Social Referencing in the Domestic Horse
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Ethics Statement
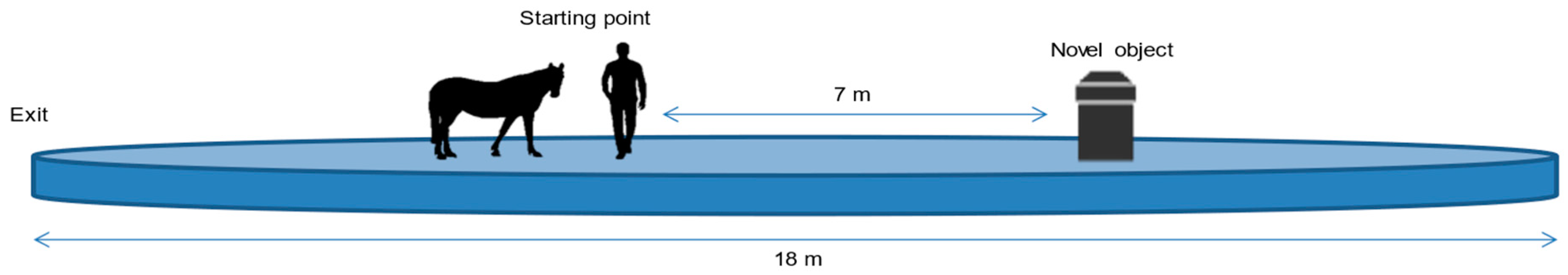
2.3. Procedure
2.4. Data Scoring and Analyses
3. Results
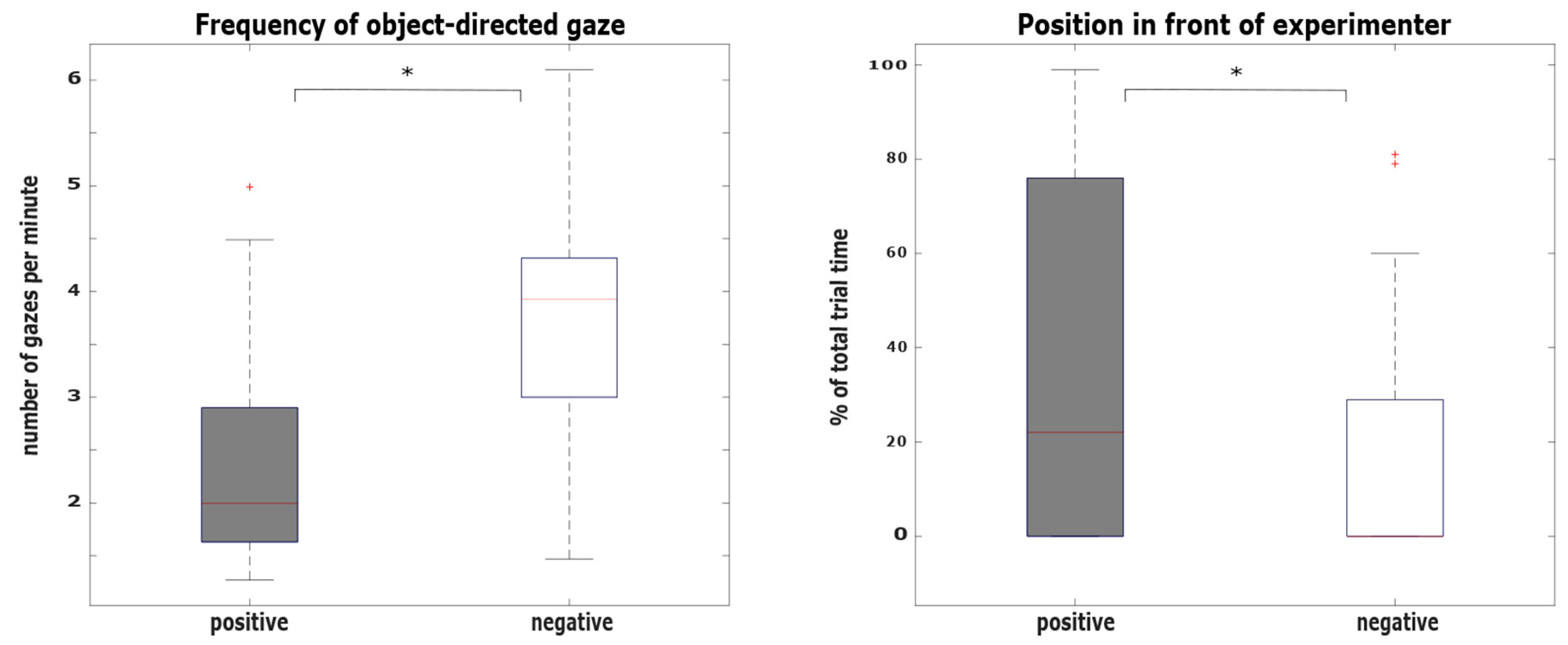
3.1. Hypothesis 1: Behavioral Differences between the Positive and the Negative Condition
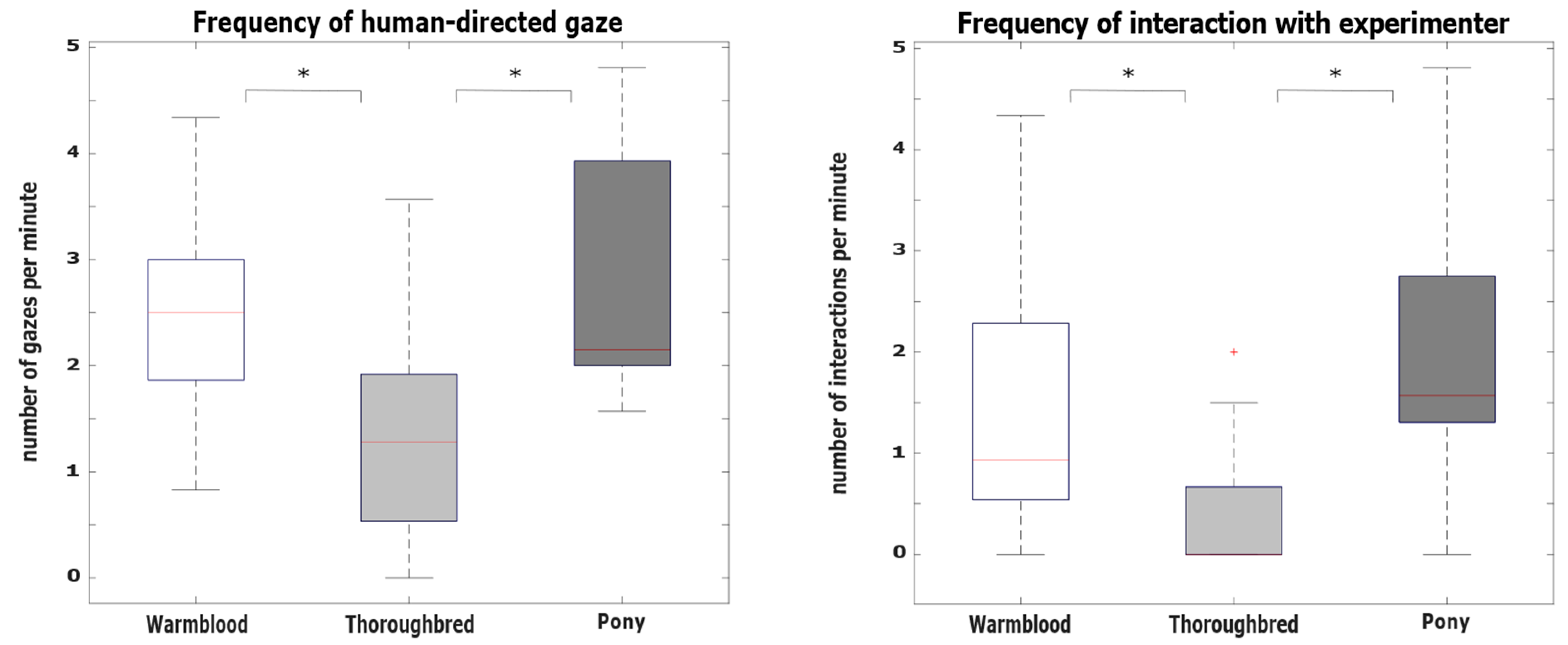
3.2. Hypothesis 2: Behavioral Differences between Breeds
3.3. Behavioral Differences between Sexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Outram, A.K.; Stear, N.A.; Bendrey, R.; Olsen, S.; Kasparov, A.; Zaibert, V.; Thorpe, N.; Evershed, R.P. The earliest horse harnessing and milking. Science 2009, 323, 1332–1335. [Google Scholar] [CrossRef]
- Schubert, M.; Jónsson, H.; Chang, D.; Der Sarkissian, C.; Ermini, L.; Ginolhac, A.; Albrechtsen, A.; Dupanloup, I.; Foucal, A.; Petersen, B.; et al. Prehistoric genomes reveal the genetic foundation and cost of horse domestication. Proc. Natl. Acad. Sci. USA 2014, 111, E5661–E5669. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Udell, M.A. Cognition and learning in horses (Equus caballus): What we know and why we should ask more. Behav. Process. 2016, 126, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Roche, H.; Henry, S.; Visser, E.K. A review of the human–horse relationship. Appl. Anim. Behav. Sci. 2008, 109, 1–24. [Google Scholar] [CrossRef]
- Feinman, S. Social Referencing in Infancy. Merrill-Palmer Q. 1982, 28, 445–470. [Google Scholar]
- Walden, T.A.; Ogan, T.A. The development of social referencing. Child. Dev. 1988, 59, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Klinnert, M.D.; Emde, R.N.; Butterfield, P.; Campos, J.J. Social referencing: The infant’s use of emotional signals from a friendly adult with mother present. Dev. Psychol. 1986, 22, 427–432. [Google Scholar] [CrossRef]
- Hoehl, S.; Wiese, L.; Striano, T. Young infants’ neural processing of objects is affected by eye gaze direction and emotional expression. PLoS ONE 2008, 3. [Google Scholar] [CrossRef]
- Frank, H. Evolution of canine information processing under conditions of natural and artificial selection. Zeitschrift Für Tierpsychologie 1980, 53, 389–399. [Google Scholar] [CrossRef]
- Hare, B.; Rosati, A.; Kaminski, J.; Bräuer, J.; Call, J.; Tomasello, M. The domestication hypothesis for dogs’ skills with human communication: A response to Udell et al. (2008) and Wynne et al. (2008). Anim. Behav. 2010, 79, e1–e6. [Google Scholar] [CrossRef]
- Riedel, J.; Schumann, K.; Kaminski, J.; Call, J.; Tomasello, M. The early ontogeny of human-dog communication. Anim. Behav. 2008, 75, 1003–1014. [Google Scholar] [CrossRef]
- Soproni, K.; Miklósi, Á.; Topál, J.; Csányi, V. Dogs’ (Canis familiaris) responsiveness to human pointing gestures. J. Comp. Psychol. 2002, 116, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gácsi, M.; Miklód, Á.; Varga, O.; Topál, J.; Csányi, V. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human’s attention. Anim. Cognit. 2004, 7, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chijiiwa, H.; Kuroshima, H.; Hori, Y.; Anderson, J.R.; Fujita, K. Dogs avoid people who behave negatively to their owner: Third-party affective evaluation. Anim. Behav. 2015, 106, 123–127. [Google Scholar] [CrossRef]
- Kundey, S.M.A.; de Los Reyes, A.; Royer, E.; Molina, S.; Monnier, B.; German, R.; Coshun, A. Reputation-like inference in domestic dogs (Canis familiaris). Anim. Cognit. 2011, 14, 291–302. [Google Scholar] [CrossRef]
- Duranton, C.; Bedossa, T.; Gaunet, F. When facing an unfamiliar person, pet dogs present social referencing based on their owners’ direction of movement alone. Anim. Behav. 2016, 113, 147–156. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Social referencing in dog-owner dyads? Anim. Cognit. 2012, 15, 175–185. [Google Scholar] [CrossRef]
- Buttelmann, D.; Tomasello, M. Can domestic dogs (Canis familiaris) use referential emotional expressions to locate hidden food? Anim. Cognit. 2013, 16, 137–145. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Lazzaroni, M.; Marshall-Pescini, S. Dogs’ comprehension of referential emotional expressions: Familiar people and familiar emotions are easier. Anim. Cognit. 2014, 17, 373–385. [Google Scholar] [CrossRef]
- Yong, M.H.; Ruffman, T. Is that fear? Domestic dogs’ use of social referencing signals from an unfamiliar person. Behav. Process. 2015, 110, 74–81. [Google Scholar] [CrossRef]
- Miklósi, Á.; Pongrácz, P.; Lakatos, G.; Topál, J.; Csányi, V. A comparative study of the use of visual communicative signals in interactions between dogs (Canis familiaris) and humans and cats (Felis catus) and humans. J. Comp. Psychol. 2005, 119, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Galvan, M.; Vonk, J. Man’s other best friend: Domestic cats (F. silvestris catus) and their discrimination of human emotion cues. Anim. Cognit. 2016, 19, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Merola, I.; Lazzaroni, M.; Marshall-Pescini, S.; Prato-Previde, E. Social referencing and cat–human communication. Anim. Cognit. 2015, 18, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Flauger, B.; Farmer, K.; Maros, K. Horses (Equus caballus) use human local enhancement cues and adjust to human attention. Anim. Cognit. 2011, 14, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, A.; Hori, Y.; Fujita, K. Horses (Equus caballus) adaptively change the modality of their begging behavior as a function of human attentional states. Psychologia 2016, 59, 100–111. [Google Scholar] [CrossRef]
- Proops, L.; McComb, K. Attributing attention: The use of human-given cues by domestic horses (Equus caballus). Anim. Cognit. 2010, 13, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Sankey, C.; Henry, S.; André, N.; Richard-Yris, M.-A.; Hausberger, M. Do horses have a concept of person? PLoS ONE 2011, 6, 18331. [Google Scholar] [CrossRef]
- Maros, K.; Gácsi, M.; Miklósi, Á. Comprehension of human pointing gestures in horses (Equus caballus). Anim. Cognit. 2008, 11, 457–466. [Google Scholar] [CrossRef]
- Proops, L.; Walton, M.; McComb, K. The use of human-given cues by domestic horses, Equus caballus, during an object choice task. Anim. Behav. 2010, 79, 1205–1209. [Google Scholar] [CrossRef]
- Prendergast, A.; Nansen, C.; Blache, D. Responses of domestic horses and ponies to single, combined and conflicting visual and auditory cues. J. Equine Vet. Sci. 2016, 46, 40–46. [Google Scholar] [CrossRef]
- Proops, L.; Rayner, J.; Taylor, A.M.; McComb, K. The responses of young domestic horses to human-given cues. PLoS ONE 2013, 8, 67000. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, R.; Huber, L. Evidence of heterospecific referential communication from domestic horses (Equus caballus) to humans. Anim. Cognit. 2016, 19, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ringhofer, M.; Yamamoto, S. Domestic horses send signals to humans when they face with an unsolvable task. Anim. Cognit. 2017, 20, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Proops, L.; Mccomb, K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. R. Soc. B Biol. Sci. 2012, 279, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; McComb, K. Functionally relevant responses to human facial expressions of emotion in the domestic horse (Equus caballus). Biol. Lett. 2016, 12, 20150907. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, T. Can horses read emotional cues from human faces? Re-analysis of Smith et al. Biol. Lett. 2016, 12, 20160201. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.V.; Wilson, C.; McComb, K.; Proops, L. Domestic horses (Equus caballus) prefer to approach humans displaying a submissive body posture rather than a dominant body posture. Anim. Cognit. 2018, 21, 307–312. [Google Scholar] [CrossRef]
- Smith, A.V.; Proops, L.; Grounds, K.; Wathan, J.; Scott, S.K.; McComb, K. Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalisations. Sci Rep. 2018, 8, 13052. [Google Scholar] [CrossRef]
- Nakamura, K.; Takimoto-Inose, A.; Hasegawa, T. Cross-modal perception of human emotion in domestic horses (Equus caballus). Sci. Rep. 2018, 8, 8660. [Google Scholar] [CrossRef]
- Lloyd, A.S.; Martin, J.E.; Bornett-Gauci, H.L.I.; Wilkinson, R.G. Horse personality: Variation between breeds. Appl. Anim. Behav. Sci. 2008, 112, 369–383. [Google Scholar] [CrossRef]
- Gu, J.; Orr, N.; Park, S.D.; Katz, L.M.; Sulimova, G.; MacHugh, D.E.; Hill, E.W. A genome scan for positive selection in thoroughbred horses. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Górecka-Bruzda, A.; Jastrzebska, E.; Sosnowska, Z.; Jaworski, Z.; Jezierski, T.; Chruszczewski, M.H. Reactivity to humans and fearfulness tests: Field validation in Polish Cold Blood Horses. Appl. Anim. Behav. Sci. 2011, 133, 207–215. [Google Scholar] [CrossRef]
- Henriksson, J.; Sauveroche, M.; Roth, L.S.V. Effects of size and personality on social learning and human-directed behaviour in horses (Equus caballus). Anim. Cognit. 2019, 22, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Budzyńska, M.; Kamieniak, J.; Marciniak, B.; Sołtys, L. Relationships between thoroughbreds’ contribution in the pedigree and the level of fearfulness and performance in warmblood stallions. Acta Vet. 2018, 68, 288–300. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Dogs’ social referencing towards owners and strangers. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Duberstein, K.J.; Gilkeson, J.A. Determination of sex differences in personality and trainability of yearling horses utilizing a handler questionnaire. Appl. Anim. Behav. Sci. 2010, 128, 57–63. [Google Scholar] [CrossRef]
- Wulf, M.; Aurich, J.; May, A.C.; Aurich, C. Sex differences in the response of yearling horses to handling by unfamiliar humans. J. Vet. Behav. Clin. Appl. Res. 2013, 8, 238–244. [Google Scholar] [CrossRef]
- ASAB/ABS. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 159, 201–309. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Stenberg, G.; Hagekull, B. Social referencing and mood modification in 1-year-olds. Infant Behav. Dev. 1997, 20, 209–217. [Google Scholar] [CrossRef]
- Goodwin, D. Horse Behaviour: Evolution, Domestication and Feralisation. In The Welfare of Horses; Springer: Dordrecht, The Netherlands, 2007; pp. 1–18. [Google Scholar] [CrossRef]
- Leiner, L.; Fendt, M. Behavioural fear and heart rate responses of horses after exposure to novel objects: Effects of habituation. Appl. Anim. Behav. Sci. 2011, 131, 104–109. [Google Scholar] [CrossRef]
- Christensen, J.W.; Keeling, L.J.; Nielsen, B.L. Responses of horses to novel visual, olfactory and auditory stimuli. Appl. Anim. Behav. Sci. 2005, 93, 53–65. [Google Scholar] [CrossRef]
- Baba, C.; Kawai, M.; Takimoto-Inose, A. Are horses (Equus caballus) sensitive to human emotional cues? Animals 2019, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Oddie, C.; Burton, F.L.; McLean, A.N. The horse-human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet. J. 2009, 181, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, M.; Muller, C. A brief note on some possible factors involved in the reactions of horses to humans. Appl. Anim. Behav. Sci. 2002, 76, 339–344. [Google Scholar] [CrossRef]
- Hausberger, M.; Bruderer, C.; Scolan, N.L.e.; Pierre, J.S. Interplay between environmental and genetic factors in temperament/personality traits in horses (Equus caballus). J. Comp. Psychol. 2004, 118, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Sackman, J.E.; Houpt, K.A. Equine personality: Association with breed, use, and husbandry factors. J. Equine Vet. Sci. 2019, 72, 47–55. [Google Scholar] [CrossRef]
- Hori, Y.; Ozaki, T.; Yamada, Y.; Tozaki, T.; Kim, H.S.; Takimoto, A.; Endo, M.; Manabe, N.; Inoue-Murayama, M.; Fujita, K. Breed differences in dopamine receptor D4 gene (DRD4) in horses. J. Equine Vet. Sci. 2013, 24, 31–36. [Google Scholar] [CrossRef]
- Minero, M.; Dalla Costa, E.; Dai, F.; Canali, E.; Barbieri, S.; Zanella, A.; Pascuzzo, R.; Wemelsfelder, F. Using qualitative behaviour assessment (QBA) to explore the emotional state of horses and its association with human-animal relationship. Appl. Anim. Behav. Sci. 2018, 204, 53–59. [Google Scholar] [CrossRef]
- Lansade, L.; Philippon, P.; Hervé, L.; Vidament, M. Development of personality tests to use in the field, stable over time and across situations, and linked to horses’ show jumping performance. J. Equine Vet. Sci. 2016, 176, 43–51. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C. Differences in motor laterality between breeds of performance horse. J. Equine Vet. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- Momozawa, Y.; Terada, M.; Sato, F.; Kikusui, T.; Takeuchi, Y.; Kusunose, R.; Mori, Y. Assessing equine anxiety-related parameters using an isolation test in combination with a questionnaire survey. J. Vet. Med. Sci. 2007, 69, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Aurich, J.; Möstl, E.; Müller, J.; Aurich, C. Changes in cortisol release and heart rate and heart rate variability during the initial training of 3-year-old sport horses. Horm. Behav. 2010, 58, 628–636. [Google Scholar] [CrossRef]
- Le Scolan, N.; Hausberger, M.; Wolff, A. Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behav. Process. 1997, 41, 257–266. [Google Scholar] [CrossRef]
- Wolff, A.; Hausberger, M.; Le Scolan, N. Experimental tests to assess emotionality in horses. Behav. Process. 1997, 40, 209–221. [Google Scholar] [CrossRef]
- Dai, F.; Cogi, N.H.; Heinzl, E.U.L.; Dalla Costa, E.; Canali, E.; Minero, M. Validation of a fear test in sport horses using infrared thermography. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 128–136. [Google Scholar] [CrossRef]
- Hedberg, Y.; Dalin, A.M.; Öhagen, P.; Holm, K.R.; Kindahl, H. Effect of oestrous-cycle stage on the response of mares in a novel object test and isolation test. Reprod. Domest. Anim. 2005, 40, 480–488. [Google Scholar] [CrossRef]
- Ijichi, C.; Griffin, K.; Squibb, K.; Favier, R. Stranger danger? An investigation into the influence of human-horse bond on stress and behaviour. Appl. Anim. Behav. Sci. 2018, 206, 59–63. [Google Scholar] [CrossRef]
- McGreevy, P.D. Equine Behaviour: A Guide for Veterinarians and Equine Scientists; Elsevier Limited. W.B.: Saunders, UK, 2004. [Google Scholar]
| Full Sample n = 46 | Positive Condition n = 22 | Negative Condition n = 24 | |
|---|---|---|---|
| Age | 11.11 ± 7.2 (1–26) | 11.32 ± 6.8 (2–23) | 10.92 ± 7.8 (1–26) |
| Male | 58.7% (28) | 68.2% (15) | 54.2% (13) |
| Female | 39.1% (18) | 31.8% (7) | 45.8% (11) |
| Warmbloods | 43.5% (20) | 40.9% (9) | 45.8% (11) |
| Thoroughbreds | 41.3% (19) | 45.5% (10) | 37.5% (9) |
| Ponies | 15.2% (7) | 13.6% (3) | 16.7% (4) |
| Gaze | ||
| Gaze human | Frequency | Number of horse’s head orientations towards human |
| Gaze object | Frequency | Number of horse’s head orientations towards object |
| Interaction | ||
| Interaction human | Frequency | Number of horse’s physical contacts with human |
| Interaction object | Frequency | Number of horse’s physical contacts with object |
| Position | ||
| Behind experimenter | Duration | Total time of horse’s position between the experimenter and close to the door, farthest from object |
| Abreast to experimenter | Duration | Total time of horse’s position next to experimenter between door and object |
| In front of experimenter | Duration | Total time of horse’s position between the experimenter and the object |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrimpf, A.; Single, M.-S.; Nawroth, C. Social Referencing in the Domestic Horse. Animals 2020, 10, 164. https://doi.org/10.3390/ani10010164
Schrimpf A, Single M-S, Nawroth C. Social Referencing in the Domestic Horse. Animals. 2020; 10(1):164. https://doi.org/10.3390/ani10010164
Chicago/Turabian StyleSchrimpf, Anne, Marie-Sophie Single, and Christian Nawroth. 2020. "Social Referencing in the Domestic Horse" Animals 10, no. 1: 164. https://doi.org/10.3390/ani10010164
APA StyleSchrimpf, A., Single, M.-S., & Nawroth, C. (2020). Social Referencing in the Domestic Horse. Animals, 10(1), 164. https://doi.org/10.3390/ani10010164





