Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site for Forage Planting
2.2. Hay and Silage Mixture Preparation
2.3. Rumen Fluid Collection
2.4. In Vitro Batch Culturing and Sample Collection
2.5. Chemical Analysis
2.6. Calculations
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Hay and Silage Mixtures
3.2. Ensiling Characteristics of Orchardgrass and Alfalfa Silage Mixtures
3.3. Degradation Characteristics and Gas Production Kinetics of Hay and Silage Mixtures
3.4. Fermentation Characteristics of Hay and Silage Mixtures
3.5. SFAEI and MFAEI of Hay and Silage Mixtures
4. Discussions
4.1. Chemical Composition of Hay and Silage Mixtures
4.2. Ensiling Characteristics of Orchardgrass and Alfalfa Silage Mixtures
4.3. Associative Effects on the Degradation Characteristics and Gas Production Kinetics of Hay and Silage Mixtures
4.4. Associative Effects on the Fermentation Characteristics of Hay and Silage Mixtures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stewart, A.V.; Ellison, N.W. Dactylis. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–87. [Google Scholar]
- Turner, L.; Donaghy, D.; Lane, P.; Rawnsley, R. A comparison of the establishment, productivity, and feed quality of four cocksfoot (Dactylis glomerata L.) and four brome (Bromus spp.) cultivars, under leaf stage based defoliation management. Aust. J. Agric. Res. 2007, 58, 900–906. [Google Scholar] [CrossRef]
- Butkutė, B.; Lemežienė, N.; Kanapeckas, J.; Navickas, K.; Dabkevičius, Z.; Venslauskas, K. Cocksfoot, tall fescue and reed canary grass: Dry matter yield, chemical composition and biomass convertibility to methane. Biomass Bioenergy 2014, 66, 1–11. [Google Scholar] [CrossRef]
- Bourquin, L.D.; Titgemeyer, E.C.; Merchen, N.R.; Fahey, G.C. Forage level and particle size effects on orchardgrass digestion by steers.1. Site and extent of organic matter, nitrogen, and cell wall digestion. J. Anim. Sci. 1994, 72, 746. [Google Scholar] [CrossRef] [PubMed]
- Niderkorn, V.; Martin, C.; Rochette, Y.; Julien, S.; Baumont, R. Associative effects between orchardgrass and red clover silages on voluntary intake and digestion in sheep: Evidence of a synergy on digestible dry matter intake. J. Anim. Sci. 2015, 93, 4967–4976. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Olmos Colmenero, J.D.J.; Winters, A.L.; Scollan, N.D.; Minchin, F.R. Polyphenol oxidase activity in grass and its effect on plant-mediated lipolysis and proteolysis of Dactylis glomerata (cocksfoot) in a simulated rumen environment. J. Sci. Food Agric. 2006, 86, 1503–1511. [Google Scholar] [CrossRef]
- Phelan, P.; Moloney, A.P.; McGeough, E.J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P. Forage Legumes for Grazing and Conserving in Ruminant Production Systems. Crit. Rev. Plant Sci. 2015, 34, 281–326. [Google Scholar] [CrossRef]
- Peyraud, J.; Astigarraga, L. Review of the effect of nitrogen fertilization on the chemical composition, intake, digestion and nutritive value of fresh herbage: Consequences on animal nutrition and N balance. Anim. Feed Sci. Technol. 1998, 72, 235–259. [Google Scholar] [CrossRef]
- Patra, A. Effects of supplementing low-quality roughages with tree foliages on digestibility, nitrogen utilization and rumen characteristics in sheep: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2010, 94, 338–353. [Google Scholar] [CrossRef]
- Johansen, M.; Søegaard, K.; Lund, P.; Weisbjerg, M.R. Digestibility and clover proportion determine milk production when silages of different grass and clover species are fed to dairy cows. J. Dairy Sci. 2017, 100, 8861–8880. [Google Scholar] [CrossRef]
- INRA. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 44–59. [Google Scholar]
- Niderkorn, V.; Martin, C.; Bernard, M.; Le Morvan, A.; Rochette, Y.; Baumont, R. Effect of increasing the proportion of chicory in forage-based diets on intake and digestion by sheep. Animal 2019, 13, 718–726. [Google Scholar] [CrossRef]
- Hristov, A.N.; Sandev, S.G. Proteolysis and rumen degradability of protein in alfalfa preserved as silage, wilted silage or hay. Anim. Feed Sci. Technol. 1998, 72, 175–181. [Google Scholar] [CrossRef]
- Plaizier, J.C. Replacing chopped alfalfa hay with alfalfa silage in barley grain and alfalfa-based total mixed rations for lactating dairy cows. J. Dairy Sci. 2004, 87, 2495–2505. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Z.; Li, J.; Shao, T. Ensiling characteristics, in vitro rumen fermentation, microbial communities and aerobic stability of low-dry matter silages produced with sweet sorghum and alfalfa mixtures. J. Sci. Food Agric. 2019, 99, 2140–2151. [Google Scholar] [CrossRef]
- Dal Pizzol, J.; Ribeiro-Filho, H.; Quereuil, A.; Le Morvan, A.; Niderkorn, V. Complementarities between grasses and forage legumes from temperate and subtropical areas on in vitro rumen fermentation characteristics. Anim. Feed Sci. Technol. 2017, 228, 178–185. [Google Scholar] [CrossRef]
- Getachew, G.; Robinson, P.; DePeters, E.; Taylor, S. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Zhang, D.; Yang, H. In vitro ruminal methanogenesis of a hay-rich substrate in response to different combination supplements of nitrocompounds; pyromellitic diimide and 2-bromoethanesulphonate. Anim. Feed Sci. Technol. 2011, 163, 20–32. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q. Effect of different types of fibre supplemented with sunflower oil on ruminal fermentation and production of conjugated linoleic acids in vitro. Arch. Anim. Nutr. 2006, 60, 402–411. [Google Scholar] [CrossRef]
- Verdouw, H.; Vanechteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Makkar, H.; Sharma, O.; Dawra, R.; Negi, S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Cui, J.; Yang, H.; Yu, C.; Bai, S.; Wu, T.; Song, S.; Sun, W.; Shao, X.; Jiang, L. Effect of urea fertilization on biomass yield, chemical composition, in vitro rumen digestibility and fermentation characteristics of straw of highland barley planted in Tibet. J. Agric. Sci. 2016, 154, 151–164. [Google Scholar] [CrossRef]
- Kilic, A. Silo Feed (Instruction, Education and Application Proposals); Bilgehan Press: Izmir, Turkey, 1986. [Google Scholar]
- Moore, J.E.; Undersander, D.J. Relative forage quality: An alternative to relative feed value and quality index. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 10–11 January 2002. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Mertens, D. Predicting intake and digestibility using mathematical models of ruminal function. J. Anim. Sci. 1987, 64, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Allen, M. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: Effects on dry matter intake and milk yield of dairy cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Moore, J.; Kunkle, W. Evaluation of equations for estimating voluntary intake of forages and forage-based diets. J. Anim. Sci. 1999, 1, 204. [Google Scholar]
- France, J.; Dijkstra, J.; Dhanoa, M.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, R.; Ranilla, M.; Tejido, M.; Carro, M. Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage: Concentrate ratio. Br. J. Nutr. 2005, 94, 71–77. [Google Scholar] [CrossRef]
- Niderkorn, V.; Baumont, R.; Le Morvan, A.; Macheboeuf, D. Occurrence of associative effects between grasses and legumes in binary mixtures on in vitro rumen fermentation characteristics. J. Anim. Sci. 2011, 89, 1138–1145. [Google Scholar] [CrossRef]
- Wang, J. Methods in Ruminant Nutrition Research; Modern Education Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Krawutschke, M.; Kleen, J.; Weiher, N.; Loges, R.; Taube, F.; Gierus, M. Changes in crude protein fractions of forage legumes during the spring growth and summer regrowth period. J. Agric. Sci. 2013, 151, 72–90. [Google Scholar] [CrossRef]
- Buxton, D.R.; Redfearn, D.D. Plant limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 814S–818S. [Google Scholar] [CrossRef]
- Kondo, M.; Shimizu, K.; Jayanegara, A.; Mishima, T.; Matsui, H.; Karita, S.; Goto, M.; Fujihara, T. Changes in nutrient composition and in vitro ruminal fermentation of total mixed ration silage stored at different temperatures and periods. J. Sci. Food Agric. 2016, 96, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.S.A.; Zeid, M.M.K. Hay quality evaluation of summer grass and legume forage monocultures and mixtures grown under irrigated conditions. Aust. J. Crop Sci. 2016, 11, 1543. [Google Scholar] [CrossRef]
- Favre, J.R.; Castiblanco, T.M.; Combs, D.K.; Wattiaux, M.A.; Picasso, V.D. Forage nutritive value and predicted fiber digestibility of Kernza intermediate wheatgrass in monoculture and in mixture with red clover during the first production year. Anim. Feed Sci. Technol. 2019, 258, 114298. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Yu, Z. Fermentation dynamics and bacterial diversity of mixed lucerne and sweet corn stalk silage ensiled at six ratios. Grass Forage Sci. 2019, 74, 264–273. [Google Scholar] [CrossRef]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Allen, M.S. Characteristics of plant-cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 1995, 73, 2774–2790. [Google Scholar] [CrossRef]
- Hristov, A.N.; Broderick, G.A. Synthesis of microbial protein in ruminally cannulated cows fed alfalfa silage, alfalfa hay, or corn silage. J. Dairy Sci. 1996, 79, 1627–1637. [Google Scholar] [CrossRef]
- Reid, R.L.; Templeton, W.C., Jr.; Ranney, T.S.; Thayne, W.V. Digestibility, intake and mineral utilization of combinations of grasses and legumes by lambs. J. Anim. Sci. 1987, 64, 1725–1734. [Google Scholar] [CrossRef]
- Bowman, J.; Asplund, J. Evaluation of mixed lucerne and caucasian bluestem hay diets fed to sheep. Anim. Feed Sci. Technol. 1988, 20, 19–31. [Google Scholar] [CrossRef]
- Hobson, P.N.; Stewart, C.S. The Rumen Microbial Ecosystem, 2nd ed.; Blackie Academic & Professional: London, UK, 1997. [Google Scholar]
- Millen, D.D.; Arrigoni, M.D.B.; Pacheco, R.D.L. Rumenology, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Liu, J.; Zhang, M.; Xue, C.; Zhu, W.; Mao, S. Characterization and comparison of the temporal dynamics of ruminal bacterial microbiota colonizing rice straw and alfalfa hay within ruminants. J. Dairy Sci. 2016, 99, 9668–9681. [Google Scholar] [CrossRef]
- Kammes, K.L.; Allen, M.S. Nutrient demand interacts with forage family to affect digestion responses in dairy cows. J. Dairy Sci. 2012, 95, 3269–3287. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.A.; Bowman, J.G.P.; Firkins, J.L.; Grove, A.V.; Hunt, C.W. Effect of intake level and alfalfa substitution for grass hay on ruminal kinetics of fiber digestion and particle passage in beef cattle. J. Anim. Sci. 2008, 86, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University press: Ithaca, NY, USA, 1994. [Google Scholar]
- Chen, K.J.; Jan, D.F.; Chiou, P.W.S.; Yang, D.W. Effects of dietary heat extruded soybean meal and protected fat supplement on the production, blood and ruminal characteristics of Holstein cows. Asian-Australas. J. Anim. Sci. 2002, 15, 821–827. [Google Scholar] [CrossRef]
- Brask, M.; Lund, P.; Hellwing, A.L.F.; Poulsen, M.; Weisbjerg, M.R. Enteric methane production, digestibility and rumen fermentation in dairy cows fed different forages with and without rapeseed fat supplementation. Anim. Feed Sci. Technol. 2013, 184, 67–79. [Google Scholar] [CrossRef]
- Oba, M.; Mewis, J.L.; Zhining, Z. Effects of ruminal doses of sucrose, lactose, and corn starch on ruminal fermentation and expression of genes in ruminal epithelial cells. J. Dairy Sci. 2015, 98, 586–594. [Google Scholar] [CrossRef]
- Menke, K.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Pen, B.; Sar, C.; Mwenya, B.; Kuwaki, K.; Morikawa, R.; Takahashi, J. Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim. Feed Sci. Technol. 2006, 129, 175–186. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Kim, J.; Oh, Y.; Kim, B.; Kim, C.; Kim, K. Methane production potential of feed ingredients as measured by in vitro gas test. Asian Australas. J. Anim. Sci. 2003, 16, 1143–1150. [Google Scholar] [CrossRef]
- Cronje, P.B. Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Jayanegara, A.; Wina, E.; Soliva, C.; Marquardt, S.; Kreuzer, M.; Leiber, F. Dependence of forage quality and methanogenic potential of tropical plants on their phenolic fractions as determined by principal component analysis. Anim. Feed Sci. Technol. 2011, 163, 231–243. [Google Scholar] [CrossRef]
- Zhang, S.J.; Chaudhry, A.S.; Osman, A.; Shi, C.Q.; Edwards, G.R.; Dewhurst, R.J.; Cheng, L. Associative effects of ensiling mixtures of sweet sorghum and alfalfa on nutritive value, fermentation and methane characteristics. Anim. Feed Sci. Technol. 2015, 206, 29–38. [Google Scholar] [CrossRef]
- Mehrez, A.; Ørskov, E.; McDonald, I. Rates of rumen fermentation in relation to ammonia concentration. Br. J. Nutr. 1977, 38, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Satter, L.; Slyter, L. Effect of ammonia concentration on rumen microbial protein production in vitro. Br. J Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, J.; Xing, F.; Yang, L. Alfalfa as a supplement of dried cornstalk diets: Associative effects on intake, digestibility, nitrogen metabolisation, rumen environment and hematological parameters in sheep. Livest. Sci. 2008, 113, 87–97. [Google Scholar] [CrossRef]
- Eun, J.S.; Beauchemin, K.; Schulze, H. Use of exogenous fibrolytic enzymes to enhance in vitro fermentation of alfalfa hay and corn silage. J. Dairy Sci. 2007, 90, 1440–1451. [Google Scholar] [CrossRef]
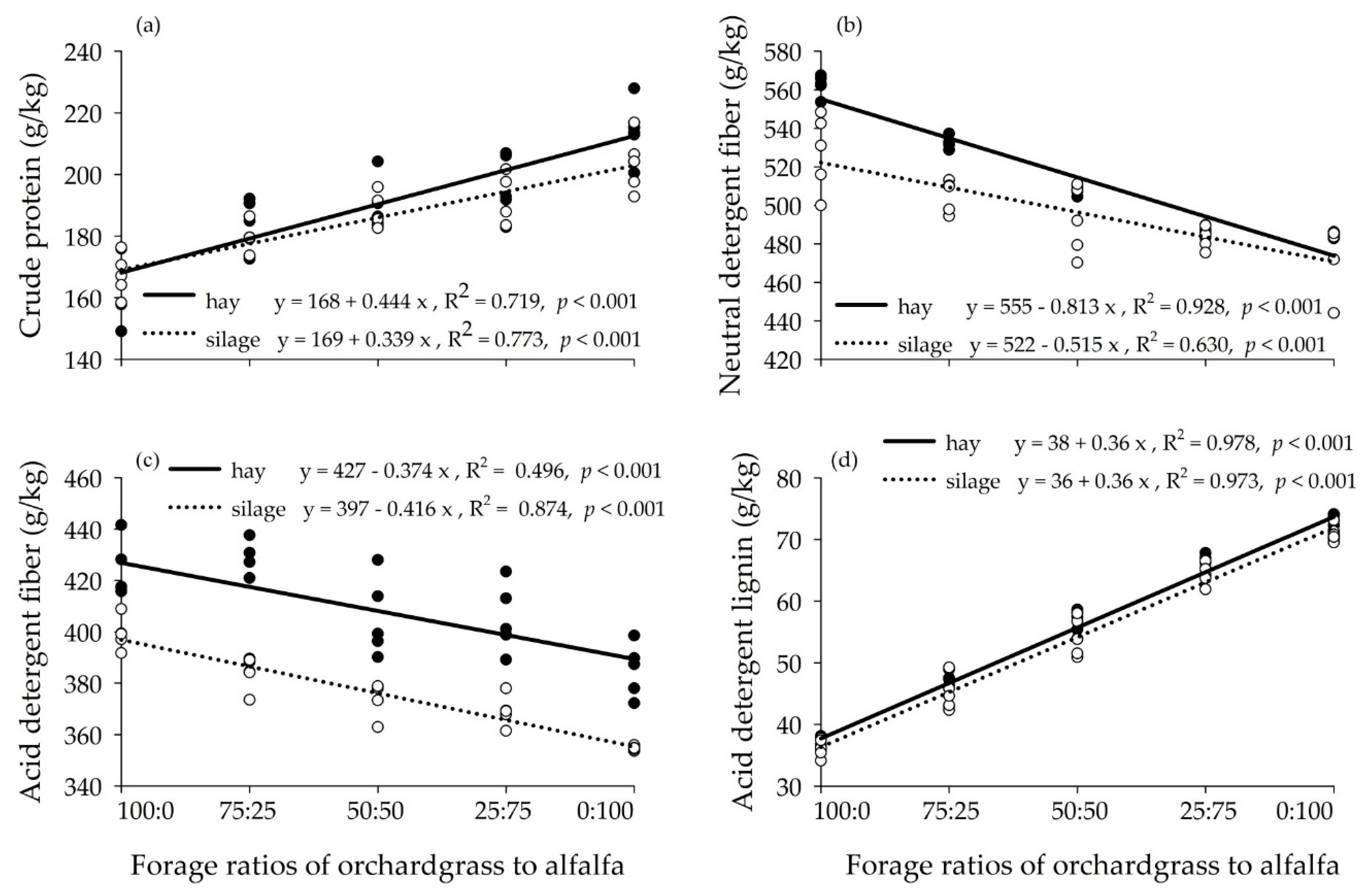
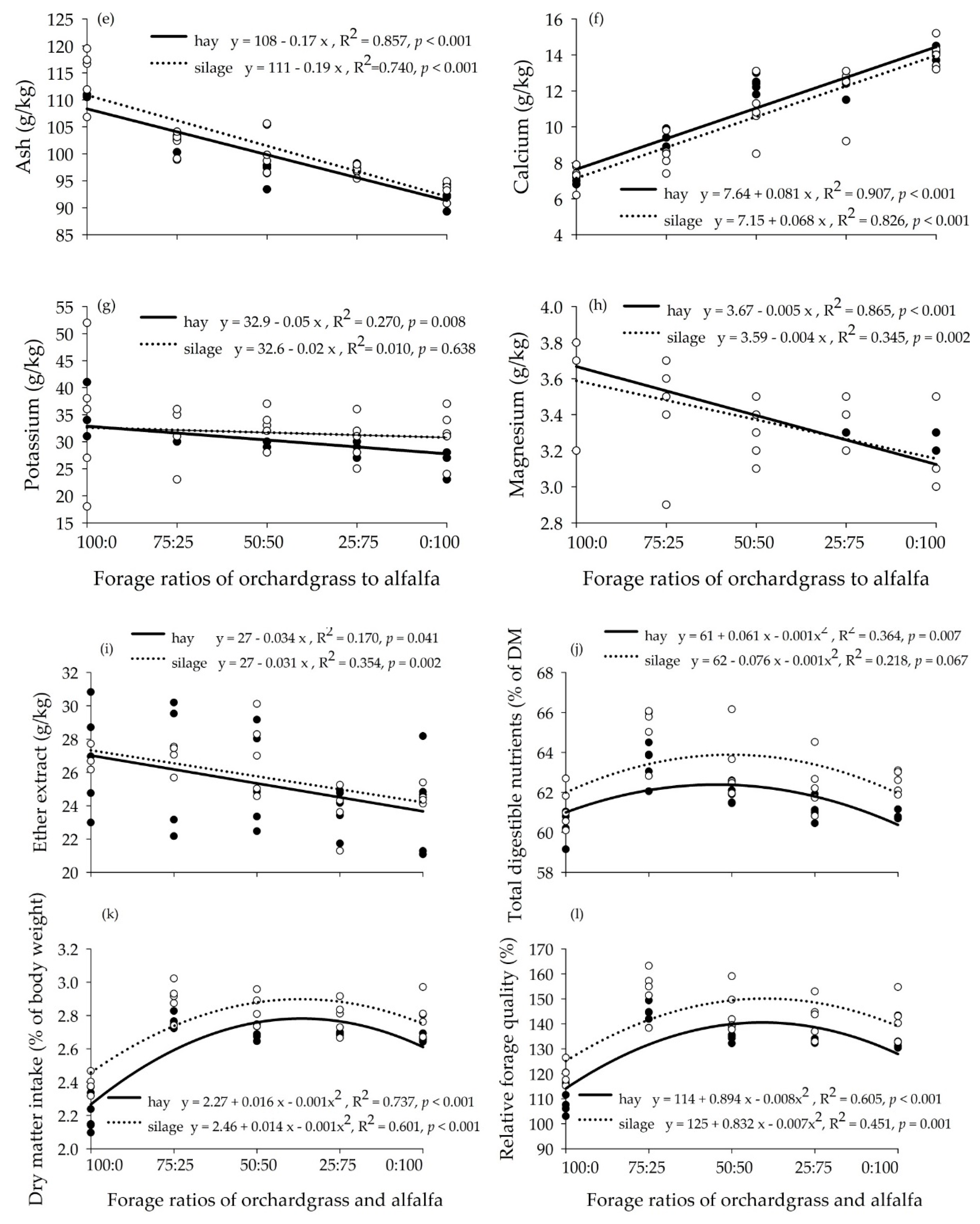
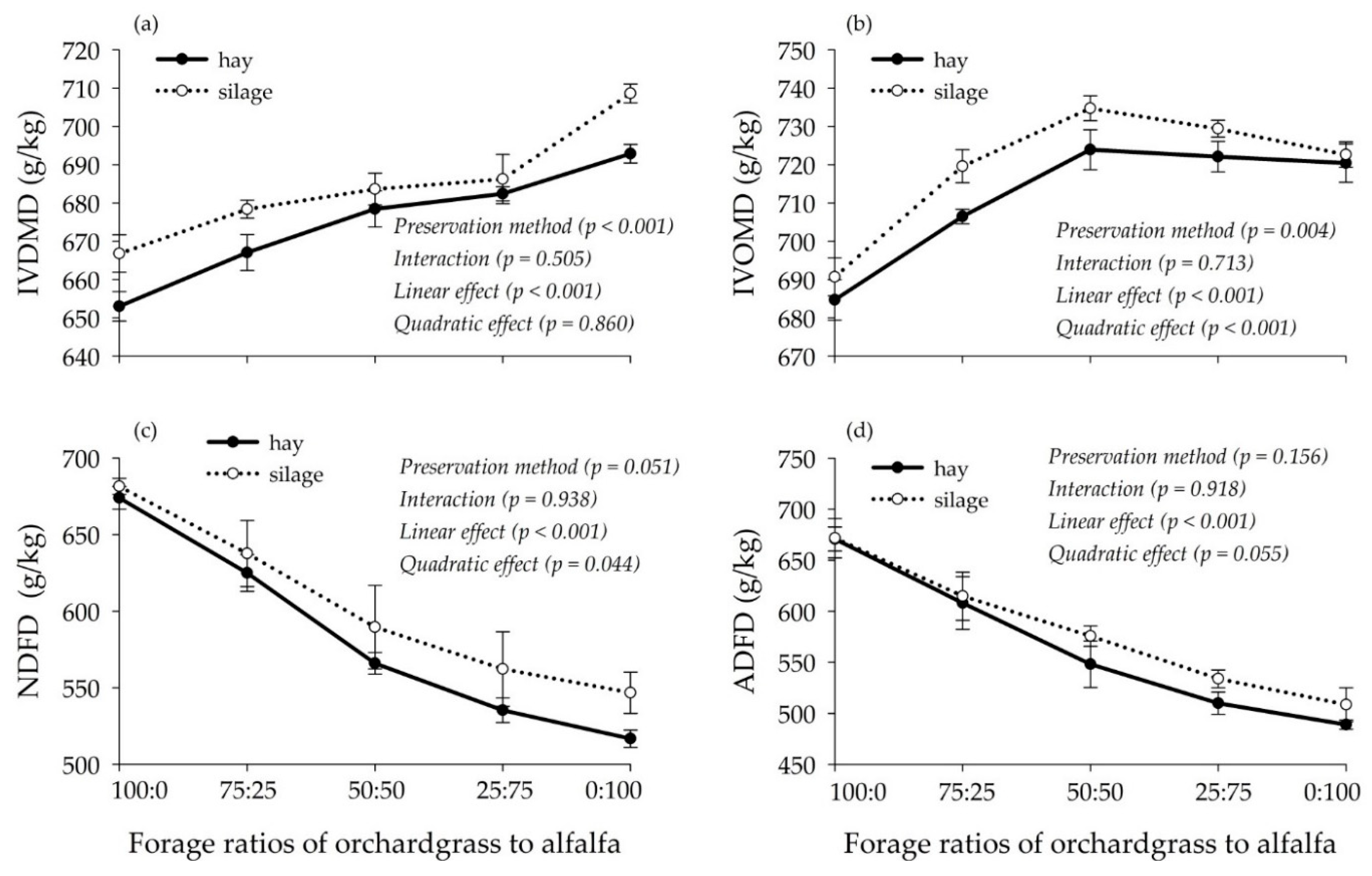
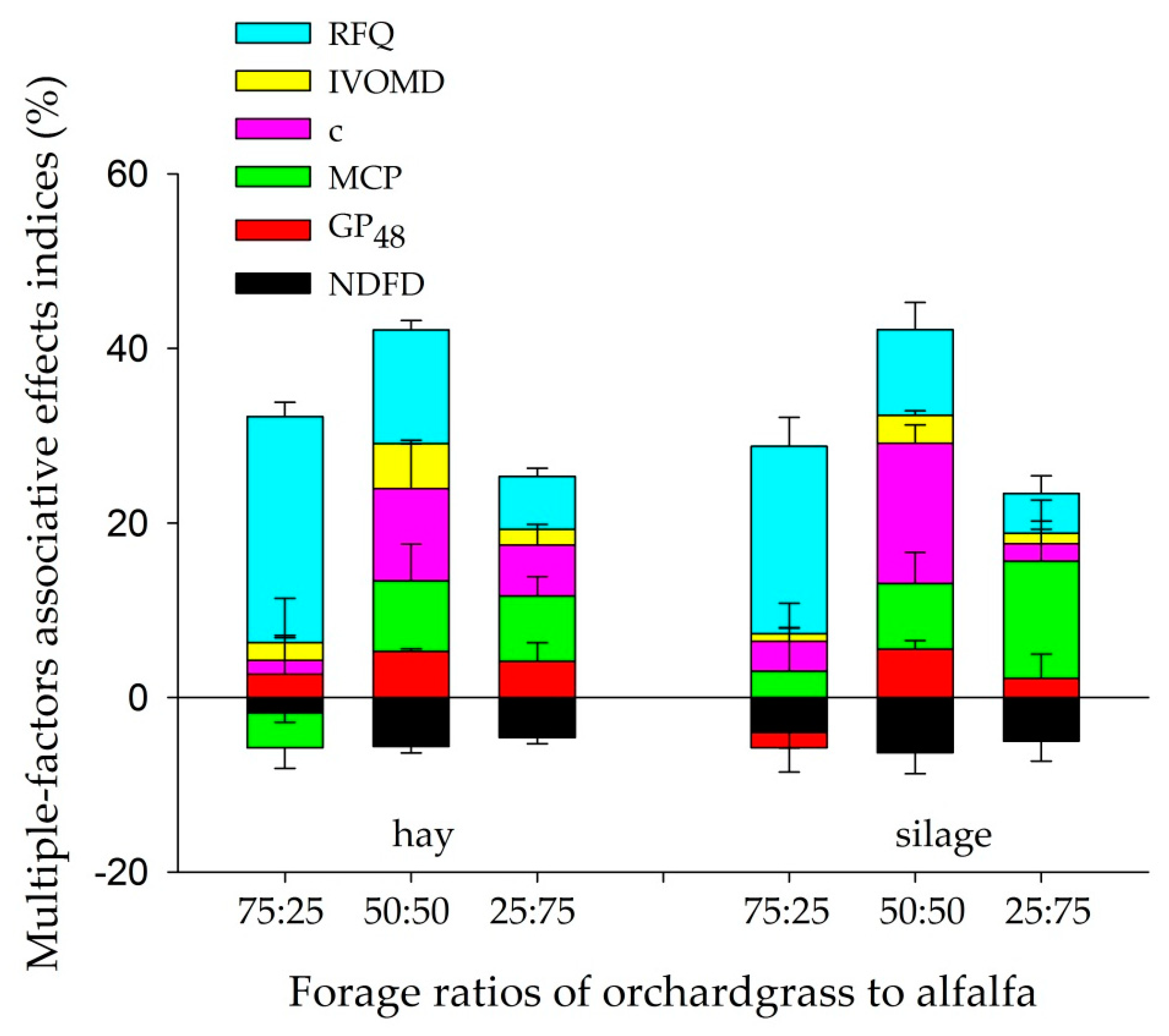
| Item | Orchardgrass | Alfalfa |
|---|---|---|
| Dry matter | 260 | 268 |
| Crude protein | 167 | 214 |
| Neutral detergent fiber | 562 | 484 |
| Acid detergent fiber | 424 | 385 |
| Acid detergent lignin | 37.0 | 72.2 |
| Ash | 111 | 92.0 |
| Ether extract | 26.8 | 24.0 |
| Calcium | 7.04 | 14.1 |
| Phosphorus | 3.42 | 3.36 |
| Potassium | 33.6 | 27.8 |
| Magnesium | 3.72 | 3.16 |
| Item | Forage Ratios of Orchardgrass to Alfalfa | SEM 2 | p3 | |||||
|---|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | Linear | Quadratic | ||
| pH | 4.50 | 4.83 | 4.75 | 5.03 | 4.96 | 0.049 | <0.001 | <0.001 |
| Ammonia N (mmol/L) | 5.36 | 7.96 | 9.96 | 11.2 | 11.1 | 0.585 | <0.001 | <0.001 |
| Lactic acid (mmol/L) | 963 | 998 | 969 | 754 | 608 | 50.7 | 0.004 | 0.005 |
| Acetic acid (mmol/L) | 1.58 | 2.55 | 7.54 | 7.88 | 8.63 | 0.860 | <0.001 | <0.001 |
| Propionic acid (mmol/L) | 0.38 | 0.30 | 0.29 | 0.31 | 0.14 | 0.023 | 0.002 | 0.005 |
| Butyric acid (mmol/L) | 0.12 | 0.10 | 0.13 | 0.14 | 0.15 | 0.008 | 0.081 | 0.159 |
| Flieg’s score 1 | 95.6 | 89.2 | 87.6 | 79.3 | 76.8 | 1.67 | <0.001 | <0.001 |
| Single-factor associative effects indices (%) of ensiling characteristics 4 | ||||||||
| pH | - | 4.6 | 0.5 | 3.8 | - | 0.76 | ||
| Ammonia N (mmol/L) | - | 17.0 | 20.6 | 15.4 | - | 4.09 | ||
| Lactic acid (mmol/L) | - | 14.2 | 23.4 | 8.2 | - | 5.76 | ||
| Acetic acid (mmol/L) | - | −23.7 | 12.8 | 14.8 | - | 10.2 | ||
| Propionic acid (mmol/L) | - | −6.4 | 11.0 | 51.1 | - | 11.93 | ||
| Item 1 | Preservation Method | Forage Ratios of Orchardgrass to Alfalfa | SEM 2 | p3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | Preservation Method | Linear | Quadratic | |||
| GP48 (mL/g DM) | Hay Silage | 122 124 | 128 124 | 133 136 | 134 134 | 131 134 | 1.1 | 0.847 | <0.001 | 0.026 |
| GP kinetics | ||||||||||
| A (mL/g DM) | Hay Silage | 123 124 | 128 124 | 134 136 | 135 134 | 131 134 | 1.1 | 0.837 | <0.001 | 0.025 |
| c | Hay Silage | 0.13 0.13 | 0.14 0.14 | 0.16 0.16 | 0.16 0.14 | 0.15 0.14 | 0.002 | 0.165 | 0.011 | 0.004 |
| Half-time (h) | Hay Silage | 2.71 2.72 | 2.69 2.66 | 2.55 2.57 | 2.60 2.65 | 2.60 2.65 | 0.012 | 0.339 | 0.005 | 0.005 |
| AGPR (mL/h) | Hay Silage | 23.4 24.2 | 25.1 24.1 | 28.4 28.4 | 29.2 28.5 | 29.8 29.1 | 0.37 | 0.319 | <0.001 | 0.025 |
| Fermentation gas pattern (mL/g OM digested) | ||||||||||
| CO2 | Hay Silage | 174 176 | 176 166 | 178 178 | 180 175 | 176 177 | 0.8 | 0.081 | 0.150 | 0.675 |
| CH4 | Hay Silage | 31.6 34.6 | 34.5 37.5 | 36.2 37.8 | 36.4 39.0 | 37.1 39.2 | 0.62 | 0.045 | 0.006 | 0.287 |
| H2 | Hay Silage | 0.59 2.00 | 0.36 0.84 | 0.82 0.94 | 0.76 0.66 | 1.51 0.40 | 0.121 | 0.476 | 0.513 | 0.164 |
| Item 1 | Preservation Method | Forage Ratios of Orchardgrass to Alfalfa | SEM 2 | p3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | Preservation method | Linear | Quadratic | |||
| Ammonia N (mg/dL) | Hay Silage | 20.0 20.0 | 20.0 20.2 | 20.4 21.2 | 21.5 21.7 | 21.5 21.8 | 0.31 | 0.635 | 0.029 | 0.996 |
| MCP (μg/mL) | Hay Silage | 270 282 | 276 296 | 329 315 | 345 338 | 338 303 | 5.2 | 0.555 | <0.001 | 0.034 |
| Total VFAs (mmol/L) | Hay Silage | 110 113 | 116 110 | 118 115 | 117 110 | 120 119 | 1.2 | 0.208 | 0.049 | 0.688 |
| VFA pattern (mmol/L) | ||||||||||
| Acetate | Hay Silage | 71.2 74.5 | 76.6 70.1 | 78.4 72.6 | 77.4 68.8 | 77.8 76.2 | 1.10 | 0.085 | 0.309 | 0.809 |
| Propionate | Hay Silage | 18.8 17.2 | 17.6 17.9 | 17.4 19.5 | 18.9 17.4 | 19.0 18.4 | 0.17 | 0.297 | 0.136 | 0.631 |
| Butyrate | Hay Silage | 9.09 9.45 | 9.76 9.56 | 9.51 9.82 | 9.33 10.3 | 10.2 10.4 | 0.31 | 0.620 | 0.300 | 0.887 |
| Isobutyrate | Hay Silage | 2.14 2.36 | 2.63 2.34 | 2.56 2.64 | 2.14 2.63 | 2.20 2.83 | 0.084 | 0.257 | 0.900 | 0.811 |
| Valerate | Hay Silage | 2.61 2.80 | 3.02 2.88 | 3.10 2.94 | 2.81 3.25 | 3.50 3.40 | 0.093 | 0.801 | 0.015 | 0.710 |
| Isovalerate | Hay Silage | 6.04 6.60 | 6.20 6.76 | 6.68 7.18 | 6.54 7.23 | 7.16 7.99 | 0.185 | 0.097 | 0.024 | 0.704 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Liu, N.; Wang, Y.; Yang, H.; Wei, Y.; Moriel, P.; Palmer, E.; Zhang, Y. Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay. Animals 2020, 10, 59. https://doi.org/10.3390/ani10010059
Xue Z, Liu N, Wang Y, Yang H, Wei Y, Moriel P, Palmer E, Zhang Y. Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay. Animals. 2020; 10(1):59. https://doi.org/10.3390/ani10010059
Chicago/Turabian StyleXue, Zhulin, Nan Liu, Yanlu Wang, Hongjian Yang, Yuqi Wei, Philipe Moriel, Elizabeth Palmer, and Yingjun Zhang. 2020. "Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay" Animals 10, no. 1: 59. https://doi.org/10.3390/ani10010059
APA StyleXue, Z., Liu, N., Wang, Y., Yang, H., Wei, Y., Moriel, P., Palmer, E., & Zhang, Y. (2020). Combining Orchardgrass and Alfalfa: Effects of Forage Ratios on In Vitro Rumen Degradation and Fermentation Characteristics of Silage Compared with Hay. Animals, 10(1), 59. https://doi.org/10.3390/ani10010059






