1. Introduction
Standing on the data proposed by the Food and Agriculture Organization in 2018 [
1], eggs are the second-fastest growing industry in the world, with more than 50% growth forecast in the next 2 decades. Europe production in 2019 amounts to 7.5 million tonnes of eggs thanks to the 400 million laying hens kept throughout the European Union (27 Member States) [
2]. Italy is the fourth producer with approximately 817,000 tons of eggs consumed as a whole or destined to the egg industry. Indeed, despite eggs play a very important role in human nutrition as a precious source of proteins, essential amino acids, lipid and several trace elements, the industry is continuously asking for egg products, such as liquid, powdered, concentrated whole egg or its separated main components, yolk and albumen. For instance, the egg allowance directed toward processing chains has been estimated at 32% of the whole Italian production [
3]. This interest is linked to the unique functional properties of eggs, such as foaming, gelling and emulsification. The foaming capacity belongs to the egg white protein and it is defined as “the ability to rapidly adsorb on the air-liquid interface during whipping or bubbling and by its ability to form a cohesive viscoelastic film by way of intermolecular interactions” [
4]. Thanks to these characteristics, albumen is widely utilized as an ingredient in bakery products, like bread, cakes and meringues, ice creams and several other processed foods.
The increase in egg and egg-derived product demand is however dependent on the laying hen farming, a sector that is affected by some critical aspects, such as feed supplying. As one of the main concerns of the last decade, the unsustainability of feed, especially soybean meal, is quite debated. Indeed, despite being an important source of essential amino acids, soybean production for feed directly competes with human nutrition and is largely associated with water pollution and exploitation of the lands [
5].
A big opportunity for companies looking at more sustainable protein sources has been recently identifying in insect meals. Although processed animal proteins (PAPs) are still banned as livestock feeds in Europe, the European Commission voted in 2017 to introduce seven insect species, processed as meal or oil, into fish feeds and pet food [
6]. That is why the stakeholders are confident that these protein sources would be allowed in other feeds in the coming years. As the interest in this alternative ingredient has grown, researchers are checking the feasibility of partial or complete substitution of the conventional protein sources with insect meal in poultry feed. Among the other species,
Hermetia illucens meal (HI) has deserved major attention for laying hen diets. Indeed, in addition to the other advantages related to the rearing process of this species of insect, HI is a valuable protein source (40–44 g/100 g of dry matter, DM) and it provides calcium and phosphorous (50 to 80 g/kg dry matter, 6 to 15 g/kg dry matter, respectively), which are fundamental for laying nutrition [
7]. HI has been proved to be a feasible substitute for vegetable protein sources in the hen diet when considering animal growth and performance [
8,
9,
10] and egg quality [
11,
12,
13]. However, the authors focused on the egg deposition, quality characteristics as the albumen height and the yolk color and its chemical composition, more than the albumen technological properties, which could be of interest to the egg industry. Thus, this study aimed to test the effect of partial substitution of the conventional protein sources with a partially defatted
Hermetia illucens larva meal on the overall egg quality and on the technological properties of the albumen from the eggs produced by Hy-line Brown laying hens and collected at three different hen ages.
3. Results
The egg, eggshell and yolk weights significantly increased due to the inclusion of HI in the feed for laying hens, irrespective of the insect inclusion level utilized (
Table 2). Despite this, while looking at the percentage of the main components of the egg, only the eggshell resulted significantly diminished in the HI50 eggs compared to the C and HI25 groups. Accordingly, the thickness of the eggshell was reduced while increasing HI in the feed.
Furthermore, the eggshell percentage varied (
p < 0.05) along with the hen age, showing the highest value at A27. These modifications in the eggshell characteristics cannot be attributed to the different ash content since it resulted unaffected by both D and A and equal to 76.4 ± 2.5 g/100 g of eggshell. Since L*, a*, and b* values resulted significantly affected by the interaction D × A at
p < 0.026, 0.0001, and 0.049, respectively, we summarised the means obtained for this interaction in
Table 3. No difference in the lightness of yolks was found as affected by the diet in the A20 group (
Table 3). Conversely, the H50 eggs laid at A27 and A35 showed L* value significantly different from the C ones. The major differences were in the a* value, found significantly higher in the C yolks than in the H25 and H50 ones at A20 and A27. A greater decrease of the a* value was recorded in the C group increasing the time of laid, whereas the H50 raised its maximum a* value in the yolk from the eggs collected at A35. The b* index appeared scarcely affected by the diet, indeed the H50 yolks were significantly less yellow than the other two groups only in the A20 group. Furthermore, the same group significantly increased the b* index from the A27.
Table 4 shows the results of the technological properties and chemical composition analyses of the albumen. The FC was decreased by the presence of HI in the diet of laying hens, whereas the FS ad the pH value, as well as the water and protein contents were unaffected. A deep effect of the A can be observed for pH and chemical components of the albumen. For instance, a significant decrease in the pH value (
p < 0.01) was found after the A27. The albumen of the eggs collected at the A35 showed a significantly (
p = 0.05) lower protein content than those collected at the A20, while their water content increased with the aging.
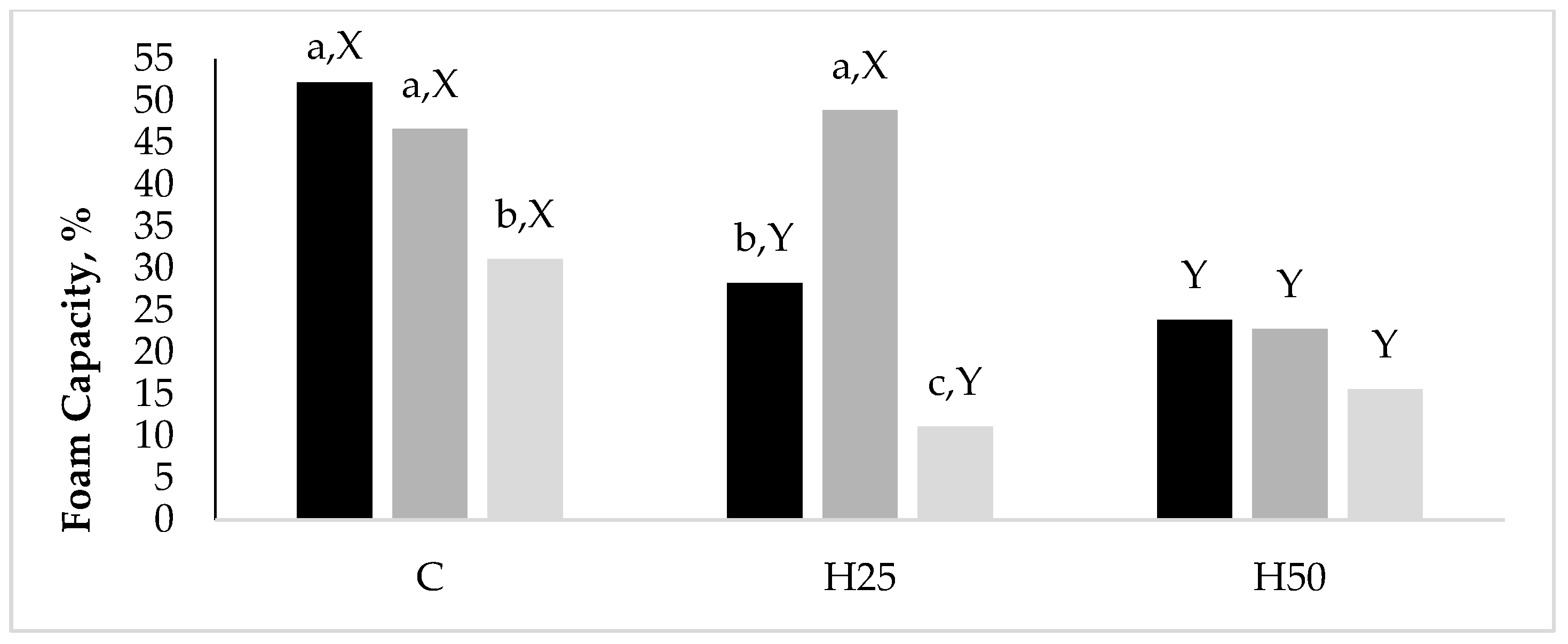
Data related to the significant interaction between D and A emerged for the FC are reported in
Figure 1. Although the H50 albumens had a halved (
p < 0.05) FC than the C ones, they kept this property constant while increasing the hen age, while both the C and H25 groups reduced their FC between the A20 and the A35.
The FC of the egg white was even determined by the analyses of physical parameters conducted on the angel cakes (
Table 5). The textural properties of the angel cake were not affected by the dietary inclusion of
Hermetia illucens, while A20 induced a significant reduction of the chewiness.
A significant interaction (D × A) emerged for height, b* value and baking loss of the samples (
Table 6). The height of the angel cakes produced from the C egg whites was unaffected by the A, whereas the angel cakes obtained by the H25 eggs collected during the A35 significantly lost their height compared to those produced from the H25 eggs of the A20. While looking at the difference among the dietary treatments, the angel cake from the C eggs was higher than the H50 only considering the A20. In addition, the H25 egg whites collected at the A27 and A35 significantly decreased the height of the angel cake compared both to the C and H50 groups. Concerning the baking loss, the age did not affect the water retention ability of the angel cake made with the C albumen, while the highest inclusion of HI in the diet increased the ability of the batter to retain water, especially at the A35. The b* value of the angel cake obtained with the C albumens was unaffected by the age, while the yellowness was significantly higher using the albumen from the H25 eggs collected at the A35 than that collected at the A20. Conversely, the H50 angel cake was slight but significantly discoloured when prepared with the egg whites from the A27 and A35. Regarding the differences among the diets, the angel cakes produced with the H25 albumens collected at the A20 had a lower (
p < 0.001) b* value than those of the C and H50 groups. In contrast, the H50 cakes prepared with the eggs collected at the A27 and A35 showed a lower (
p < 0.001) b* index than the C and HI25.
Finally,
Table 7 depicts that the batters prepared with the egg whites collected at the A27 had a slight but significantly lower water content than those from the egg whites collected at the other two considered ages.
4. Discussion
The main factors affecting the egg weight are the dietary metabolized energy [
22] and the size of the yolk that, in turn, is influenced by the body weight of the hens [
23]. Since the administered diets in the present trial were isoenergetic, it seems clear to attribute the major changes in HI egg weights to the increase in the size of their yolks. Nevertheless, this modification cannot be attributable to the different body weight of the hens, resulted unaffected by the dietary intervention as well as the feeding intake and the feed conversion ratio [
14]. Thus, the diet could have influenced the egg and yolk weights. Leeson and Summers [
23] suggested that egg weight is very sensitive to methionine and total sulphur amino acid levels, which however were balanced in all the three administered diets. Our hypothesis is that the significantly (
p = 0.032) augmented length of the jejunum found in the HI25 and HI50 hens, being respectively 4.68 and 4.64 (as percentage of the live weight), compared to the C one (3.45% live weight), [
14] could have promoted the absorption of the amino acids, hence resulting in a higher assumption of these nutrients. This possible explanation strongly needs the support of other comprehensive studies, since the effect of HI meal on hen egg weight is still scarcely investigated and conflicting in results [
13,
24]. The increased egg weight found with the HI meal inclusion in the hens’ diet may require an increase in eggshell weight because of a rising in calcium deposition. Notwithstanding, authors underlined that egg-laying birds have a limited amount of calcium available to produce the shell, approximately 2.0–2.5 g Ca
2+, irrespective of the egg size weight [
25]. Hence, the production of heavier eggs in the HI50 group might explain the significant reduction in shell percentage and even in its thickness. Since the eggshell weight and thickness are physical variables correlated with the egg strength, resistance to physical and pathogenic challenges from laying to the transportation and selling phase [
26], our results should be considered when evaluating the suitability to include the HI meal in the laying hens’ feed.
The egg internal quality, determined by several parameters (as albumen height and Haugh Unit), is even associated with the yolk quality, whose color is a fundamental characteristic [
27]. The pigments contained in feeds mainly derive by plant ingredients, however, Secci et al. [
13] underlined that even the HI larva meal may contain carotenoids and tocopherols. These pigments have a strong affinity for non-polar molecules, such as lipid, and they generally absorb wavelengths ranging from 400 to 550 nm, coloring as yellow, orange, or red. Thus, carotenoids can be easily accumulated in the egg yolk, which is rich in fat, producing the major modification of the yolk redness index, as we found.
Although the egg production and quality as affected by
H. illucens in laying hen diets have been recently examined [
11,
12,
13,
24], the focus areas were the egg deposition and quality characteristics as the albumen height, the yolk color and its chemical composition. Nonetheless, the albumen has relevant importance for the egg industry mainly because of its unique functional properties, such as foaming that promote its extensive use as an ingredient in several processed food. In the present study, the diet did not affect the egg white pH and protein content, thus supporting previous findings [
13]. The protein concentration as well as pH and cooking temperature, in addition to the physical-chemical properties of proteins, are well-established factors affecting the foaming ability [
28]. Although in the present study no significant difference among the diets was found for pH and protein values, the foam ability of the HI25 and HI50 egg whites was decreased compared to that of the C ones. Since the temperature was controlled during the experiment, we hypothesize that the HI diets could modify the concentration, or the proportion of the single protein fractions contained in the egg white. As previously noted [
4], the albumen foaming properties are the result of the interaction among the different proteins, such as globulins, ovalbumin, ovotransferrin, lysozyme, ovomucoid and ovomucin. Each fraction has its own ability to form a voluminous foam (foam capacity), to maintain it (foam stability) and to increase the volume of the batter containing it (i.e., angel cake) which differs from the overall foaming ability of the whole albumen [
4]. For instance, globulins have the highest foaming index, around 4.71 cm
3/g min, more than 7 times higher than that of the ovalbumin (0.59), while ovomucin and ovomucoid show no foaming ability [
4]. Nevertheless, Mine [
4] reviewed that these last two proteins contributed to the final volume of the angel cakes made with them, whereas the ovalbumin produced cakes with a volume comparable to the one obtained by globulins, being 308 and 330 cm
3, respectively [
4]. Standing on the mentioned literature, the possible role of the diet on protein matrix composition should be investigated to support or not our results about the technological properties of the egg white.
The albumen chemical composition varied while increasing the hen age, in line with previous findings [
29] which proposed that the increase in hen age and weight led to a reduction in the crude protein content of the albumen. Changes in pH and protein content are consistent with the reduction of the foam capacity while using eggs from the 35th week of age. Instead, the maximum height raised by the angel cake varied due to the interaction D × WDA, thus supporting the previous hypothesis on the induced modification of proteins.
Baking loss is an important technological characteristic of a batter and plays a key role both for the quality of the final baked products and their shelf life. Indeed, the water content can promote microbiological growth during the storage, inducing a loss of the shelf life length. Considering that proteins may affect the water retention during cooking, the high affinity to the water of the white from eggs laid by hens fed the HI deserves other studies.
It has been previously demonstrated that the HI inclusion in the diet affects the yolk color values of the eggs [
11,
12,
13], probably because of the pigments contained in the insect meal [
13]. Standing on our knowledge, this is the first time that a difference in color emerged in a baked product made by egg white derived by hens fed with HI and, for this reason, little comparisons are possible.
The textural attributes of food are strictly related to its quality and consumers’ acceptance. In the present study, the HI meal presence in the diet did not affect the textural properties of the angel cake, similarly to other authors who found that different dietary types of protein (i.e., soybean meal, cottonseed protein, double-zero rapeseed meal, individually or in combination with equal crude protein) administered to the Jinghong laying hens did not affect the cooked yolk hardness and springiness [
30]. Furthermore, the deep reduction in the chewiness that occurred between the eggs laid at 20 and 35 weeks of hens’ age agrees with the finite effect of the substitution of egg white by vegetable protein on angel cake chewiness [
19]. Since chewiness can be enhanced due to a reinforcement of the protein entanglement in the networks [
19], it is possible that variations in the egg white protein-gluten-starch connections occurred with hen aging, although more studies are necessary to define what kind of interaction is eventually developed.