3D Geometric Morphometrics Reveals Convergent Character Displacement in the Central European Contact Zone between Two Species of Hedgehogs (Genus Erinaceus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
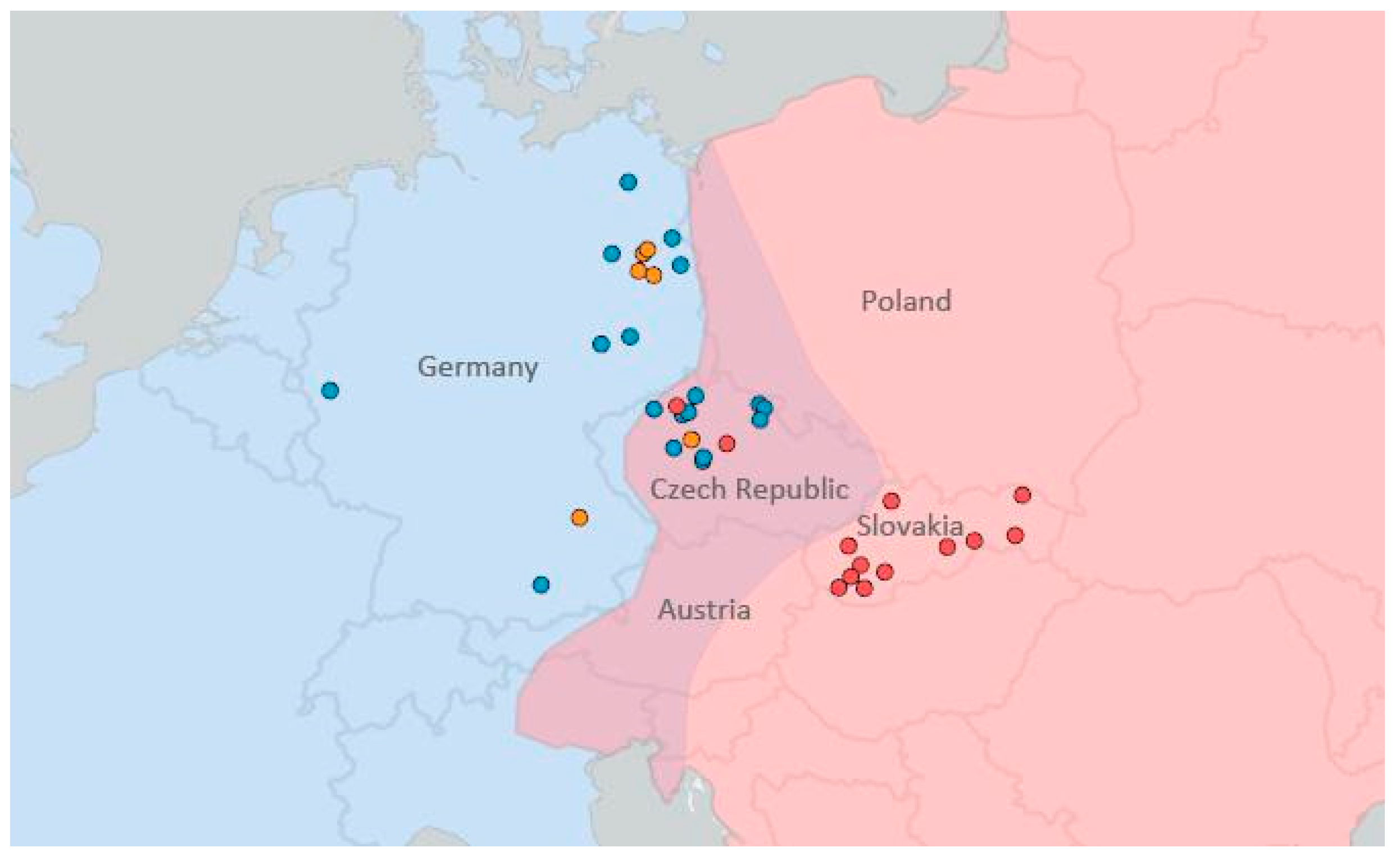
2.1. Specimens
2.2. Genetic Analyses
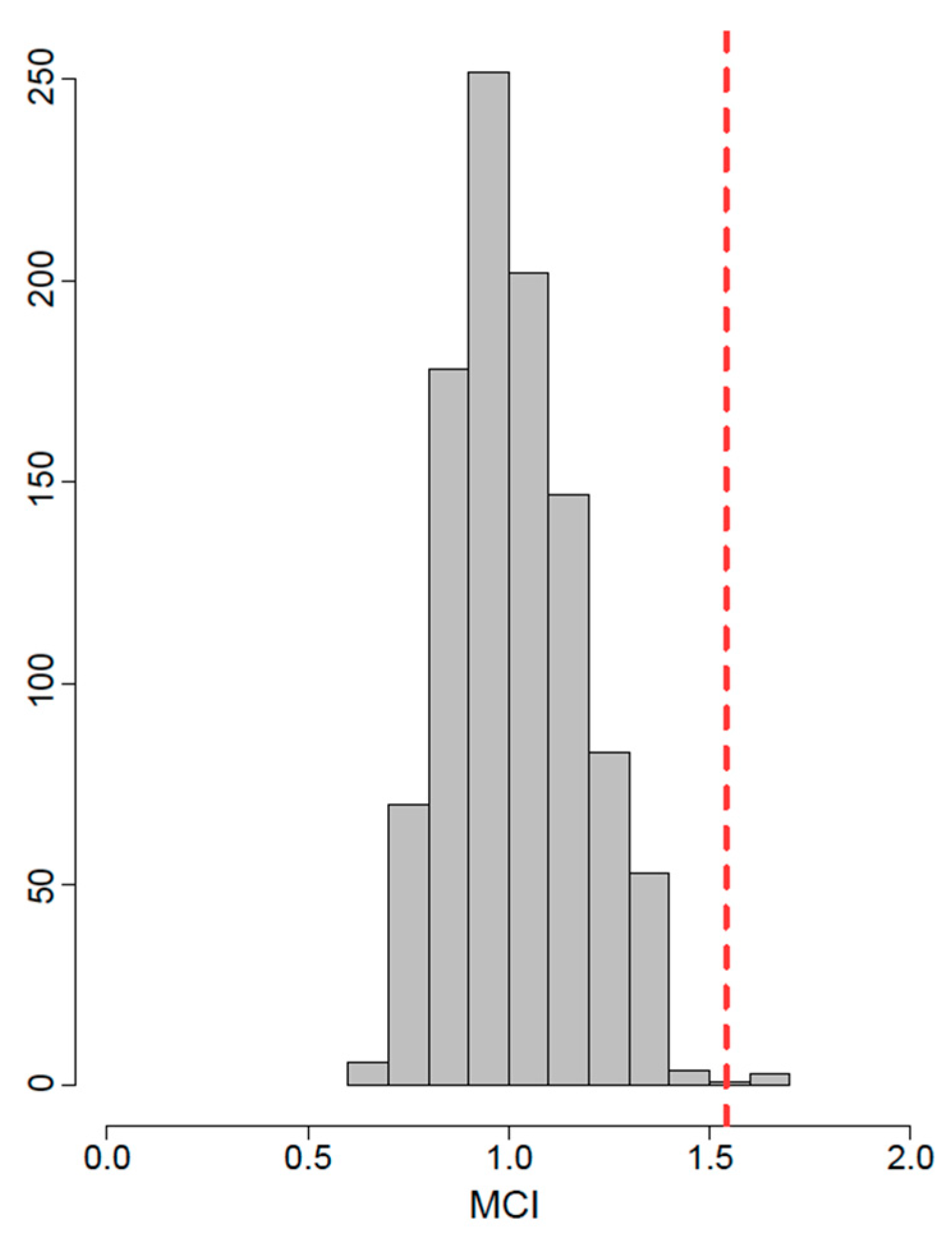
2.3. 3D Geometric Morphometrics Approach and Statistical Analyses
3. Results
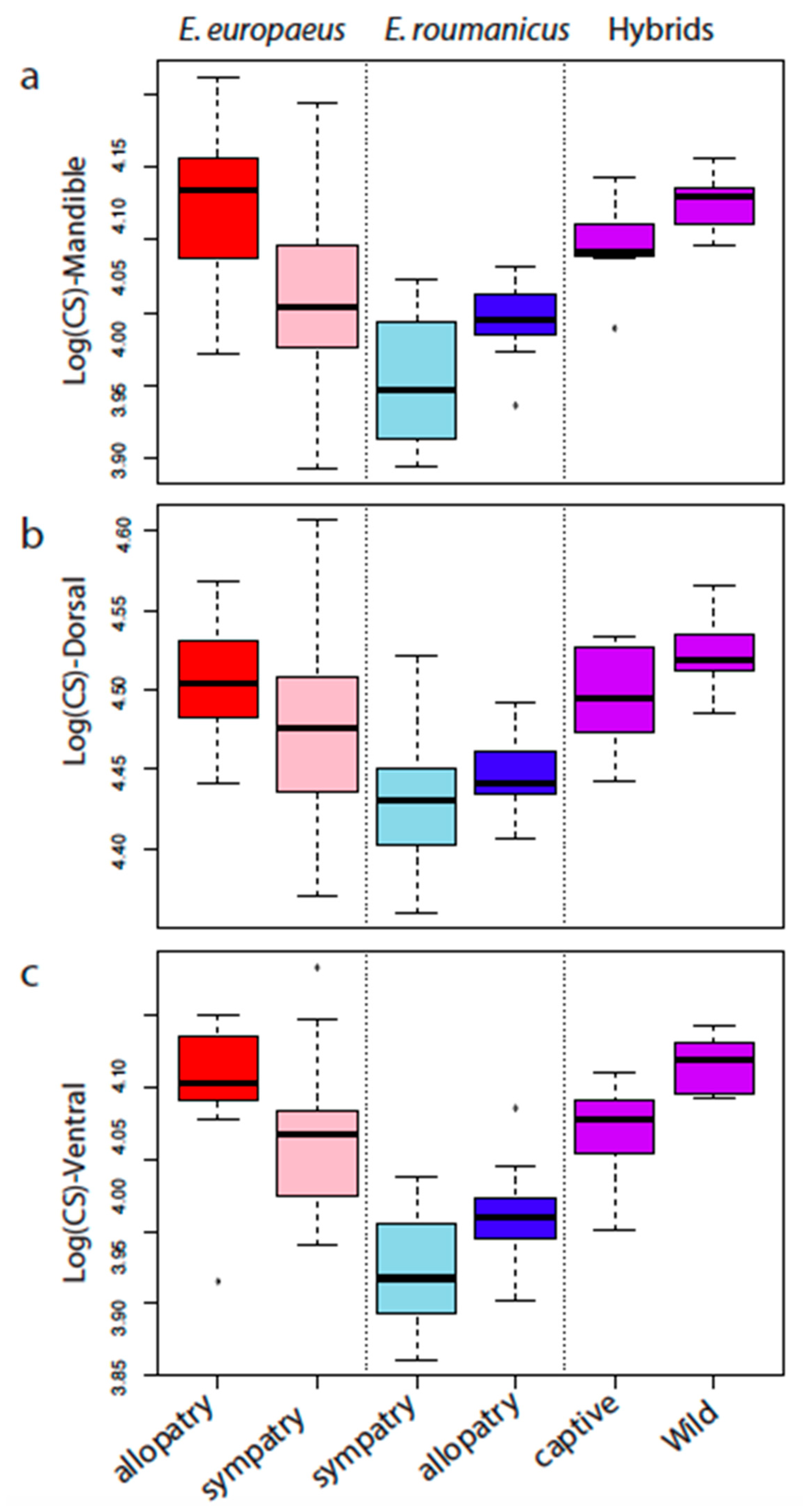
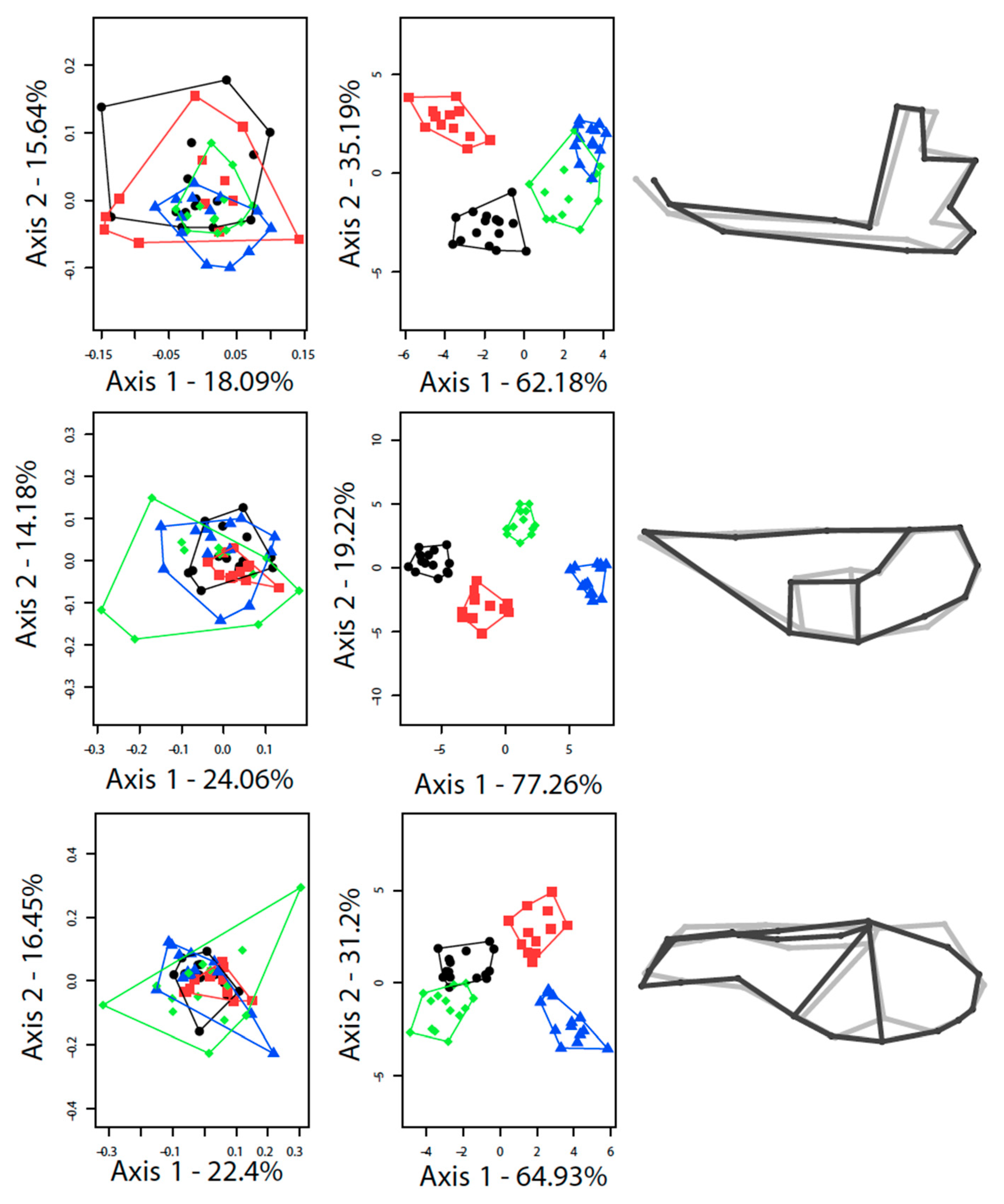
3.1. Differences between the Species
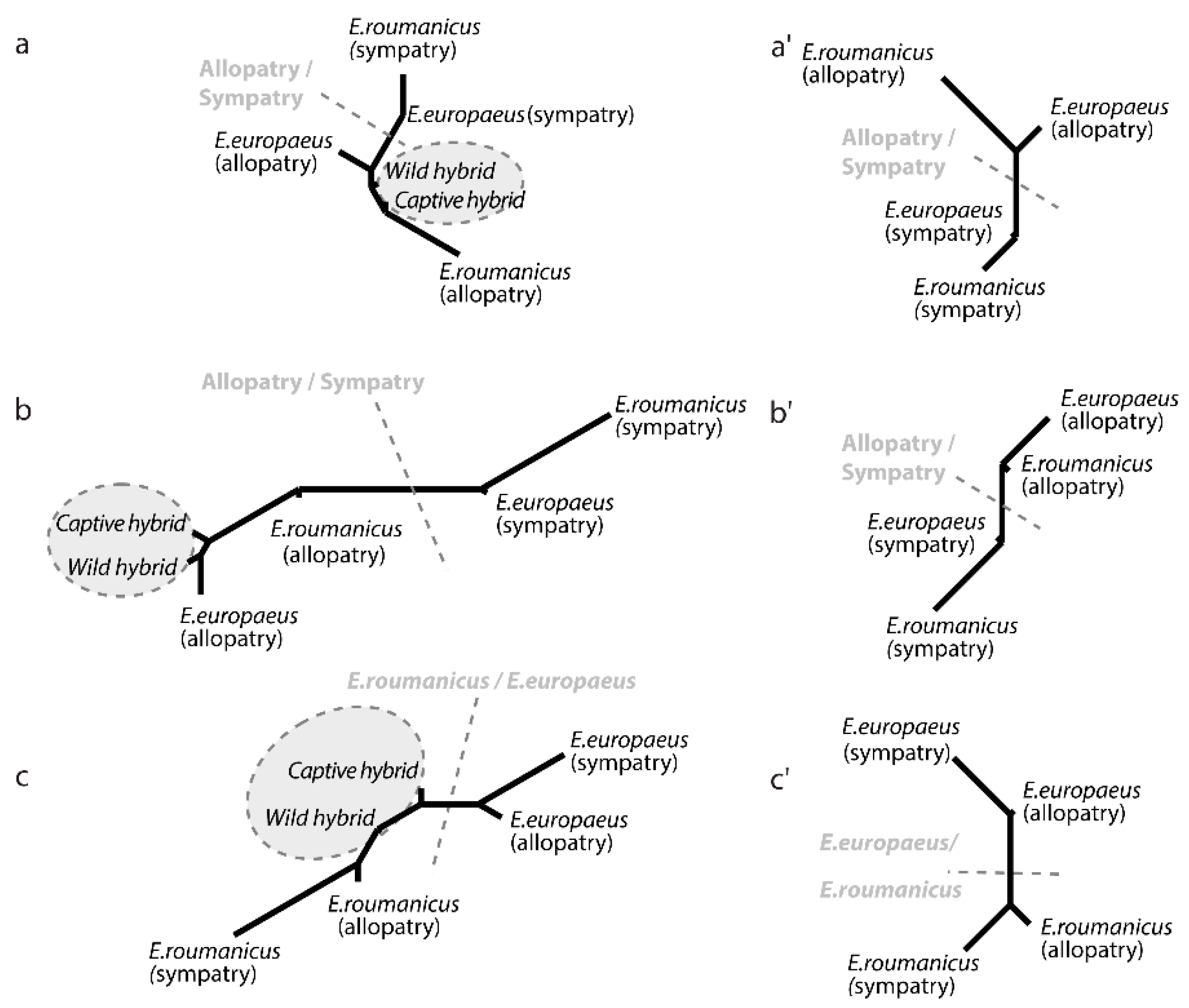
3.2. Contrasted Species Differentiation in Allopatry and Sympatry
3.3. Comparison of Allopatric and Sympatric Populations
3.4. Differences between the Two Hybrid Populations
3.5. Geographical Structure
4. Discussion
4.1. Convergent Character Displacement in Size and Shape
4.2. Hybridisation and Introgression
4.3. Clinal Variation
4.4. Ecological Species Interactions—Competitive and Predator Pressure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hewitt, G.M. Post-Glacial Re-Colonization of European Biota. Biol. J. Linn. Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Seddon, J.M.; Santucci, F.; Reeve, N.J.; Hewitt, G.M. DNA Footprints of European Hedgehogs, Erinaceus europaeus and E. concolor: Pleistocene Refugia, Postglacial Expansion and Colonization Routes. Mol. Ecol. 2001, 10, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Bolfíková, B.; Hulva, P. Microevolution of Sympatry: Landscape Genetics of Hedgehogs Erinaceus europaeus and E. roumanicus in Central Europe. Heredity 2012, 108, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Černá Bolfíková, B.; Eliášová, K.; Loudová, M.; Kryštufek, B.; Lymberakis, P.; Sándor, A.D.; Hulva, P. Glacial Allopatry vs. Postglacial Parapatry and Peripatry: The Case of Hedgehogs. PeerJ 2017, 5, e3163:1–e3163:21. [Google Scholar] [CrossRef]
- Curto, M.; Winter, S.; Seiter, A.; Schmid, L.; Scheicher, K.; Barthel, L.M.F.; Plass, J.; Meimberg, H. Application of a SSR-GBS Marker System on Investigation of European Hedgehog Species and Their Hybrid Zone Dynamics. Ecol. Evol. 2019, 9, 2814–2832. [Google Scholar] [CrossRef]
- Evin, A.; Horáček, I.; Hulva, P. Phenotypic Diversification and Island Evolution of Pipistrelle Bats (Pipistrellus pipistrellus Group) in the Mediterranean Region Inferred from Geometric Morphometrics and Molecular Phylogenetics. J. Biogeogr. 2011, 38, 2091–2105. [Google Scholar] [CrossRef]
- Coyne, J.; Orr, H. Speciation; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Reifová, R.; Reif, J.; Antczak, M.; Nachman, M.W. Ecological Character Displacement in the Face of Gene Flow: Evidence from Two Species of Nightingales. BMC Evol. Biol. 2011, 11, 138:1–138:11. [Google Scholar] [CrossRef]
- Kryštufek, B.; Tvrtković, N.; Paunović, M.; Özkan, B. Size Variation in the Northern White-Breasted Hedgehog Erinaceus roumanicus: Latitudinal Cline and the Island Rule. Mammalia 2009, 73, 299–306. [Google Scholar] [CrossRef]
- Reeve, N. Hedgehogs; T & AD Poyser: London, UK, 1994. [Google Scholar]
- Ruprecht, A. Correlation Structure of Skull Dimensions in European Hedgehogs. Acta Theriol. 1972, 17, 419–442. [Google Scholar] [CrossRef]
- Holz, H.; Niethammer, J. Erianceus concolor Martin, 1838—Weissbrustigel, Ostigel. In Handbuch der Säugetiere Europas 3/1; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1990; pp. 50–64. [Google Scholar]
- Holz, H.; Niethammer, J. Erinaceus europaeus Linnaeus, 1758—Braunbrustigel, Westigel. In Handbuch der Säugetiere Europas 3/1; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1990; pp. 26–49. [Google Scholar]
- Kryštufek, B. Cranial Variability in the Eastern Hedgehog Erinaceus concolor (Mammalia: Insectivora). J. Zool. 2002, 258, 365–373. [Google Scholar] [CrossRef]
- Hrabě, V. Variation in Cranial Measurement of Erinaceus concolor roumanicus (Insectivora, Mammalia). Zool. List. 1976, 25, 315–326. [Google Scholar]
- Niethammer, J.; Krapp, F. Handbuch Der Säugetiere Europas. Bd. 3/1, Insektenfresser-Insectivora, Herrentiere-Primates; Niethammer, J., Krapp, F., Eds.; Aula-Verlag: Wiesbaden, Germany, 1990. [Google Scholar]
- Hrabě, V. Variation in Somatic Characters of Two Species of Erinaceus (Insectivora, Mammalia) in Relation to Individual Age. Zool. List. 1975, 24, 335–351. [Google Scholar]
- Wolff, P. Unterscheidungsmerkmale am Unterkiefer von Erinaceus europaeus L. und Erinaceus concolor Martin. Ann. Naturhist. Mus. Wien 1976, 80, 337–341. [Google Scholar] [CrossRef]
- Kratochvíl, J. Die Hirnmasse Der Mitteleuropäischen Arten Der Gattung Erinaceus (Insectivora, Mamm.). Folia Zool. 1980, 29, 1–20. [Google Scholar]
- Hrabě, V. Notes on the Dentition of Two Erinaceus Spp. from Czechoslovakia (Insectivora, Mammalia). Folia Zool. 1981, 30, 311–316. [Google Scholar]
- Hanken, J.; Hall, B.K. The Skull, Volume 3: Functional and Evolutionary Mechanisms; University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Škoudlín, J. Zur Altersbestimmung Bei Erinaceus europaeus Und Erinaceus concolor (Insectivora: Erinaceidae). Věst. Českosl. Spol. Zool. 1976, 40, 300–306. [Google Scholar]
- Herter, K. Studien Zur Verbreitung Der Europäischen Igel. Arch. Naturgesch. 1934, 3, 21–382. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Goodall, C.R. Procrustes Methods in the Statistical Analysis of Shape Revisited. In Current Issues in Statistical Shape Analysis; Mardia, K.V., Gill, C.A., Eds.; Leeds University Press: Leeds, UK, 1995; pp. 18–33. [Google Scholar]
- Bookstein, F. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Evin, A.; Cucchi, T.; Cardini, A.; Strand Vidarsdottir, U.; Larson, G.; Dobney, K. The Long and Winding Road: Identifying Pig Domestication through Molar Size and Shape. J. Archaeol. Sci. 2013, 40, 735–743. [Google Scholar] [CrossRef]
- Monteiro, L.R. Multivariate Regression Models and Geometric Morphometrics: The Search for Causal Factors in the Analysis of Shape. Syst. Biol. 1999, 48, 192–199. [Google Scholar] [CrossRef]
- Schlager, S. Morpho and Rvcg—Shape Analysis in R. In Statistical Shape and Deformation Analysis: Methods, Implementation and Applications; Zheng, G., Li, S., Szekely, G., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 217–256. [Google Scholar]
- Baylac, M.; Frieß, M. Fourier Descriptors, Procrustes Superimposition, and Data Dimensionality: An Example of Cranial Shape Analysis in Modern Human Populations. In Modern Morphometrics in Physical Anthropology; Slice, D.E., Ed.; Springer: Boston, MA, USA, 2005; pp. 145–162. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mahalanobis, P. On the Generalized Distance in Statistics. Proc. Natl. Acad. Sci. India 1936, 2, 49–55. [Google Scholar]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Co.: New York, NY, USA, 1995. [Google Scholar]
- Stayton, C.T. The Definition, Recognition, and Interpretation of Convergent Evolution, and Two New Measures for Quantifying and Assessing the Significance of Convergence. Evolution 2015, 69, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Stayton, C.T. Testing Hypotheses of Convergence with Multivariate Data: Morphological and Functional Convergence among Herbivorous Lizards. Evolution 2006, 60, 824–841. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Adams, D.; Collyer, M.; Kaliontzopoulou, A.; Sherratt, E. Geomorph: Software for Geometric Morphometric Analyses. R Package Version 3.1.0. 2019. Available online: https://github.com/geomorphR/geomorph (accessed on 20 December 2019).
- Dray, S.; Dufour, A.-B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.r-project.org (accessed on 29 February 2020).
- Poduschka, W.; Poduschka, C. Kreuzungsversuche an Mitteleuropäischen Igeln. Säugetierk. Mitt. 1983, 31, 1–12. [Google Scholar]
- O’Regan, H.J.; Kitchener, A.C. The Effects of Captivity on the Morphology of Captive, Domesticated and Feral Mammals. Mamm. Rev. 2005, 35, 215–230. [Google Scholar] [CrossRef]
- Evin, A.; Dobney, K.; Schafberg, R.; Owen, J.; Strand Vidarsdottir, U.; Larson, G.; Cucchi, T. Phenotype and Animal Domestication: A Study of Dental Variation between Domestic, Wild, Captive, Hybrid and Insular Sus scrofa. BMC Evol. Biol. 2015, 15, 6. [Google Scholar] [CrossRef]
- Meiri, S.; Simberloff, D.; Dayan, T. Community-Wide Character Displacement in the Presence of Clines: A Test of Holarctic Weasel Guilds. J. Anim. Ecol. 2011, 80, 824–834. [Google Scholar] [CrossRef]
- Škoudlín, J. Craniometric Analysis of a Czechoslovak and a Polish Population of Erinaceus concolor (Mammalia: Erinaceidae). Věst. Českosl. Spol. Zool. 1982, 46, 304–316. [Google Scholar]
- Smith, K.K. The Form of the Feeding Apparatus in Terrestrial Vertebrates: Studies of Adaptation and Constraint. In The Skull; Hanken, J., Hall, B.K., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 150–196. [Google Scholar]
- Zelditch, M.L.; Ye, J.; Mitchell, J.S.; Swiderski, D.L. Rare Ecomorphological Convergence on a Complex Adaptive Landscape: Body Size and Diet Mediate Evolution of Jaw Shape in Squirrels (Sciuridae). Evolution 2017, 71, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P.; Kinnison, M.T. Perspective: The Pace of Modern Life: Measuring Rates of Contemporary Microevolution. Evolution 1999, 53, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.P.; Hendry, A.P.; Reznick, D.N.; Fox, C.W. Evolution on Ecological Time-Scales. Funct. Ecol. 2007, 21, 387–393. [Google Scholar] [CrossRef]
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Van Damme, R.; Irschick, D.J. Rapid Large-Scale Evolutionary Divergence in Morphology and Performance Associated with Exploitation of a Different Dietary Resource. Proc. Natl. Acad. Sci. USA 2008, 105, 4792–4795. [Google Scholar] [CrossRef]
- Lovegrove, B.G. The Evolution of Body Armor in Mammals: Plantigrade Constraints of Large Body Size. Evolution 2001, 55, 1464–1473. [Google Scholar] [CrossRef]
- Morris, P.J.R.; Cobb, S.N.F.; Cox, P.G. Convergent Evolution in the Euarchontoglires. Biol. Lett. 2018, 14, 20180366:1–20180366:4. [Google Scholar] [CrossRef]
- Cheverud, J.M. Phenotypic, Genetic, and Environmental Morphological Integration in the Cranium. Evolution 1982, 36, 499–516. [Google Scholar] [CrossRef]
- Goswami, A. Cranial Modularity Shifts during Mammalian Evolution. Am. Nat. 2006, 168, 270–280. [Google Scholar] [CrossRef]
- Alroy, J. Cope’s Rule and the Dynamics of Body Mass Evolution in North American Fossil Mammals. Science 1998, 280, 731–734. [Google Scholar] [CrossRef]
- Stankowich, T.; Campbell, L.A. Living in the Danger Zone: Exposure to Predators and the Evolution of Spines and Body Armor in Mammals. Evolution 2016, 70, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Baker, P.J.; Thomas, E.; Wilson, G.; Judge, J.; Yarnell, R.W. Reduced Occupancy of Hedgehogs (Erinaceus europaeus) in Rural England and Wales: The Influence of Habitat and an Asymmetric Intra-Guild Predator. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.R.; Bright, P.W. Quantifying the Long-Term Decline of the West European Hedgehog in England by Subsampling Citizen-Science Datasets. Eur. J. Wildl. Res. 2016, 62, 407–413. [Google Scholar] [CrossRef]
- Hulva, P.; Černá Bolfíková, B.; Woznicová, V.; Jindřichová, M.; Benešová, M.; Mysłajek, R.W.; Nowak, S.; Szewczyk, M.; Niedźwiecka, N.; Figura, M.; et al. Wolves at the Crossroad: Fission–Fusion Range Biogeography in the Western Carpathians and Central Europe. Divers. Distrib. 2018, 24, 179–192. [Google Scholar] [CrossRef]
- Ploi, K.; Curto, M.; Bolfíková, B.Č.; Loudová, M.; Hulva, P.; Seiter, A.; Fuhrmann, M.; Winter, S.; Meimberg, H. Evaluating the Impact of Wildlife Shelter Management on the Genetic Diversity of Erinaceus europaeus and E. roumanicus in Their Contact Zone. Animals 2020, 10, 1452. [Google Scholar] [CrossRef]

| Trait | Shape | Size | |||||
|---|---|---|---|---|---|---|---|
| F(numDf, denDf) | p | CVP | W | p | CVP | ||
| Mandible | Overall | F(22,30) = 5.94 | 5 × 10−6 | 83.3% (80–88.1%) | 16.648 | 4 × 10−5 | 72.5% (70–76%) |
| Sympatry | F(15,10) = 2.16 | 0.11 | - | 134 | 0.01 | 69.2% (69.2–69.2%) | |
| Allopatry | F(13,13) = 11.4 | 4 × 10−5 | 89.8% (79–95.8%) | 164 | 1 × 10−4 | 85.7% (79–87.7%) | |
| Ventral | Overall | F(23,27) = 8.68 | 2 × 10−7 | 88.7% (84–92.1%) | 25.12 | 5 × 10−7 | 85.3% (84–88%) |
| Sympatry | F(19,5) = 4.47 | 0.05 | - | 133 | 0.04 | 66.6% (61.5–69.2%) | |
| Allopatry | F(10,15) = 7.32 | 3 × 10−4 | 92.3% (91.7–95.8%) | 155 | 7 × 10−5 | 92.2% (91.7–95.8%) | |
| Dorsal | Overall | F(29,24) = 12.41 | 1 × 10−8 | 95.0% (92–98%) | 15.713 | 7 × 10−5 | 78.2% (72–82%) |
| Sympatry | F(19,7) = 17 | 4 × 10−4 | 90.8% (84.6–100%) | 145 | 7 × 10−5 | 79.5% (79.2–83.3%) | |
| Allopatry | F(15,11) = 6.6 | 1 × 10−3 | 84.25% (83.3–87.5%) | 167 | 4 × 10−5 | 84.7% (83.3–87.5%) | |
| Trait | Mandible | Ventral | Dorsal | |||
|---|---|---|---|---|---|---|
| Tests | CVP | Tests | CVP | Tests | CVP | |
| Shape | F(2,12) = 0.6, p = 0.56 | - | F(2, 12) = 2.8, p = 0.09 | - | F(10,4) = 4.08, p = 0.09 | - |
| Size | W = 46, p = 0.04 | 73.9% (64.3–85.7%) | W = 52, p = 0.004 | 79.4% (71.4–92.9%) | W = 40, p = 0.19 | - |
| Trait | E. europaeus | E. roumanicus | ||||||
|---|---|---|---|---|---|---|---|---|
| obs | p-Value | adj. R2 | p-value | obs | p-Value | adj. R2 | p-Value | |
| Mandible | 0.283 | 0.036 | 0.167 | 0.039 | 0.221 | 0.005 | 0.274 | 0.011 |
| Ventral | −0.09 | 0.775 | 0.04 | 0.232 | −0.066 | 0.762 | 0.18 | 0.042 |
| Dorsal | −0.070 | 0.626 | −0.01 | 0.44 | −0.014 | 0.53 | −0.02 | 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černá Bolfíková, B.; Evin, A.; Rozkošná Knitlová, M.; Loudová, M.; Sztencel-Jabłonka, A.; Bogdanowicz, W.; Hulva, P. 3D Geometric Morphometrics Reveals Convergent Character Displacement in the Central European Contact Zone between Two Species of Hedgehogs (Genus Erinaceus). Animals 2020, 10, 1803. https://doi.org/10.3390/ani10101803
Černá Bolfíková B, Evin A, Rozkošná Knitlová M, Loudová M, Sztencel-Jabłonka A, Bogdanowicz W, Hulva P. 3D Geometric Morphometrics Reveals Convergent Character Displacement in the Central European Contact Zone between Two Species of Hedgehogs (Genus Erinaceus). Animals. 2020; 10(10):1803. https://doi.org/10.3390/ani10101803
Chicago/Turabian StyleČerná Bolfíková, Barbora, Allowen Evin, Markéta Rozkošná Knitlová, Miroslava Loudová, Anna Sztencel-Jabłonka, Wiesław Bogdanowicz, and Pavel Hulva. 2020. "3D Geometric Morphometrics Reveals Convergent Character Displacement in the Central European Contact Zone between Two Species of Hedgehogs (Genus Erinaceus)" Animals 10, no. 10: 1803. https://doi.org/10.3390/ani10101803
APA StyleČerná Bolfíková, B., Evin, A., Rozkošná Knitlová, M., Loudová, M., Sztencel-Jabłonka, A., Bogdanowicz, W., & Hulva, P. (2020). 3D Geometric Morphometrics Reveals Convergent Character Displacement in the Central European Contact Zone between Two Species of Hedgehogs (Genus Erinaceus). Animals, 10(10), 1803. https://doi.org/10.3390/ani10101803





