1. Introduction
In the EU, more than 80 million male piglets are castrated every year within the first week of life, mostly without pain relief [
1]. Castration is performed to prevent boar taint, to minimize aggressive and sexual behavior associated with intact males and to gain a constant quality of meat [
2,
3,
4]. The majority of the EU member states are still carrying out surgical castration with or without anesthesia. The exceptions are Ireland, the United Kingdom, the Netherlands, Portugal and Spain, where 20% or less of the male pigs are castrated [
5]. According to EU Directive 2008/120/EC, better practices are demanded [
6]. In Germany, the Animal Welfare Act prohibits the surgical castration of male piglets without anesthesia, even under the age of eight days, as of 1 January 2021 [
7]. In addition to a complete waiver of surgical castration by implementing the practices of boar fattening or GnRH (gonadotropin releasing hormone) vaccination, an improved practice of male piglet castration requires sufficient anesthesia and analgesia to avoid acute and postoperative pain. Several possibilities to ensure these requirements are discussed: injectable anesthesia, inhalation anesthesia and local anesthesia. The possibility of castration with local anesthetics is currently not permitted in Germany, because the evidence of the efficacy of this method is still controversially discussed. Kluivers-Poodt et al. [
8], Hansson et al. [
9] and Leidig et al. [
10] concluded that the use of local anesthetics reduces pain responses during castration, whereas Perez-Pedraza et al. [
11] did not show any effect. Bonastre et al. [
12] as well as Rauh et al. [
13] indicated that local anesthetics lead to a reduction in pain-related responses, without eliminating the pain. These previous studies differ in terms of used local anesthetics, application methods and obtained pain associated parameters and are therefore difficult to compare. Procaine is the local anesthetic with the lowest anesthetic potency [
14] and the only approved local anesthetic for pigs in Germany at the moment. Lidocaine is currently used for piglet castration in Norway, Sweden and Italy [
5]. The effects of procaine and lidocaine in piglets undergoing castration have been investigated in various studies [
8,
9,
10,
11,
12,
13,
15] but showed contradictory results. Furthermore, Bonastre et al. [
12] investigated the effect of the combination of lidocaine and bupivacaine. Bupivacaine is described with the highest anesthetic potency [
14]. The local anesthetic mepivacaine is commonly used in horses. To the authors’ knowledge, there has been no investigation on mepivacaine in conscious piglets undergoing castration so far.
To assess pain in piglets undergoing castration, different methods are available. As physiological parametersi.e., blood parameters, such as cortisol [
12,
16,
17,
18,
19,
20] adrenalin and adrenocorticotropic hormones [
16,
18,
19] and changes in heart rate and blood pressure [
21] can be assessed. Furthermore, behavioral variables, such as defensive movements [
10,
21,
22] and vocalization [
8,
20,
23,
24,
25,
26,
27] can be used for pain assessment. Additionally, Bilsborrow et al. [
28] described an objective test to assess pain in piglets where the navigation time through a handling chute is determined.
In a first already published study part, the four local anesthetics, procaine, lidocaine, bupivacaine and mepivacaine, were evaluated based on pain-related physiological parameters and limb movements under a minimal anesthesia model using low doses of isoflurane [
21]. Under light anesthesia, all four local anesthetics were highly effective at reducing signs of nociception during castration.
The aim of the present study was to investigate the effect of four local anesthetics (procaine, lidocaine, bupivacaine, mepivacaine) on pain relief during surgical castration under standardized conditions in conscious piglets as a second part of a comprehensive study. To evaluate pain, defensive movements and vocalization were assessed. To detect side effects locomotor activity, postoperative bleeding, wound healing, weight gain and mortality were evaluated.
2. Materials and Methods
Data of four groups of piglets castrated under local anesthesia were collected and compared with piglets castrated without local anesthesia and with only handled piglets. The study was performed in accordance with the EU Directive 2010/63/EU on the protection of animals used for scientific purposes and the German Animal Welfare Act (2019). The research protocol was approved by the Ethical Committee for Animal Experiments of the Government of Upper Bavaria, Munich, Germany (reference number ROB-55.2-2532.Vet_02-19-11).
2.1. Animals and Housing
The present randomized, double-blinded study was conducted in an experimental farrow-to-finish farm in Bavaria, Germany, with 80 sows farrowing in a three-week batch. The piglets (Piétrain × Large White/Landrace) were housed in farrowing units on partially slatted floor, except for the nest area, which was made up of concrete floor and provided with shavings and an infrared heat lamp. All piglets in the study received 1 mL of an intramuscular iron supplementation (Ursoferran 200 mg/mL solution for injection for pigs, Serumwerk Bernburg AG, Bernburg, Germany) the day before castration. Besides, no other painful procedures, such as tail docking or ear tagging, were carried out before the start of the study.
Data were collected from 71 male piglets between three to seven days of life (5.2 ± 1.09 days, 2.2 ± 0.48 kg (mean ± standard deviation)) of 16 litters. Inclusion criteria were a good general condition, a healthy sow and a minimum weight of 1.4 kg on the day of castration. Piglets with any deviation from the normal anatomical condition (e.g., hernia scrotalis or inguinalis) were excluded.
2.2. Experimental Design
On the day before castration, each piglet was weighed (MS weighing plateau max. 100 kg, Schippers GmbH, Kerken, Germany), the general conditions of the piglets were checked and a computer-generated simple randomization was used to distribute the animals to one of the six experimental groups (
Table 1). Additionally, the handling chute, according to Bilsborrow et al. [
28] was trained four times with each piglet (two times without and two times with hurdles) (
Figure 1).
On the morning of the castration day, piglets passed through the handling chute three consecutive times to determine the baseline. During injection and castration, all piglets, including the handling group, were fixed in a castration cradle (Schippers GmbH, Kerken, Germany). The piglets were given at least five minutes to rest before the injection.
All piglets except those of the handling group (H, only fixation) were injected with a total volume of 2 mL with the respective local anesthetic (procaine hydrochloride (P), lidocaine hydrochloride (L), bupivacaine hydrochloride (B) and mepivacaine hydrochloride (M)) or 0.9% sodium chloride (NaCl) according to the study group. Then, 0.5 mL were injected intratesticularly and 0.5 mL subscrotally for each testicle. For the injection an automatic self–filling system 1 mL syringe (HSW ECO–MATIC®, Henke-Sass, Wolf GmbH, Tuttlingen, Germany) with a 25 G sized cannula (0.5 × 16 mm, B. Braun TravaCare GmbH, Hallbergmoos, Germany) was used. The intratesticular injection was performed by fixing the testicle between the thumb and index finger caudally. Directly following the intratesticular injection the subscrotal single point injection was performed by releasing the fixed testicle, retracting the cannula from it but leaving the cannula under the skin and making a skin fold of the scrotum. The intratesticular and subscrotal injection was repeated for the second testicle. Thereafter, the piglets were returned into the farrowing pen for a period of 20 min.
Afterwards, all piglets were removed again from the farrowing pen and, with the exception of group H (only fixation and simulated interventions), castrated. Therefore, the scrotal area was cleaned with an antiseptic solution (octenisept® aqueous wound and mucous membrane antiseptic, colorless, Schuelke & Mayr GmbH, Norderstedt, Germany). Using a sterile scalpel (scalpel blades carbon steel, sterile 21, Heinz Herenz Medizinalbedarf GbmH, Hamburg, Germany; scalpel handle no. 4, AESCULAP AG & CO. KG, Tuttlingen, Germany) for every piglet, scrotal tissue and tunica vaginalis were opened by a skin incision parallel to the raphe scroti and the first testicle was removed by severing of the spermatic cord with the scalpel. Skin incision and severing of the spermatic cord were repeated for the second testicle. The injection and castration were performed by three trained veterinarians. After surgery, no disinfectant was applied on the scrotal area to avoid behavioral changes by irritating substances. Group NaCl served as positive control for pain-related behavior induced by injection and castration without pain relief. As negative control, piglets from group H underwent simulated injection and castration to measure stress related behavior. In order to avoid an analgesic effect on the pain-related behavior during and after injection and castration, meloxicam (0.4 mg/kg) (Metacam® 5 mg/mL, solution for injection for cattle and pigs, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany) was not administered until one day after castration.
2.3. Data Sampling
For data sampling, evaluation of recordings and also data analysis for all persons involved were blinded at any time with the exception for group H (handling).
2.3.1. Defensive Behavior
To evaluate defensive movements, piglets were filmed during injection and castration procedure (Samsung Galaxy S6-Samsung Electronics GmbH, Schwalbach, Germany). Analyzing of the defensive movements according to a score modified by Leidig et al. [
10] (
Table S1) was always performed by the same blinded person. “Injection”, “skin incision” and “severing of the spermatic cord” were evaluated separately according to intensity (0–4) and duration (0–3). The intensity was scaled into 0 = no movements, 1 = moving of one limb, 2 = moving of two limbs, 3 = moving of three limbs, 4 = moving of all four limbs. The duration was scaled into 0 = no movements, 1 = one single movement, 2 = repeated movements (2–4), 3 = continuous movements (>4). Defensive movements were evaluated for the right and left testicle separately. The single scores resulted in a maximum of seven for each testicle and step. The scores of both testicles and each step were summed up for evaluation. This results in a combined total score for “injection”, “skin incision” and “severing of the spermatic cord” from 0 to 14. On the basis of the audio of these recordings, a blinded person evaluated vocalization with “Yes” or “No”. Vocalization was assessed with “Yes”, if there was an acute onset of increased vocalization during the injection and/or castration procedure. Moreover, two additional blinded persons evaluated the recordings of defensive movements independently to ensure the reliability of the observer.
2.3.2. Handling Chute Behavior
To evaluate the navigation time according to Bilsborrow et al. [
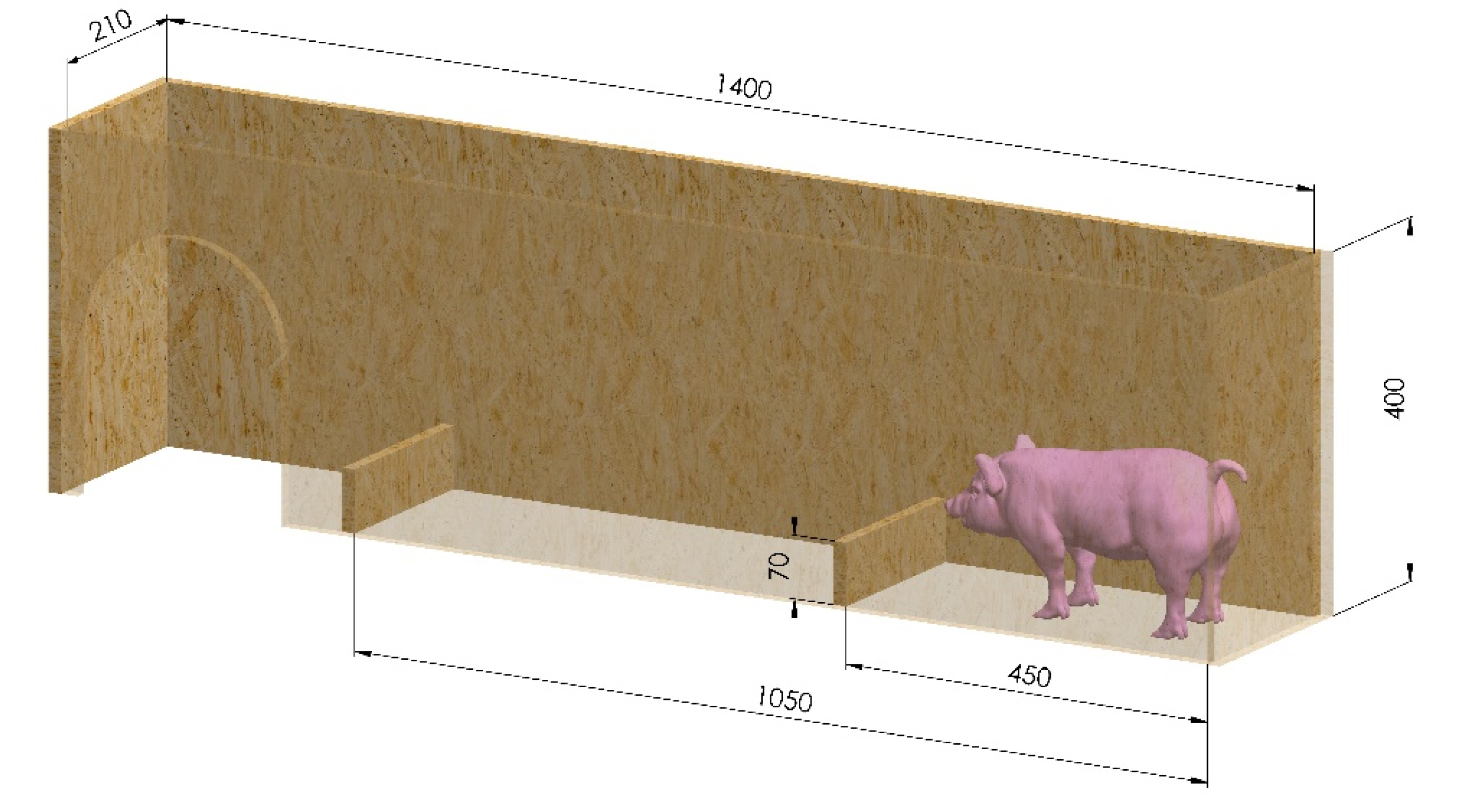
28], piglets passed through the handling chute after injection and castration, two hours after castration and one day after castration. The handling chute had a length of 1400 mm, width of 210 mm, height of 400 mm, two 70 mm hurdles (distance between the hurdles: 600 mm) and an open top to record the duration and quality of the run (Panasonic LUMIX DMC-FZ8, Matsushita Electric Industrial Co., Ltd., Osaka, Japan) (
Figure 1). Additionally, a structured thermal mat was used as a base for the handling chute to avoid slipping. The navigation time was determined, based on the recordings, starting when the piglets began running and ending when all four limbs were over the last hurdle. Particular attention was paid to impairments of the locomotor activity (unsteady gait, stumble upon the hurdles, sitting/lying in the handling chute).
2.3.3. Postoperative Assessment
Piglets were weighed and wound healing was scored according to Zankl [
29] (
Table S2) on days 1, 7, 14 and 21 after castration to assess the postoperative period up to day 21. Castration wounds were ranked on the basis of wound healing (0 = without specific findings (dry, fully closed wound), 1 = minor findings (swelling, redness), 2 = open wound), wound secretion (0 = no secretion, 1 = serous/bloody secretion, 2 = purulent secretion) and texture and size of the spermatic cord (0 = hardly palpable, 1 = up to 1 cm, soft to rough and elastic, 2 = stronger than 1cm, soft to rough and elastic, 3 = stronger than 1 cm, rough or fluctuating). The score was summed up for evaluation with a maximum of seven. Postoperative bleeding was quantified two hours after castration using a score adapted by Enz et al. [
30] (
Table S3). The score ranged from 0 (no bleeding) to a maximum of 3 (severe bleeding, perineal area and hind limbs bloodstained). In addition, mortality was recorded until day 21 post castration.
2.4. Statistical Analysis
For the statistical analysis, all experimental groups were tested for age and weight on day of castration, defensive movements, vocalization, navigation time, wound healing, postoperative bleeding, and average daily weight gain. The distribution of all continuous parameters was tested using Shapiro–Wilk normality test. For non-normally distributed parameters defensive movements, wound healing and postoperative bleeding a Kruskal–Wallis test was performed. Dunn test for multiple comparisons between the groups P, L, B, M, H and NaCl with Benjamin–Hochberg corrections followed the Kruskal–Wallis test. For normally distributed parameters (average daily weight gain) ANOVA was used with Student’s t-test for pairwise comparisons. Nonparametric bootstrap was used for obtaining confidence limits for the population mean without assuming normality. For mostly normal distributed parameters age and weight on day of castration a Mann–Whitney-U test was performed. Vocalization was analyzed using pairwise Fisher’s tests with Benjamin–Hochberg corrections. Navigation time was analyzed using mixed-effects linear model due to the presence of repeated measures, with time, group and interaction between them as fixed effects and a random effect of individual piglet. Due to the non-normality and heteroscedasticity of the residuals, the response variable was log-transformed.
Statistical significance was considered at
p < 0.05. These statistical analyses were performed using R statistical software version 3.6.1, mainly with “ggstatsplot” [
31], “lme4” [
32] and “emmeans” [
33] packages and IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). For the score used to evaluate the defensive movements, the Intraclass Correlation Coefficients (ICC) and their 95% confident intervals were calculated using IBM SPSS Statistics for Windows, Version 26.0, based on mean rating, absolute agreement and 2-way mixed-effects model. The ICC values ranged between 0 and 1, with values closer to 1 representing stronger reliability.
4. Discussion
Several studies have already been conducted on local anesthesia as an alternative to piglet castration without anesthesia but they differed in study designs, local anesthetics used and yielded heterogeneous results [
8,
9,
10,
11,
12,
13]. This second part of a comprehensive study examined the four local anesthetics, procaine, lidocaine, bupivacaine and mepivacaine, in conscious piglets during castration in the first week of life. Part one of the comprehensive study showed that the intratesticular injection with a subcutaneous depot of a local anesthetic prior to castration reduces nociception-related cardiovascular responses and limb movements in piglets under anesthesia [
21].
Similar to Leidig et al. [
10] and Rauh et al. [
13], we used defensive movements as a suitable parameter to evaluate pain responses in conscious piglets. In the present study, the ICC values for the score used to evaluate defensive movements were more than 0.9 for “injection”, “skin incision” and “severing of the spermatic cord”, which indicates an excellent Interrater Reliability [
34].
The highest mean score of defensive movements occurred in group NaCl (castration without anesthesia) during severing of the spermatic cord. This is in line with the findings of Rauh et al. [
13] and thus it confirms the suggestion based on the vocalization of Taylor and Weary [
35] that pulling and severing of the spermatic cords are the most painful components of castration. Almost the same score was observed in group NaCl during skin incision, which is consistent with several studies that the whole castration procedure without anesthesia causes severe pain [
8,
10,
35,
36]. Prior studies showed, based on defensive movements, that the application of lidocaine intratesticularly and subscrotally reduces pain responses during the castration procedure [
9,
13]. In the present study, the injection of procaine, lidocaine, bupivacaine or mepivacaine led to a significant reduction in defensive movements compared to castration without anesthesia during the severing of the spermatic cord, whereas during skin incision a significant reduction was observed only after the injection of lidocaine or mepivacaine. An explanation for the different effects of local anesthesia on skin incision and severing of the spermatic cord could be that with the subscrotal and intratesticular applicated depots used in the present study, the local anesthetics did not readily diffuse into the skin. Ranheim et al. [
37] discovered, through autoradiograms, that radiolabeled lidocaine injected into the testis and subcutaneously into the scrotum does not readily diffuse through the tunica vaginalis. However, the assumption that the local anesthetics did not readily diffuse into the skin does not explain the different extent of the reduction in defensive movements between local anesthetics during skin incision. The injection of lidocaine and mepivacaine led to a significant reduction in defensive movements during skin incision, while procaine and bupivacaine application did not. Another possible explanation lies in the physiochemical properties of the used local anesthetics. Local anesthetics are weak bases and thus exist in equilibrium between the neutral, non-ionized, lipid-soluble and the ionized, water-soluble form. The position of equilibrium can be defined using pKa. The pKa of a molecule represents the pH at which 50% exists in the non-ionized (lipid-soluble) and 50% in the ionized form (water-soluble) [
38]. The main access of local anesthetics to the cell is by penetration of the lipophilic neutral form through the lipid membrane [
14,
39]. Therefore, a local anesthetic with a low pKa (mepivacaine: 7.72; lidocaine: 7.77; bupivacaine: 8.1; procaine: 8.89) will have a greater proportion of the non-ionized lipid-soluble form at physiological pH, resulting in better diffusion into the surrounding tissue and a more rapid onset of action. Moreover, the relative anesthetic potency is of importance as well. The main determinant of local anesthetic potency is the lipid solubility [
40,
41]. However, we could not prove that the local anesthetic with the highest lipid solubility and the highest relative anesthetic potency, in this case bupivacaine, produced the greatest reduction in pain related behavior. According to these results, we assume that the spermatic cord was anesthetized more efficiently than the scrotal skin. For future research, optimizing the application method is needed to improve the anesthesia of the scrotal skin.
To evaluate pain caused by the injection, irrespective of the acidity of local anesthetic solutions, sodium chloride (NaCl) was injected into the testicles of the piglets castrated without anesthesia. In the present study, piglets that received an intratesticular and subscrotal injection tended to have higher scores of defensive movements and vocalization than piglets that were handled only (H). The lack of significance between the control groups H and NaCl could result from the stress and fear provoked by the fixation of the piglet in a supine position and the fixation of the testicles, which may have overlapped the defensive behavior caused by the injection. Moreover, piglets injected with bupivacaine showed significantly more defensive movements than piglets injected with lidocaine or piglets that were only fixated. However, this result is contradictory to the assumption that the pain induced by injection of local anesthetics is believed in part to be related to the acidity, since Bupivacain 0.5% with epinephrine 0.0005% JENAPHARM® (mibe GmbH Arzneimittel, Brehna, Germany) and Xylocitin® 2% (mibe GmbH Arzneimittel, Brehna, Germany) with epinephrine 0.001% have the same pH. The results of the present study indicate that the injection of bupivacaine seemed to be painful itself. Additionally, the sole fixation of the testicles might induce defensive movements, as seen in the handling group, but to a lesser extent compared to injecting any substances.
Weary et al. [
23] validated vocal measures as a reliable indicator of acute pain due to castration. Several studies confirmed that the vocalization of piglets castrated without anesthesia differs from piglets that were sham castrated [
24] or castrated with anesthesia [
8,
25,
26]. White et al. [
25] assessed vocalization during the castration procedure of piglets with frequency of highest energy (HEF). Weary et al. [
23], Taylor et al. [
24] and Puppe et al. [
27] used high frequency calls. In contrast, Marx et al. [
26] classified calls during castration into three different call types (grunt, squeal, scream) and demonstrated that piglets castrated without anesthesia produced almost twice as many screams as piglets castrated with local anesthesia. It is necessary to mention that, in their studies, for the recordings of the vocalization separate rooms were used to prevent interfering noises [
23,
26,
27]. Furthermore, collected data were elaborately analyzed with special programs [
23,
24,
25,
26,
27]. In the present study, vocalizations were recorded in the nursery and not in a separate room, so the occurrence of interfering noises could not be prevented. As a practicable method to assess vocalization, despite the interfering noises, an acute onset of increased vocalization, comparable with the call type “scream” was evaluated in the present study, in orientation to the classification of Marx et al. [
26]. Since no computer program was used for the evaluation of the vocalization in the present study, it is difficult to compare the results with prior studies. Nonetheless the results obtained are similar to former studies [
8,
10,
23,
24,
25,
26,
35]. Piglets castrated without anesthesia (NaCl) reacted significantly more often with an acute onset of increased vocalization compared to piglets that were handled (H). In contrast to Marx et al. [
26] and Kluivers-Poodt et al. [
8] no significant difference occurred between the control groups and piglets castrated with local anesthesia during the castration procedure, with the exception of piglets injected with mepivacaine during the severing of the spermatic cord. This could be due to the used measurement of vocalization in the present study and the resulting lower sensitivity.
The navigation time through the handling chute was determined with the assumption that the presence of pain would result in longer navigation times [
13,
28]. According to Bilsborrow et al. [
28], piglets castrated without anesthesia had a significantly longer navigation time through a handling chute 0 and 15 min after castration than sham castrated piglets [
28]. Davis et al. [
42] and Rauh et al. [
13] validated these results. However, no significant difference regarding navigation time was found between control groups castrated without anesthesia and sham castrated in the present study. In contrast, piglets injected with mepivacaine had a significantly shorter navigation time after injection, castration and two hours after castration, possibly because of significant weight differences. Piglets injected with mepivacaine were significantly heavier. The significant differences regarding the weight on day of castration could be caused by the small number of piglets. Since no significant difference was found between both control groups in the present study, the handling chute was not suitable to assess pain objectively following castration in the present study. However, the recordings were not only used to measure navigation time, but also to get evidence of impairments of locomotor activity. The impairments occurred, as shown in
Table 5, as an unsteady gait, kyphotic spine, tripping, sitting or lying in the handling chute or refusing the hurdles. Two piglets injected with bupivacaine showed such impairments after injection. This is in line with the results of defensive movements, where piglets injected with bupivacaine showed the highest score during injection. However, we could not explain these results. Furthermore, after castration, one piglet injected with sodium chloride, one piglet injected with lidocaine and two piglets injected with bupivacaine showed impairments. In addition, one piglet injected with bupivacaine showed an impairment two hours after castration. The impairments of individual piglets of groups NaCl, L and B after castration could be explained by pain itself. Moreover, local anesthetics could cause side effects, such as impairments of locomotor activity. Since every piglet was injected with a standardized dosage of local anesthetic regardless of its weight, an overdose in lighter piglets might be conceivable. Furthermore, it is possible that heavier piglets had a loss of effectiveness, although we could not observe an effect. However, the piglets concerned were not among the lightest. Further studies are necessary to investigate how different dosages, based on individual weights, influence the effectiveness of local anesthesia and the occurrence of side effects. Moreover, an inadvertent intravenous injection of the local anesthetics could result in central nervous system and cardiovascular toxicity. Lipid-soluble local anesthetics—i.e., bupivacaine followed by lidocaine or mepivacaine—are more potent and therefore cause more systemic toxicity than less lipid-soluble agents, such as procaine, which is described with a minimal potential for systemic toxicity [
14]. Two piglets of the same litter (group NaCl and group L) stood out when they were supposed to complete the handling chute two hours after castration. Their general condition was severely disturbed two hours after castration and they were unable to walk or stand. Both piglets died the same day. Prior studies on local anesthesia, which applied lidocaine [
8,
9,
12,
15] or procaine [
15], did not show any effect on mortality due to local anesthesia. Bonastre et al. [
12] also did not show any effect on mortality caused by the local anesthetics, lidocaine and bupivacaine. Prior to the present study, no data were available on the mortality of piglets that were injected with the local anesthetic mepivacaine prior to castration. In the present study, the deceased lidocaine injected piglet was examined histopathologically. The autopsy showed a severe hemorrhage into the abdomen, probably associated with an increased bleeding propensity. Furthermore, we cannot exclude that the severe hemorrhage into the abdomen could have been caused by the surgical castration itself. The histology of the lungs showed a hemorrhagic and necrotizing pneumonia, which is most likely compatible with an infarction. Thus, in addition to the bleeding-induced loss of procoagulatory substances, an iatrogenic introduction of local anesthetic into the bloodstream with thrombus formation and a pronounced cardiotoxicity should also be considered. Since one piglet of group NaCl died as well, and both piglets were from the same litter, it is more likely that a coagulopathy may have caused their death. Unrestricted locomotor activity, especially in suckling piglets, is essential in order to avoid losses for example due to crushing by the sow. Moreover, it can be a sign of side effect of the injected bupivacaine or lidocaine. Further research is needed to investigate if it is possible to reduce the dosage without reducing the effect of the anesthesia.
As described by Hofmann et al. [
15] and Zankl et al. [
43], local anesthetics have no impact on wound healing after castration. In the present study, only one piglet injected with bupivacaine showed a heightened score one day after castration. This piglet defecated during castration, which possibly led to a contamination of the wound and consequently to an increased wound score, demonstrating that the castration should be performed as sterile as possible.
In the present study piglets were castrated only using a scalpel and no severe postoperative bleeding occurred in the scrotal area. The use of local anesthetics did not influence bleeding score two hours after castration, though local anesthetics contain different amounts of epinephrine or none (Mepidor 20 mg/mL). It was noticed that piglets castrated without any pain relief ranked the lowest considering postoperative bleeding. An explanation could be that, due to the pain caused by castration without pain relief, the adrenaline, which was released from adrenal glands, led to a greater vasoconstriction than the epinephrine, which is contained in the drugs. Moreover, neither castration without anesthesia nor castration with local anesthesia had a negative impact on daily growth performance until 21 days after castration. This is in line with the findings of Kluivers-Poodt et al. [
8] and Bonastre et al. [
12], who described that neither anesthetics nor meloxicam have an effect on mortality or growth performance in piglets. In contrast, Telles et al. [
44] demonstrated that the use of local anesthetic prior to castration appears to have positive effects on long-term weight gain of pigs. Whether local anesthesia has an influence on long-term weight gain could not be evaluated in the present study, because the weights of the animals were only recorded until the 21st day after castration.
Limitations of the present study are the different piglet ages and weights on day of castration, use of commercially available local anesthetic products with different concentrations of epinephrine or no epinephrine at all, and the fact that the dosage was based on volume and not on weight. Furthermore, the small sample size should be mentioned as a limitation of the study.