842 |
| SD | 0.291 | 0.030 | 0.029 | 0.029 | 0.019 | |||
| CAN | Mean | 29.6 | 5.040 | 0.642 | 0.634 | 0.645 | −0.025 | 0.176 |
| SD | 0.426 | 0.031 | 0.027 | 0.028 | 0.042 | |||
| PEL | Mean | 29.6 | 4.960 | 0.646 | 0.620 | 0.630 | −0.049 | 0.105 |
| SD | 0.344 | 0.030 | 0.027 | 0.028 | 0.027 |
| Source of Variation | DF | SS | MS | Var. Comp. | %T | F (p-Value) |
|---|---|---|---|---|---|---|
| Between populations | 2 | 110.817 | 55.480 | 0.714 | 8 | FST = 0.082 (0.001) 1 |
| Between individuals | 97 | 800.858 | 8.257 | 0.234 | 3 | FIT = 0.108 (0.001) 2 |
| Within individuals | 100 | 779.000 | 7.790 | 7.790 | 89 | FIS = 0.029 (0.042) 3 |
| Total | 199 | 1690.775 | 0.027 | 8.738 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo de Carvalho, D.; Martínez Martínez, A.; Carolino, I.; Barros, M.C.; Camacho Vallejo, M.E.; Santos-Silva, F.; de Oliveira Almeida, M.J.; Carolino, N.; Delgado Bermejo, J.V.; Sarmento, J.L.R. Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil. Animals 2020, 10, 1857. https://doi.org/10.3390/ani10101857
Araújo de Carvalho D, Martínez Martínez A, Carolino I, Barros MC, Camacho Vallejo ME, Santos-Silva F, de Oliveira Almeida MJ, Carolino N, Delgado Bermejo JV, Sarmento JLR. Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil. Animals. 2020; 10(10):1857. https://doi.org/10.3390/ani10101857
Chicago/Turabian StyleAraújo de Carvalho, Débora, Amparo Martínez Martínez, Inês Carolino, Maria Claudene Barros, María Esperanza Camacho Vallejo, Fátima Santos-Silva, Marcos Jacob de Oliveira Almeida, Nuno Carolino, Juan Vicente Delgado Bermejo, and José Lindenberg Rocha Sarmento. 2020. "Diversity and Genetic Relationship of Free-Range Chickens from the Northeast Region of Brazil" Animals 10, no. 10: 1857. https://doi.org/10.3390/ani10101857






