Variation in Milk Composition and Fatty Acid Profile during the Lactation of Araucana Creole Ewes in a Pasture-Based System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Site
2.2. Milk Samples
2.3. Milk Composition Analysis
2.4. Milk FA Analysis
2.5. Statistical Analysis
3. Results
3.1. BCS, Ewe Weight (EW), and Lamb Liveweight
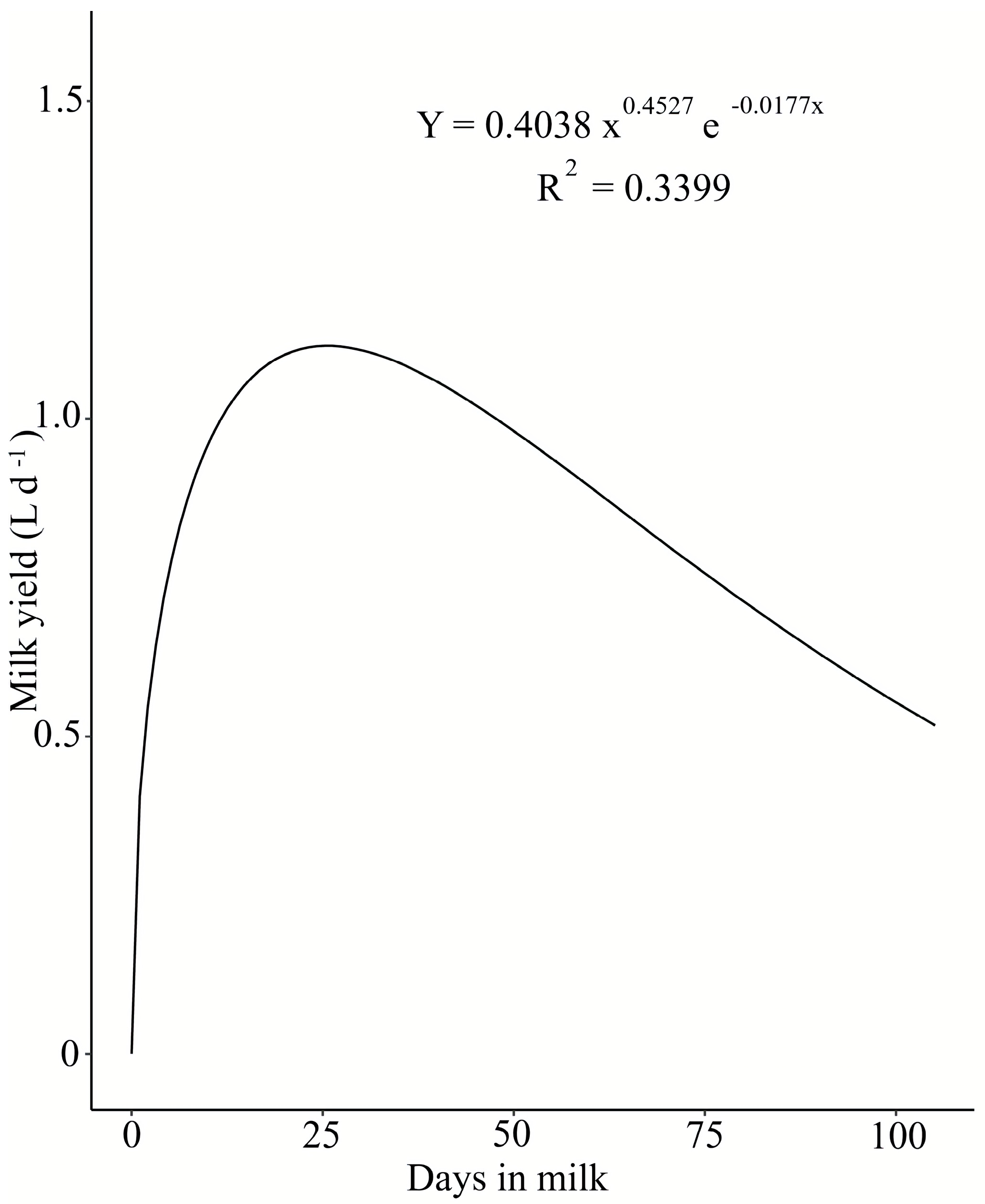
3.2. Milk Yield and Composition
3.3. FA Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ermias, E.; Rege, J. Characteristics of live animal allometric measurements associated with body fat in fattailed sheep. Livest. Prod. Sci. 2003, 81, 271–281. [Google Scholar] [CrossRef]
- Zaitoun, I.; Tabbaa, M.; Bdour, S. Differentiation of native goat breeds of Jordan on the basis of morphostructural characteristics. Small Rumin. Res. 2005, 56, 173–182. [Google Scholar] [CrossRef]
- Sepúlveda, N. Características productivas de los rebaños ovinos de ganaderos indígenas Mapuches en la IX Región de Chile. El Arca 1999, 3, 47–52. [Google Scholar]
- Bravo, S.; Larama, G.; Quiñonez, J.; Paz, E.; Rodero, E.; Sepúlveda, N. Genetic diversity and phylogenetic among Araucana creole sheep and Spanish sheep breeds. Small Rumin. Res. 2019, 172, 23–30. [Google Scholar] [CrossRef]
- Bravo, S.; Larama, G.; Ortíz, M.; Sepúlveda, N. Genetic differentiation between “Araucana” creole and “Hampshire Down” sheeps in Chile. Chil. J. Agric. Res. 2015, 75, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Bravo, S.; Larama, G.; Paz, E.; Inostroza, K.; Montaldo, H.; Sepúlveda, N. Polymorphism of the GDF9 gene associated with litter size in Araucana creole sheep. Anim. Genet. 2016, 47, 390–391. [Google Scholar] [CrossRef]
- Bravo, S.; Sepúlveda, N. Índices zoométricos en ovejas criollas Araucanas. Int. J. Morphol. 2010, 28, 489–495. [Google Scholar] [CrossRef]
- Bravo, S.; Fabres, M.; Schnettler, B.; Sepúlveda, N. Corporal composition and characteristics of carcass of Araucano creole lambs. Int. J. Morphol. 2010, 28, 1107–1111. [Google Scholar] [CrossRef] [Green Version]
- Quiñonez, J.; Bravo, S.; Paz, E.; Sepúlveda, N. Detection of polymorphism in the melatonin receptor gene (MT1) in Araucana sheep. Int. J. Morphol. 2012, 30, 546–549. [Google Scholar] [CrossRef] [Green Version]
- Paz, E.; Quiñonez, J.; Bravo, S.; Montaldo, H.; Sepúlveda, N. Genotyping of BMPR1-B, BMP-15 and GDF-9 genes in Chilean sheep breeds and association with prolificacy. Anim. Genet. 2015, 46, 98–99. [Google Scholar] [CrossRef]
- Ostrovský, I.; Pavlíková, E.; Blasko, J.; Górová, R.; Kubinec, R.; Margetín, M.; Soják, L. Variation in milk fatty acid composition of ewes’ milk during continuous transition from dry winter to natural pasture diet. Int. Dairy J. 2009, 19, 545–549. [Google Scholar] [CrossRef]
- Górová, R.; Pavlíková, E.; Blasko, J.; Mel’uchová, B.; Kubinec, R.; Margetín, M.; Soják, L. Temporal variations in fatty acid composition of individual ewes during first colostrum day. Small Rumin. Res. 2011, 95, 104–112. [Google Scholar] [CrossRef]
- Soják, L.; Blasko, J.; Kubinec, R.; Górová, R.; Addová, G.; Ostrovský, I.; Margetín, M. Variation among individuals, breeds, parities and milk fatty acid profile and milk yield of ewes grazed on pasture. Small Rumin. Res. 2013, 109, 173–181. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep. Practice 1984, 6, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Court, J.; Webb-Ware, J.; Hides, S. Sheep Farming for Meat & Wool; CSIRO Publishing, Department of Primary Industries: Victoria, Australia, 2010. [Google Scholar]
- Canseco, C.; Demanet, R.; Balocchi, O.; Parga, J.; Anwandter, V.; Abarzúa, A.; Teuber, N.; Lopetegui, J. Determinación de la disponibilidad de materia seca de praderas en pastoreo. In Manejo del Pastoreo; Teuber, N., Balocchi, O., Parga, J., Eds.; Fundación para la Innovación Agraria (FIA) Publishing: Santiago, Chile, 2007. [Google Scholar]
- Burja, A.; Armenta, R.; Radianingtyas, H.; Barrow, C. Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J. Agric. Food Chem. 2007, 55, 4795–4801. [Google Scholar] [CrossRef]
- McCane, I. The determination of milk yield in the Merino ewe. Aust. J. Agric. Res. 1959, 10, 839–853. [Google Scholar] [CrossRef]
- Bencini, R.; Hartmann, P.; Lightfoot, J. Comparative dairy potential of Merino and Awassi x Merino ewes. Proc. Aust. Assoc. Anim. Breed Genet. 1992, 10, 114–117. [Google Scholar]
- Doney, J.; Peart, J.; Smith, W.; Louda, F. A consideration of the techniques for estimation of milk yield by suckled sheep and a comparison of estimated obtained by two methods in relation to the effect of breed, level of production and stage of lactation. J. Agric. Sci. 1979, 92, 123–132. [Google Scholar] [CrossRef]
- ISO. Milk and Liquid Milk Products—Guidelines for the Application of Mid-Infrared Spectrometry (ISO: 9622-2013), 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Wood, P.D. Algebraic model of the lactation curve in cattle. Nature 1967, 216, 164–165. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Molik, E.; Murawski, M.; Bonczar, G.; Wierzchos, E. Effect of genotype on yield and chemical composition of sheep milk. Anim. Sci. Pap. Rep. 2008, 26, 211–218. [Google Scholar]
- Fuertes, J.A.; Gonzalo, C.; Carriedo, J.A. Parameters of test day milk yield and milk components for dairy ewes. J. Dairy Sci. 1998, 81, 1300–1307. [Google Scholar] [CrossRef]
- Martínez, M.; Calderón, C.; De la Barra, R.; De la Fuente, F.; Gonzalo, C. Udder morphological traits and milk yield of Chilota and Suffolk Down sheep breeds. Chil. J. Agric. Res. 2011, 71, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Cabiddu, A.; Decandia, M.; Addis, M.; Piredda, G.; Pirisi, A.; Molle, G. Managing Mediterranean pastures in order to enhance the level of beneficial fatty acids in sheep milk. Small Rumin. Res. 2005, 59, 169–180. [Google Scholar] [CrossRef]
- Nudda, A.; Battacone, G.; Boaventura Neto, O.; Cannas, A.; Helena, A.; Francesconi, D.; Stanislao Atzori, A.; Pulina, G. Feeding strategies to design the fatty acid profile of sheep milk and cheese. R. Bras. Zootec. 2014, 43, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Bencini, R.; Purvis, I.W. The yield and composition of milk from Merino sheep. Wool Technol. Sheep Breed. 1990, 38, 71–73. [Google Scholar]
- Kay, J.K.; Weber, W.J.; Moore, C.E.; Bauman, D.E.; Hansen, L.B.; Chester-Jones, H.; Crooker, B.A.; Baumgard, L.H. Effects of week of lactation and genetic selection for milk yield on milk fatty acid composition in Holstein cows. J. Dairy Sci. 2005, 88, 3886–3893. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef] [Green Version]
- Avendaño, J.; Fernández, F.; Sandoval, C. Milk production comparison of different breeds of ewes in the central-south zone of Chile. Agric. Technol. 2002, 62, 530–540. [Google Scholar]
- Garnsworthy, P.C.; Masson, L.L.; Lock, A.L.; Mottram, T.T. Variation of milk citrate with stage of lactation and de novo fatty acid synthesis in dairy cows. J. Dairy Sci. 2006, 89, 1604–1612. [Google Scholar] [CrossRef] [Green Version]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Pulina, G.; Nudda, A.; Battacone, G.; Cannas, A. Effects of nutrition on the contents of fat, protein, somatic cells, aromatic compounds, and undesirable substances in sheep milk. Anim. Feed Sci. Technol. 2006, 131, 255–291. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Beaulieu, A.D.; Barbano, D.M. Feed and animal factors influencing milk fat composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Bitman, J.; Wood, D.L. Changes in milk fat phospholipids during lactation. J. Dairy Sci. 1990, 73, 1208–1216. [Google Scholar] [CrossRef]
- Rukkwamsuk, T.; Geelen, M.J.; Kruip, T.A.; Wensing, T. Interrelation of fatty acid composition in adipose tissue, serum and liver of dairy cows during the development of fatty liver postpartum. J. Dairy Sci. 2000, 83, 52–59. [Google Scholar] [CrossRef]
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition. Lipids Health Dis. 2007, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Addis, M.; Cabiddu, A.; Pinna, G.; Decandia, M.; Piredda, G.; Pirisi, A.; Molle, G. Milk and cheese fatty acid composition in sheep fed Mediterranean forages with reference to conjugated linoleic acid cis-9, trans-11. J. Dairy Sci. 2005, 88, 3443–3454. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.P.; San Primitivo, F.; Barbosa, E.; Varona, L.; De la Fuente, L.F. Genetic determination of fatty acid composition in Spanish Churra sheep milk. J. Dairy Sci. 2010, 93, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.L. Conjugated linoleic acids in milk fat. Variation and physiological effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Kelsey, J.A.; Corl, B.A.; Collier, R.; Bauman, D.E. The effect of breed, parity, and stage of lactation on Conjugated Linoleic Acid (CLA) in milk fat from dairy cows. J. Dairy Sci. 2003, 86, 2588–2597. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Greupner, T.; Kutzner, L.; Pagenkopf, S.; Kohrs, H.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Effects of a low and a high dietary LA/ALA ratio on long-chain PUFA concentrations in red blood cells. Food Funct. 2018, 9, 4742–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierlita, D. Fatty acid profile and health lipid indices in the raw milk of ewes grazing part-time and hemp seed supplementation of lactating ewes. S. Afr. J. Anim. Sci. 2016, 46, 237–246. [Google Scholar]
- Roy, S.J.; Lapierre, S.S.; Baker, B.D.; Delfausse, L.A.; Machin, D.R.; Tanaka, H. High dietary intake of whole Milk and full-fat dairy products does not exert hypotensive effects in adults with elevated blood pressure. Nutr. Res. 2019, 64, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; De la Fuente, M.A. Milk fatty acids and potential health benefits: An update vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef] [Green Version]
| Species | July | August | September | October |
|---|---|---|---|---|
| Lolium perenne (%) | 49.5 | 43.0 | 42.1 | 40.6 |
| Hypochaeris radicata (%) | 2.4 | 8.9 | 10.2 | 15.8 |
| Trifolium repens (%) | 15.4 | 9.9 | 8.5 | 7.9 |
| Rumex acetosella (%) | 1.2 | 5.3 | 6.9 | 7.7 |
| Others (%) | 31.5 | 32.9 | 32.3 | 28 |
| Saturated fatty acids (%) | 24.0 | 25.3 | 25.8 | 28.3 |
| Monounsaturated fatty acids (%) | 3.5 | 4.8 | 8.3 | 10.1 |
| Polyunsaturated fatty acids (%) | 72.5 | 69.9 | 65.9 | 61.6 |
| Dry matter (kg ha−1) | 309.1 | 600.0 | 1450.0 | 1931.6 |
| Variable | Days in Milk | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 90 | |||
| EW | 53.88 | 50.54 | 53.17 | 54.58 | 1.14 | 0.28 |
| BCS | 2.18 | 2.21 | 2.50 | 2.58 | 0.05 | 0.08 |
| Lamb weight | 5.97 d | 10.18 c | 16.02 b | 21.00 a | 0.83 | 0.00 |
| Variable | Days in Milk | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 90 | |||
| Production (L day−1) | 0.95 b | 1.40 a | 0.74 b | 0.68 b | 0.07 | 0.02 |
| Protein (%) | 4.09 c | 4.30 c | 4.77 b | 5.24 a | 0.11 | 0.001 |
| Fat (%) | 7.09 a | 6.31 a,b | 5.76 b | 7.07 a | 0.21 | 0.001 |
| Lactose (%) | 5.28 a,b | 5.36 a,b | 5.46 a | 5.16 b | 0.04 | 0.001 |
| Total solids (%) | 18.19 a,b | 17.68 b | 17.59 b | 19.28 a | 0.26 | 0.001 |
| Solid non-fat (%) | 10.48 b | 10.80 b | 11.30 a | 11.58 a | 0.10 | 0.001 |
| Urea (g 100 mL−1) | 0.04 b | 0.05 a | 0.04 b | 0.05 a | 0.001 | 0.001 |
| Fatty Acid | Days in Milk | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 10 | 30 | 60 | 90 | |||
| C6:0 | 0.17 a | 0.09 b | 0.09 b | 0.09 b | 0.01 | 0.01 |
| C8:0 | 0.28 | 0.19 | 0.24 | 0.25 | 0.08 | ns |
| C10:0 | 1.65 c | 1.99 b,c | 2.87 a,b | 3.24 a | 0.09 | 0.01 |
| C12:0 | 1.35 c | 1.71 b,c | 2.19 ab | 2.57 a | 0.06 | 0.02 |
| C13:0 | 0.06 c | 0.08 c | 0.13 b | 0.17 a | 0.005 | 0.01 |
| C14:0 | 5.20 c | 6.26 b,c | 6.87 b | 9.30 a | 0.17 | 0.01 |
| C14:1 | 0.55 d | 0.83 c | 1.25 b | 1.57 a | 0.04 | 0.01 |
| C15:0 | 0.15 | 0.20 | 0.18 | 0.15 | 0.01 | ns |
| C16:0 | 24.14 b | 24.80 b | 24.30 b | 26.75 a | 0.16 | 0.02 |
| C16:1 | 1.10 b | 1.02 b | 1.30 a | 1.07 b | 0.03 | 0.04 |
| C17:0 | 1.17 a | 1.01 ab | 0.95 b | 0.98 b | 0.02 | 0.01 |
| C17:1 | 0.27 a | 0.15 b,c | 0.11 c | 0.18 b | 0.01 | 0.01 |
| C18:0 | 20.50 b,c | 23.11 b | 24.86 a | 18.35 c | 0.38 | 0.03 |
| C18:1n9t | 0.16 b | 0.31 a | 0.34 a | 0.17 b | 0.01 | 0.02 |
| C18:1n9c | 38.74 a | 33.19 b | 29.90 c | 29.39 c | 0.48 | 0.01 |
| C18:2n6c | 1.96 a | 1.54 b | 1.21 c | 1.47 b | 0.02 | 0.01 |
| C18:3n6 | 0.15 d | 0.22 c | 0.27 b | 0.43 a | 0.01 | 0.01 |
| C18:3n3 | 0.94 | 0.92 | 0.83 | 0.89 | 0.01 | ns |
| Total CLA 1 | 1.28 b | 1.80 ab | 1.91 a | 1.71 a,b | 0.04 | 0.04 |
| C21:0 | 0.04 c | 0.10 a | 0.06 b | 0.06 b | 0.003 | 0.01 |
| C20:5 n3 | 0.13 a | 0.09 b | 0.13 a | 0.14 a | 0.01 | 0.02 |
| C24:1n9 | 0.02 c | 0.02 c | 0.04 b | 0.09 a | 0.002 | 0.02 |
| C22:6 n3 | 0.03 b | 0.03 b | 0.03 b | 0.05 a | 0.001 | 0.03 |
| SCFA | 2.10 c | 2.27 c | 3.20 b | 3.58 a | 0.02 | 0.01 |
| MCFA | 33.97 c | 36.06 b | 37.28 b | 42.74 a | 0.05 | 0.01 |
| LCFA | 63.95 a | 61.33 a | 59.58 a | 52.75 b | 0.06 | 0.01 |
| SFA | 54.70 c | 59.55 b | 62.69 a,b | 63.34 a | 0.53 | 0.00 |
| MUFA | 40.83 a | 35.82 b | 32.94 c | 31.91 c | 0.52 | 0.00 |
| PUFA | 4.48 | 4.63 | 4.37 | 4.75 | 0.68 | ns |
| IA | 1.02 c | 1.27 b,c | 1.47 a,b | 1.73 a | 0.04 | 0.01 |
| IT | 1.12 b | 1.40 ab | 1.58 a | 1.64 a | 0.03 | 0.01 |
| PUFA/SFA | 0.08 a | 0.08 a | 0.07 b | 0.07 b | 0.001 | 0.001 |
| MUFA/SFA | 0.75 a | 0.60 b | 0.53 c | 0.50 c | 0.01 | 0.001 |
| n-6/n-3 PUFA | 1.92 a | 1.69 b | 1.49 c | 1.76 a,b | 0.03 | 0.02 |
| HFA/UFA | 0.68 c | 0.81 b | 0.89 a | 1.05 a | 0.02 | 0.00 |
| h/H | 1.47 a | 1.22 b | 1.09 c | 0.95 c | 0.03 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inostroza, K.; Bravo, S.; Larama, G.; Saenz, C.; Sepúlveda, N. Variation in Milk Composition and Fatty Acid Profile during the Lactation of Araucana Creole Ewes in a Pasture-Based System. Animals 2020, 10, 92. https://doi.org/10.3390/ani10010092
Inostroza K, Bravo S, Larama G, Saenz C, Sepúlveda N. Variation in Milk Composition and Fatty Acid Profile during the Lactation of Araucana Creole Ewes in a Pasture-Based System. Animals. 2020; 10(1):92. https://doi.org/10.3390/ani10010092
Chicago/Turabian StyleInostroza, Karla, Silvana Bravo, Giovanni Larama, Camila Saenz, and Néstor Sepúlveda. 2020. "Variation in Milk Composition and Fatty Acid Profile during the Lactation of Araucana Creole Ewes in a Pasture-Based System" Animals 10, no. 1: 92. https://doi.org/10.3390/ani10010092
APA StyleInostroza, K., Bravo, S., Larama, G., Saenz, C., & Sepúlveda, N. (2020). Variation in Milk Composition and Fatty Acid Profile during the Lactation of Araucana Creole Ewes in a Pasture-Based System. Animals, 10(1), 92. https://doi.org/10.3390/ani10010092






