1. Introduction
The parity of the sow can be an important factor influencing the growth and survival of piglets. Progeny of first-parity sows can be lighter at birth [
1] and later in life [
2], and they grow slowly compared with progeny of multiparous sows [
1,
3]. Gilts are also more prone to farrowing stress and more susceptible to pathogens, and they show a lower capacity to convert energy into milk production compared to multiparous sows [
4]. The colostrum quantity and quality may contribute to differences in progeny of first-parity sows compared with multiparous sows. Organic components of colostrum (proteins, fats, and carbohydrates) provide energy to the newborn; furthermore, colostrum is rich in antibodies, immunoglobulins (Igs), leukocytes, selenium, vitamin E, and oligosaccharides which are essential for immune function of the piglets [
4,
5,
6,
7] and for the establishment of the microbiota in piglets’ intestine [
8], which contribute to intestinal immune system development [
9,
10].
The effect of parity number on swine colostrum composition was mainly investigated in terms of immunoglobulin, fat, protein, lactose, and net energy concentrations [
11,
12]. Recently, the development of new technological tools allowed the description of the colostrum and milk metabolomics profile, defined as the comprehensive characterization and quantification of metabolites, which may have a bioactive and beneficial effect on the neonates [
13,
14]. Few studies investigated the swine colostrum metabolome composition [
15,
16,
17] and the relationship between colostrum metabolites and sows’ productive traits [
17,
18]. Recently, it was observed that sows’ parity number may influence the colostrum’s lipid composition in terms of free fatty acid (FFA) and de novo fatty acids (FA) related to the energy metabolism (mainly the C18 FA series) [
16]. Therefore, it was hypothesized that the colostrum metabolomics profile of different parity sows could differ due to the different degree of maturation and energy conversions of the sows, and this may help to explain the sows’ productive traits. For the purpose of this study, production traits included the sow’s number of piglets (born alive, stillborn), their weight, and their survival to 10 days of age. These production variables indicate the economic performance of a sow during its stay in the herd, and they are important reference points when making breeding and culling decisions.
Therefore, the aims of the present study were (i) to characterize the metabolomics profile of swine colostrum of different parity sows (from 1–4), (ii) to correlate the metabolomics profile with the Brix percentage estimate of colostrum immunoglobulin G (IgG) concentration, and (iii) to evaluate the association between the colostrum metabolomics profile and sow productive traits.
4. Discussion
In the present study, the
1H-NMR technique allowed the identification of a total of 33 compounds, some of which were previously identified in swine colostrum using the same technique [
17]. In addition to the compounds identified by Picone et al. [
17], in the present study, 12 new compounds were identified, including several amino acids (glycine, leucine, threonine, tyrosine, valine, and phenylalanine), nucleotides (uracil), nucleoside (inosine), organic acid (
trans-aconitate), and additional functional compounds such as betaine,
O-phosphocholine, and
O-acetylcarnitine, while glycolate,
N-acetylglutamate, succinate, and uridine diphosphate (UDP)-glucose were not identified. Differences in the detected compounds may be due to the difference in the animals’ health status, in the animals’ diet and management, and in animal genetics among studies. The
1H-NMR technique represents one of the most sensitive techniques that allows detection of metabolites in bio-fluids with high sensitivity, and it is quantitative, highly reproducible, and straightforward to use [
29,
30]. The present study investigated if the parity of the sow can influence the metabolomics profile of swine defatted colostrum and explain differences in the productive performance.
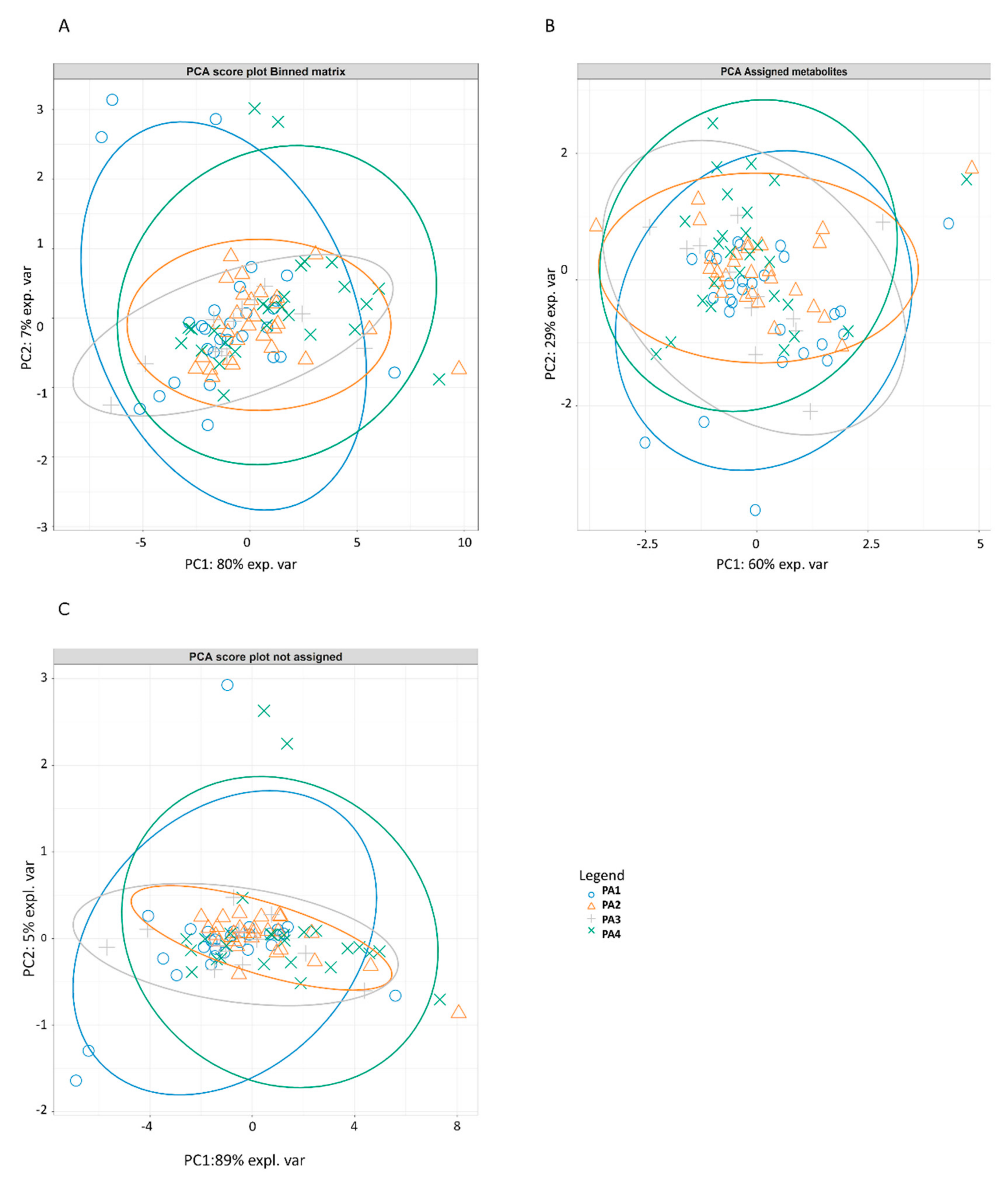
The characterization of the colostrum metabolomics profile showed that colostrum soluble compounds were only slightly affected by the sow’s parity number; indeed, poorly separated clusters due to parity number were observed using the PCA analysis, both in the whole metabolomics matrix (identified and unidentified compounds) and in the matrix composed only of identified compounds. The results of the PCAs report that, although parity is not a factor capable of strongly influencing the composition of the defatted colostrum, a part of the spectrum located in the midfield region between 3.713 and 3.874 ppm, which explains 6% of the variance, still remains to be investigated for the identification of compounds distinguishing the colostrum matrix.
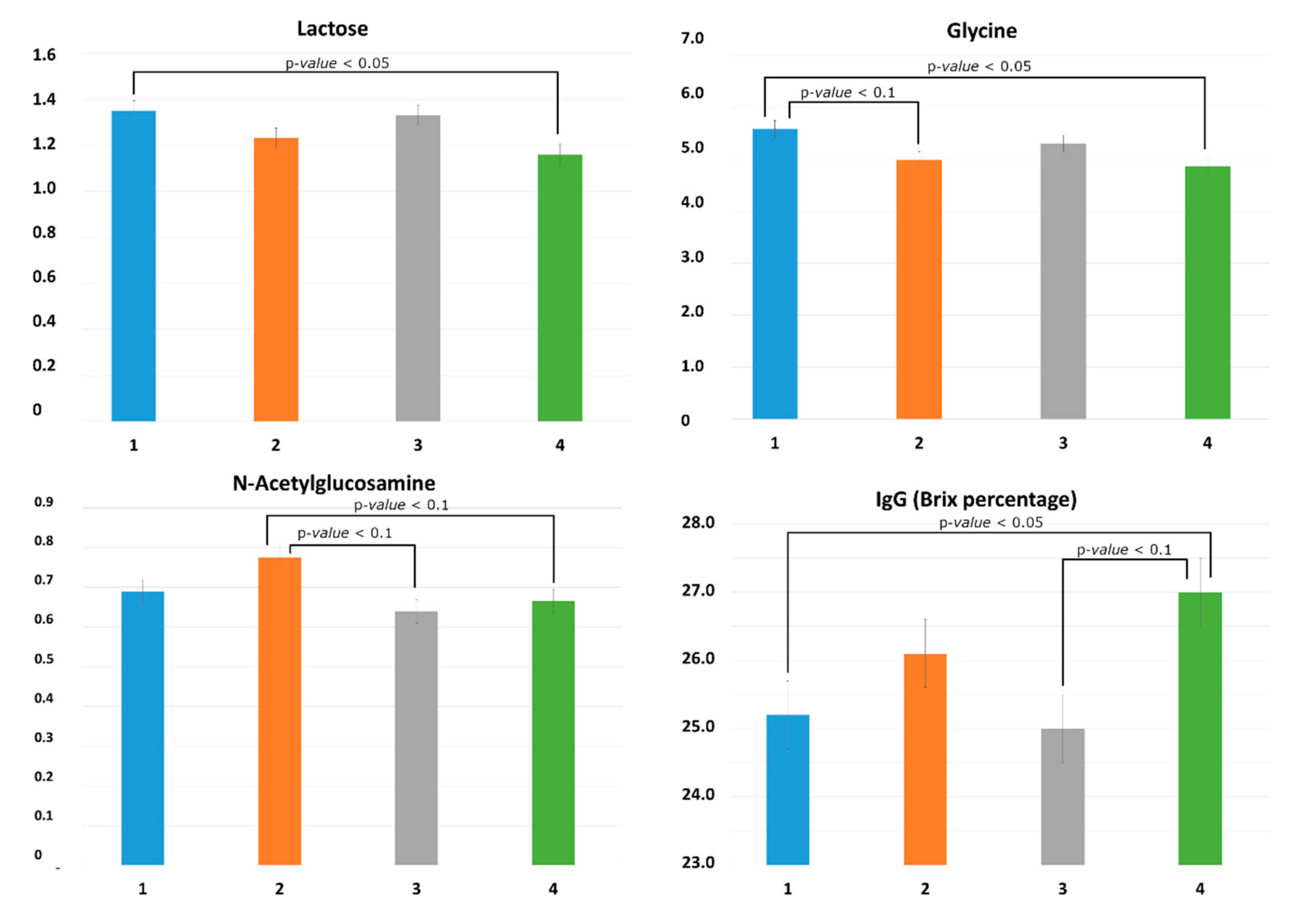
Although the parity did not strongly influence the metabolomics profile of colostrum, it significantly influenced the quantity of some compounds including lactose, glycine, and GlcNAc. Lactose quantity is generally considered stable in colostrum and milk. Previous studies did not detect differences in lactose amount among different parities [
11,
31]. In the present study, the amount of lactose was lower in the fourth-parity sows compared to gilts, which is consistent with a previous report in mature milk [
11]. Lactose is generally associated with the total amount of produced and suckled milk, as it is the principal osmotic compound in the mammary secretions [
32] and its synthesis by mammary epithelial cells is known to be stimulated by prolactin and inhibited by progesterone. Previous studies suggested that colostrum yield can vary across parity [
33,
34]. However, in the present study, colostrum yield was not measured; thus, the difference in lactose content among parities cannot be directly associated to colostrum quantity, and this hypothesis should be further investigated. Similarly to lactose, glycine content was also higher in gilts’ colostrum compared to fourth-parity sows. Glycine was recently related to a negative maternal energy balance in cows [
35]. In the present study, the sows’ energy balance was not assessed; however, it is well known that gilts have a more negative energy balance compared to multiparous sows [
36,
37], which may explain the higher level of glycine in gilt colostrum.
As expected, the colostrum of gilts had a lower Brix percentage estimate of IgG concentration compared with fourth-parity sows, which is consistent with previous reports [
12,
38]. However, the variability in Brix percentage estimate of IgG explained by the sows’ parity disappeared when the statistical model was fitted including the metabolites. In fact, the Brix percentage estimate of IgG was negatively associated with the level of lactose and positively associated with the level of
myo-inositol, creatine, and
O-phosphocholine. No previous studies investigated the relationship between IgG and other metabolite concentrations in swine colostrum, and scarce information on those metabolites is available.
Lactose is the principal osmotic compound in colostrum and milk. Since a higher milk/colostrum yield was associated with a higher lactose content [
34], it may result in a dilution of the Brix percentage estimate of IgG concentration in colostrum observed in the present study. However, since no information in colostrum yield is available in the present study, further investigation should be made to confirm the hypothesis. In addition, it was reported that the level of lactose in colostrum/milk negatively influenced the piglets’ absorption of IgG and favored gut closure, which occurs within 24–36 h of birth [
39]. The discussed hypothesis cannot be assessed in the present study and does not explain the negative relationship between lactose and Brix percentage estimate of IgG. Thus, further investigations are needed to clarify the IgG and lactose relationship in swine colostrum.
In contrast, creatine,
myo-inositol, and
O-phosphocholine were positively correlated with the Brix percentage estimate of IgG. No previous information was reported on the relationship between these compounds and the IgG content; nevertheless, beneficial proprieties were attributed to these compounds. The presence of creatine in swine colostrum and milk, as well as its importance for newborn development and maturation, is well documented [
17,
40,
41,
42]. Furthermore, it is known that creatine kinase, an enzyme that catalyzes the production of creatine, can create a macromolecular complex with IgG (IgG-CK) [
43], which may explain the association between IgG percentage and creatine amount observed in the present study; however, further elucidation is needed.
O-Phosphocholine, as a form of storage for choline, is considered a precursor for the biosynthesis of phospholipids (phosphatidylcholine and sphingomyelin), which are essential structural components of all cellular membranes [
44].
myo-Inositol, a glucose derivative generally known also as inositol, is widely used as a nutritional supplement in human preterm infants for its critical role in fetal and early neonatal life, especially for lung development [
45]. Furthermore, a genetic effect was seen for creatine and
myo-inositol in sow colostrum, with Duroc sows having lower values than Landrace and Large White sows [
17].
In the present study, metabolites identified in colostrum were associated with sow performance at farrowing and 10 days post farrowing. Although the effect of sow parity and colostrum composition on piglet performance at 10 days should be interpreted with caution due to the intense cross-fostering, the
myo-inositol in colostrum was positively associated with piglet mortality at day 10 which was not expected considering the positive properties that this compound has during neonatal life. However, since a higher level of
myo-inositol was found in human colostrum of pre-term parturition compared to normal parturition [
46], this may suggest that the increased
myo-inositol secretion in colostrum might reflect the physiological state and the immaturity of the mammary gland and the neonate immaturity, which would indirectly explain the positive association between
myo-inositol and the mortality observed in our study. In humans, the
myo-inositol quantity decreases from colostrum to milk [
46], while no information was reported in colostrum/milk transition of sows. Thus, further studies on
myo-inositol concentration across lactation are needed, to also confirm the association between
myo-inositol and piglet mortality.
Piglet mortality at day 10 was reduced by increasing levels of colostrum GlcNAc. Information on the role that the amino sugar GlcNAc plays in neonates is scarce; however, its presence in colostrum and milk was previously reported [
17,
47]. It is known that GlcNAc has important structural roles at the cell surface level (i.e., bacterial cells, fungal cells, and the extracellular matrix of animal cells) and it also exerts cell signaling activities [
48]. These properties may explain its positive effect in reducing piglet mortality; however, further studies to explain these mechanisms more thoroughly are needed.
The litter weight at birth was affected by the litter size, which was an expected result, but it was also positively associated with the taurine concentration in colostrum. Taurine is one of the main free amino acids in colostrum, and it is known to improve newborn brain development [
49]. In the study of Picone et al. [
17], taurine concentration in colostrum was associated with a lower number of dead piglets at three days; in the present study, mortality was not recorded at three days, and taurine did not affect the mortality at 10 days; thus, results cannot be compared. The result for taurine observed in the present study should be related to the metabolism of the sow rather than to the function of taurine in the neonates. Taurine in colostrum is derived from the diet or can be biosynthesized from methionine and cysteine metabolism in the liver and in other tissues, including the oviduct epithelial cells [
50]. Our results suggest that sows having colostrum richer in taurine also had a heavier litter at birth, resulting in better productive traits, and this may reflect a better health status of the sow. Further elucidations on the role that the identified compounds have in piglets’ metabolism and health are desirable. Furthermore, the relevance of sow metabolic condition for colostrum quality is also indicated by the association of backfat of the sow at farrowing with the concentration of IgA in colostrum, when equalized for the parity number of the sow [
51]. Thus, the relationship between sows’ energy balance and colostrum metabolomics composition deserves further investigations in order to improve sows’ management and, consequently, the colostrum quality.