Bioavailability of Organic Phosphorus Compounds to the Harmful Dinoflagellate Karenia mikimotoi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Growth Conditions
2.2. Cell Counts and Fv/Fm Analyses
2.3. Nutrient Analyses
2.4. Alkaline Phosphatase (APase) Activity and PDEase Activity Analyses
2.5. Pigment Analyses
2.6. Hemolytic Activity Analyses
2.7. Data Analysis
3. Results
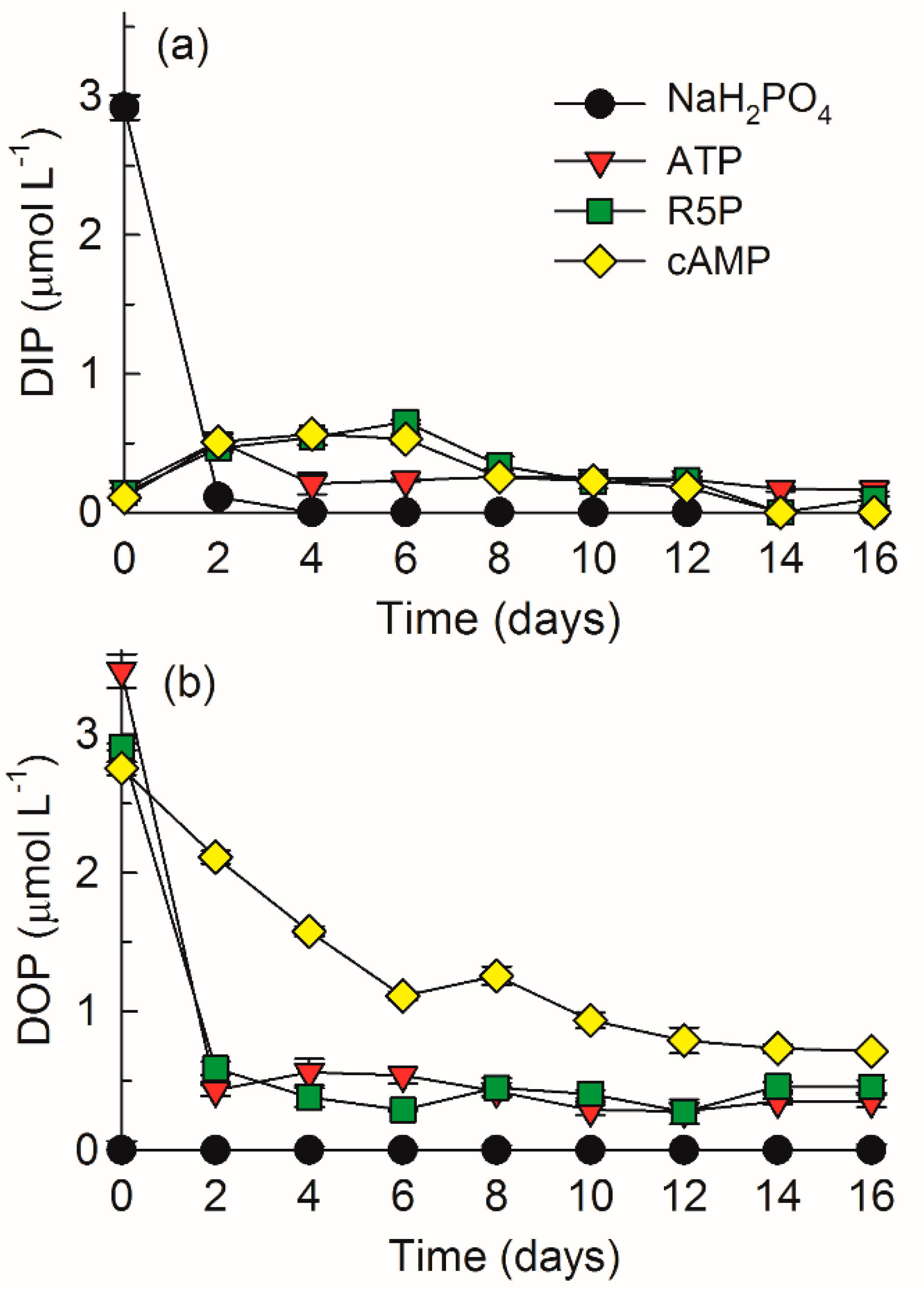
3.1. DIP and DOP Variations
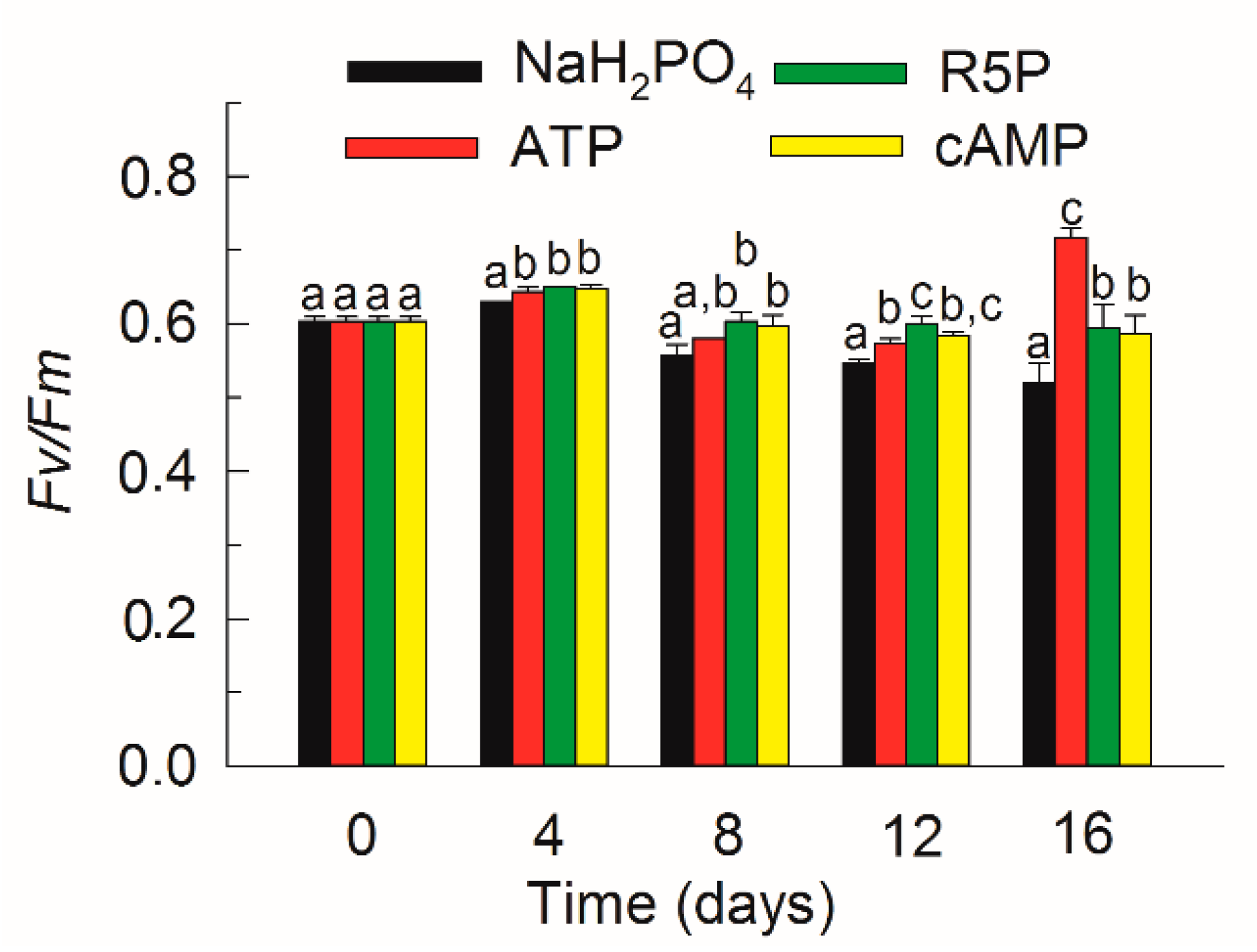
3.2. Growth and Fv/Fm
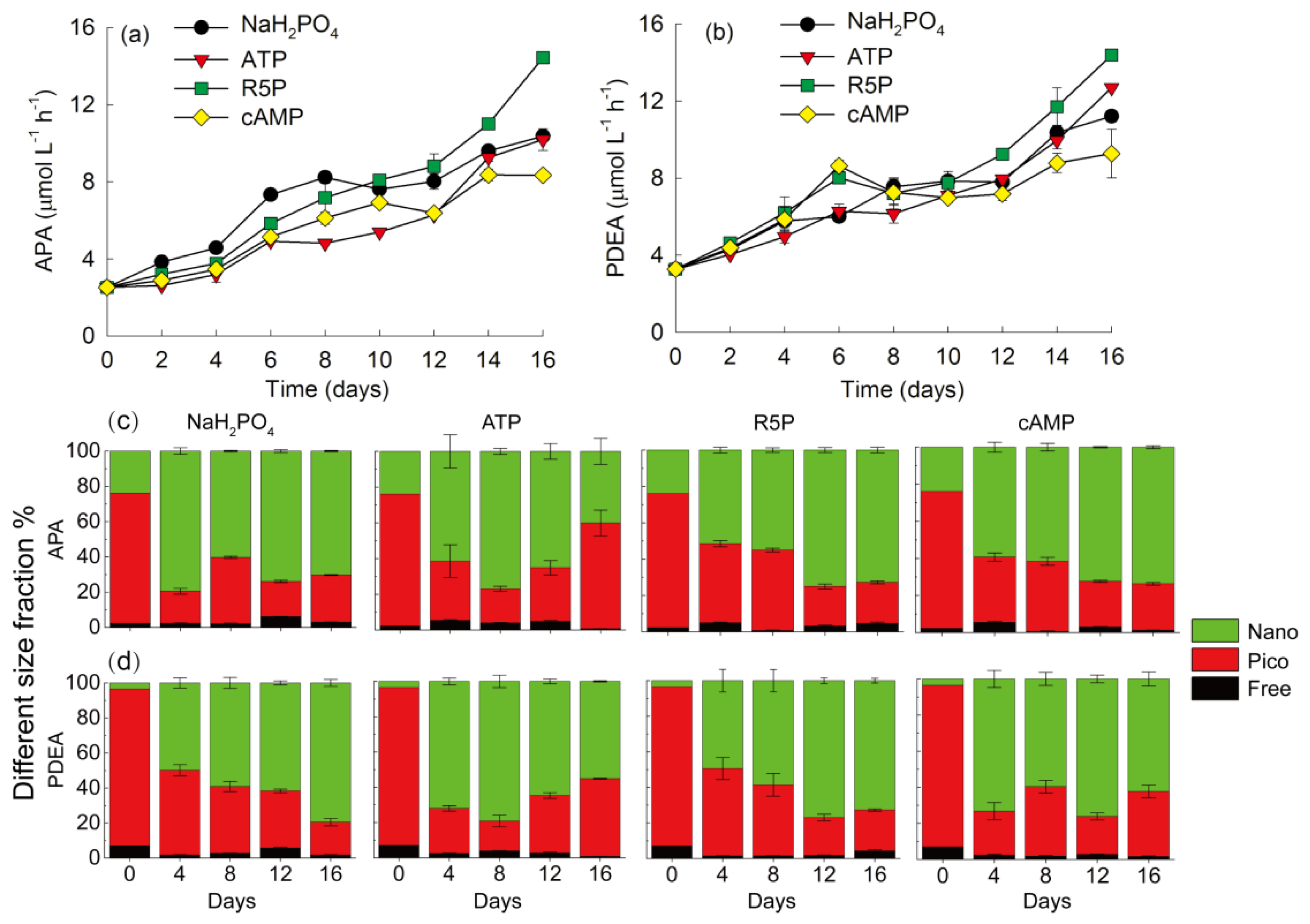
3.3. APA and PDEA
3.4. Particulate Nutrients
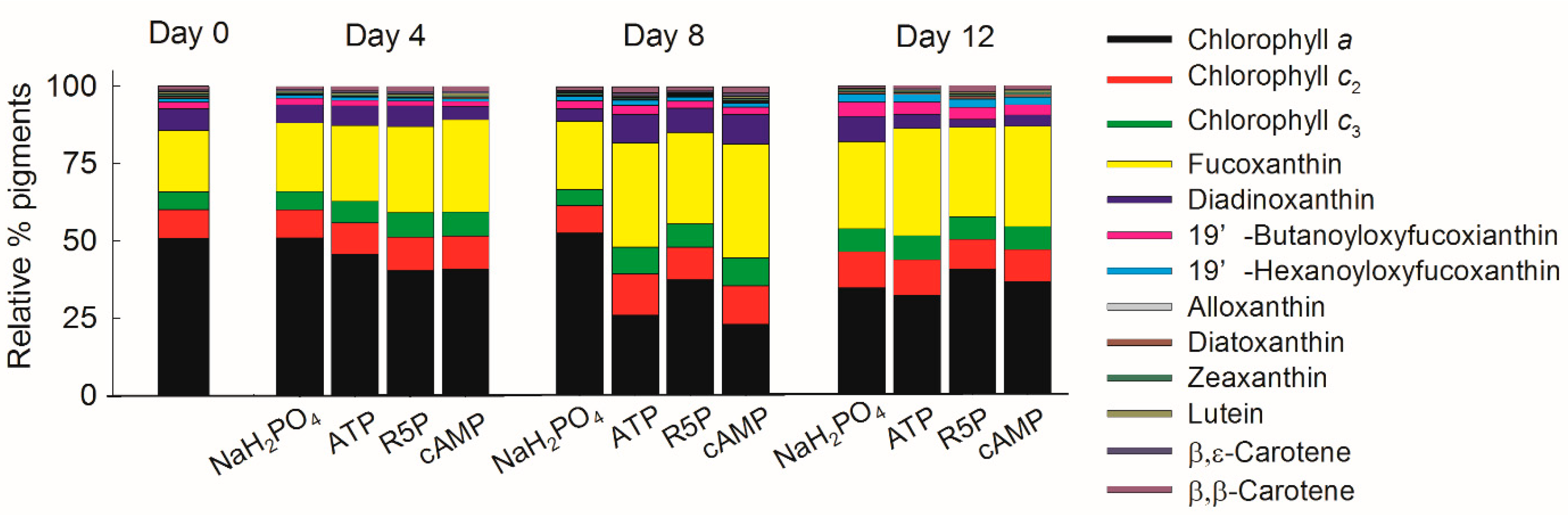
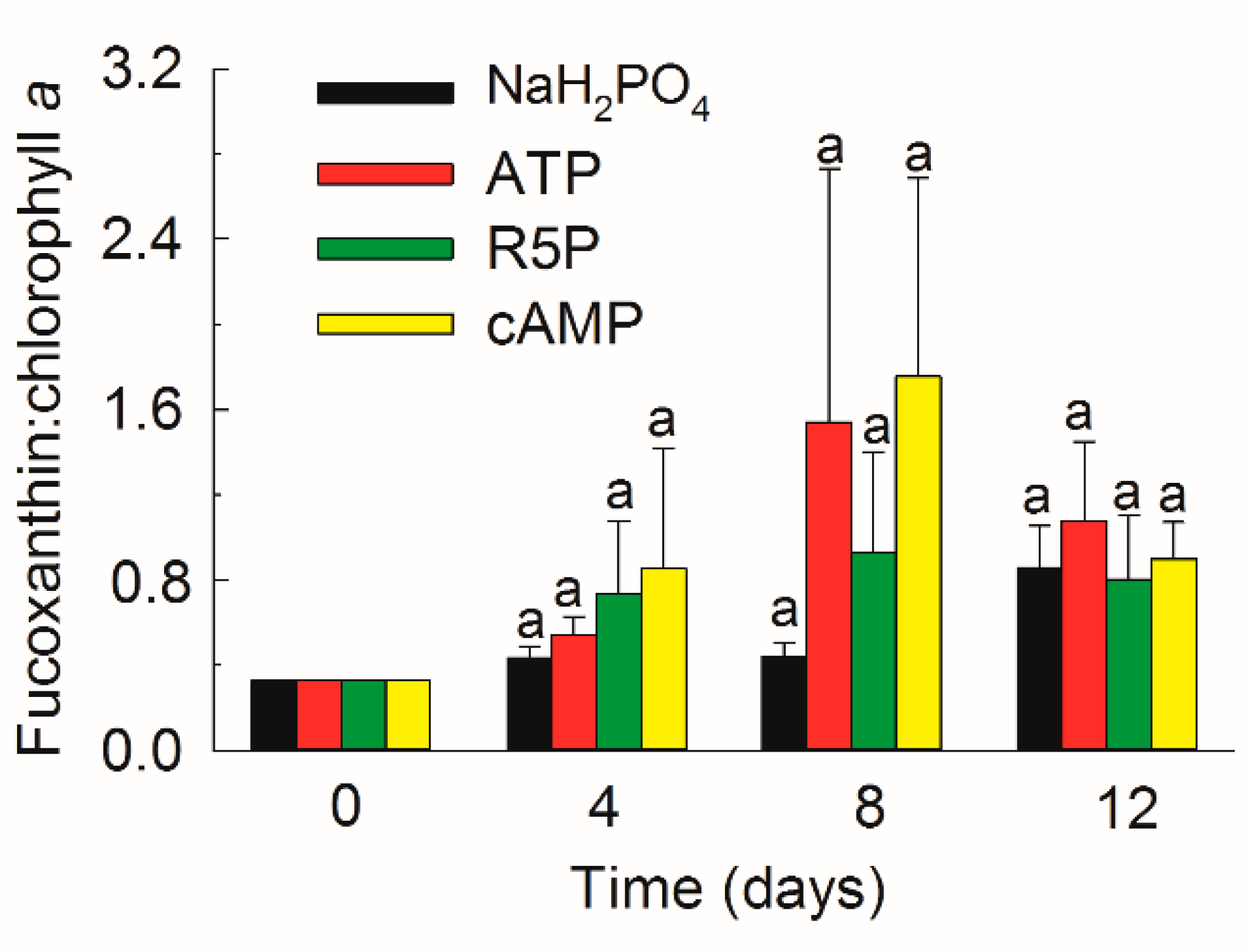
3.5. Pigments
3.6. Hemolytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Townhill, B.L.; Tinker, J.; Jones, M.; Pitois, S.; Creach, V.; Simpson, S.D.; Dye, S.; Bear, E.; Pinnegar, J.K. Harmful algal blooms and climate change: Exploring future distribution changes. ICES J. Mar. Sci. 2018, 75, 1882–1893. [Google Scholar] [CrossRef] [Green Version]
- Li, X.D.; Yan, T.; Yu, R.C.; Zhou, M.J. A review of Karenia mikimotoi: Bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 2019, 90, 101702. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Li, W.C.; Matsuyama, Y.; Matsuo, A.; Yagi, M.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Strain- dependent lethal effects on abalone and haemolytic activities of the dinoflagellate Karenia mikimotoi. Aquaculture 2020, 520, 734953. [Google Scholar] [CrossRef]
- Ohkubo, N.; Tomaru, Y.; Yamaguchi, H.; Kitatsuji, S.; Mochida, K. Development of a method to assess the ichthyotoxicity of the harmful marine microalgae Karenia spp. using gill cell cultures from red sea bream (Pagrus major). Fish Physiol. Biochem. 2017, 43, 1603–1612. [Google Scholar] [CrossRef]
- Kim, D.; Li, W.C.; Matsuyama, Y.; Cho, K.; Yamasaki, Y.; Takeshita, S.; Yamaguchi, K.; Oda, T. Extremely high level of reactive oxygen species (ROS) production in a newly isolated strain of the dinoflagellate Karenia mikimotoi. Eur. J. Phycol. 2019, 54, 632–640. [Google Scholar] [CrossRef]
- Aoki, K.; Yamatogi, T.; Hirae, S.; Yamamoto, K.; Yoshida, K.; Muta, K. Increased occurrence of red-tides of fish-killing dinoflagellate Karenia mikimotoi and related environmental conditions in Imari Bay, Japan. Reg. Stud. Mar. Sci. 2020, 39, 101470. [Google Scholar] [CrossRef]
- Chen, B.H.; Wang, K.; Guo, H.G.; Lin, H. Karenia mikimotoi blooms in coastal waters of China from 1998 to 2017. Estuar. Coast. Shelf Sci. 2021, 249, 107034. [Google Scholar]
- Sakamoto, S.; Lim, W.A.; Lu, D.D.; Dai, X.F.; Orlova, T.; Iwataki, M. Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China 2017, Japan, Korea and Russia. Harmful Algae 2021, 102, 101787. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Natural Resource of the People’s Republic of China (MNR). Bulletin of China Marine Disaster in 2017; Department of Marine Early Warning and Monitoring of MNR: Beijing, China, 2018.
- Aoki, K.; Kameda, T.; Yamatogi, T.; Ishida, N.; Hirae, S.; Kawaguchi, M.; Syutou, T. Spatio-temporal variations in bloom of the red-tide dinoflagellate Karenia mikimotoi in Imari Bay 2017, Japan, in 2014: Factors controlling horizontal and vertical distribution. Mar. Pollut. Bull. 2017, 124, 130–138. [Google Scholar] [CrossRef]
- Richardson, B.; Corcoran, A.A. Use of dissolved inorganic and organic phosphorus by axenic and nonaxenic clones of Karenia brevis and Karenia mikimotoi. Harmful Algae 2015, 48, 30–36. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Li, L.; Lin, L.X.; Zhang, C.; Lin, S.J. Transcriptomic and physiological analyses of the dinoflagellate Karenia mikimotoi reveal non-alkaline phosphatase-based molecular machinery of ATP utilization. Environ. Microbiol. 2017, 19, 4506–4518. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, K.; Shikata, T.; Kuwahara, Y.; Nishiyama, Y. Adverse effects of strong light and nitrogen deficiency on cell viability photosynthesis, and motility of the red-tide dinoflagellate Karenia mikimotoi. Phycologia 2018, 57, 525–533. [Google Scholar] [CrossRef]
- Huang, K.X.; Feng, Q.L.; Zhang, Y.; Ou, L.J.; Cen, J.Y.; Lu SHQi, Y.Z. Comparative uptake and assimilation of nitrate, ammonium, and urea by dinoflagellate Karenia mikimotoi and diatom Skeletonema costatum s.l. in the coastal waters of the East China Sea. Mar. Pollut. Bull. 2020, 155, 111200. [Google Scholar] [CrossRef]
- Niu, X.Q.; Xu, S.N.; Yang, Q.Y.; Xu, X.L.; Zheng, M.M.; Li, X.; Guan, W.C. Toxic effects of the dinoflagellate Karenia mikimotoi on zebrafish (Danio rerio) larval behavior. Harmful Algae 2021, 103, 101996. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Sun, T.; Liu, C.; Sun, Y.; Wang, Y. Using the marine rotifer Brachionus plicatilis as an endpoint to evaluate whether ROS-dependent hemolytic toxicity is involved in the allelopathy induced by Karenia mikimotoi. Toxins 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Glibert, P.M.; Zhou, M.J.; Lu, S.H.; Lu, D.D. Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar. Ecol. Prog. Ser. 2009, 383, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.K.; Tilstone, G.H.; Smyth, T.J.; Widdicombe, C.E.; Gloël, J.; Robinson, C.; Kaiser, J.; Suggett, D.J. Drivers and effects of Karenia mikimotoi blooms in the western English Channel. Prog. Oceanogr. 2015, 137, 456–469. [Google Scholar] [CrossRef]
- Glibert, P.M.; Burford, M.A. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 2017, 30, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xiao, W.P.; Landry, M.R.; Chiang, K.P.; Wang, L.; Huang, B.Q. Responses of phytoplankton communities to environmental variability in the East China Sea. Ecosystems 2016, 19, 832–849. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, K.; Lim, W.A.; Lee, E.; Dai, M.H.; Choi, Y.H.; Han, I.S.; Shin, K.; Kim, J.M.; Chae, J. Anthropogenic nitrogen is changing the East China and Yellow seas from being N deficient to being P deficient. Limnol. Oceanogr. 2020, 66, 914–924. [Google Scholar]
- Song, S.; Li, Z.; Li, C.; Yu, Z. The response of spring phytoplankton assemblage to diluted water and upwelling in the eutrophic Changjiang (Yangtze River) Estuary. Acta Oceanol. Sin. 2017, 36, 101–110. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part 1. Crit. Rev. Microbiol. 1984, 10, 317–391. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, M.; Ishikawa, K.; Ogawa, H. Characterization of dissolved organic phosphorus in coastal seawater using ultrafiltration and phosphohydrolytic enzymes. Limnol. Oceanogr. 1998, 43, 1553–1564. [Google Scholar] [CrossRef]
- Dyhrman, S.T. Nutrient and Their Acquisition: Phosphorus Physiology in Microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 159; pp. 159–187. [Google Scholar]
- Yamaguchi, M.; Itakura, S. Nutrition and growth kinetics in nitrogen- or phosphorus-limited cultures of the noxious red tide dinoflagellate Gymnodinium mikimotoi. Fish. Sci. 1999, 65, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Nishijima, T.; Nishitani, H.; Fukami, K.; Adach, M. Organic phosphorus utilization and alkaline phosphatase production of 3 red tide phytoplankton. Nippon Suisan Gakkai Shi 2004, 70, 123–130, (In Japanese with an English abstract). [Google Scholar] [CrossRef] [Green Version]
- Sunda, W.G.; Price, N.M.; Morel, F.M.M. Trace metal ion buffers and their use in culture studies. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 35–64. [Google Scholar]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Valderrama, J.C. Methods of nutrient analysis. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publ.: Paris, France, 1995; pp. 251–568. [Google Scholar]
- Jeffries, D.S.; Dieken, F.P.; Jones, D.E. Performance of the autoclave digestion method for total phosphorus analysis. Water Res. 1979, 13, 275–279. [Google Scholar] [CrossRef]
- Solórzano, L.; Sharp, J. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol. Oceanogr. 1980, 25, 754–758. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yamaguchi, M.; Fukami, K.; Adachi, M.; Nishijima, T. Utilization of phosphate diester by the marine diatom Chaetoceros ceratosporus. J. Plankton Res. 2005, 27, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.; Rodriguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton a new HPLC method using a reversed phase C8 column and phridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Eschbach, E.; Scharsack, J.P.; John, U.; Medlin, L.K. Improved erythrocyte lysis assay in microtitre plates for sensitive detection and efficient measurement of haemolytic compounds from ichthyotoxic Algae. J. Appl. Toxicol. 2001, 21, 513–519. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Arisaka, H.; Otsuka, N.; Tomaru, Y. Utilization of phosphate diesters by phosphodiesterase-producing marine diatoms. J. Plankton Res. 2014, 36, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.J.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sato, M.; Hashihama, F.; Ehama, M.; Shiozaki, T.; Takahashi, K.; Furuya, K. Basin-scale variations in labile dissolved phosphoric monoesters and diesters in the central North Pacific Ocean. J. Geophys. Res. Ocean. 2019, 124, 3058–3072. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Sakuraba, R.; Hashihama, F. Phosphate monoesterase and diesterase activities in the North and South Pacific Ocean. Biogeosciences 2013, 10, 7677–7688. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Sato, M.; Gonda, N.; Takahashi, K.; Furuya, K. Phosphate diester utilization by marine diazotrophs Trichodesmium erythraeum and Crocosphaera watsonii. Aquat. Microb. Ecol. 2020, 85, 211–218. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Ou, L.J.; Huang, X.Y.; Huang, B.Q.; Qi, Y.Z.; Lu, S.H. Growth and competition for different forms of organic phosphorus by the dinoflagellate Prorocentrum donghaiense with the dinoflagellate Alexandrium catenella and with the diatom Skeletonema costatum s.l. Hydrobiologia 2015, 754, 29–41. [Google Scholar] [CrossRef]
- Ou, L.J.; Qin, X.L.; Shi, X.Y.; Feng, Q.L.; Zhang, S.W.; Lu, S.H.; Qi, Y.Z. Alkaline phosphatase activities and regulation in three harmful Prorocentrum species from the coastal waters of East China Sea. Microb. Ecol. 2020, 79, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.G. Phosphatase activity in the sea. Hydrobiologia 2003, 493, 187–200. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Havskum, H.; Garde, K.; Riemann, B. On the strategy of “eating your competitor”: A mathematical analysis of algal mixotrophy. Ecology 1993, 77, 2108–2118. [Google Scholar] [CrossRef]
- Ou, L.J.; Huang, B.Q.; Hong, H.S.; Qi, Y.Z.; Lu, S.H. Comparative alkaline phosphatase characteristics of the algal bloom species Prorocentrum donghaiense, Alexandrium catenella and Skeletonema costatum. J. Phycol. 2010, 46, 260–265. [Google Scholar] [CrossRef]
- Moreno, A.R.; Martiny, A.C. Ecological stoichiometry of ocean plankton. Annu. Rev. Mar. Sci. 2018, 10, 43–69. [Google Scholar] [CrossRef]
- Hecky, R.E.; Campbell, P.; Hendzel, L.L. The stoichiometry of carbon, nitrogen, and phosphorus in particulate matter of lakes and oceans. Limnol. Oceanogr. 1993, 38, 709–724. [Google Scholar] [CrossRef]
- Grosse, J.; van Breugel, P.; Brussaard, C.P.D.; Boschker, H.T.S. A biosynthesis view on nutrient stress in coastal phytoplankton. Limnol. Oceanogr. 2017, 62, 490–506. [Google Scholar] [CrossRef] [Green Version]
- Tanioka, T.; Matsumoto, K. A meta-analysis on environmental drivers of marine phytoplankton C.:N:P. Biogeosciences 2020, 17, 2939–2954. [Google Scholar] [CrossRef]
- Schlüter, L.; Møhlenberg, F.; Havskum, H.; Larsen, S. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: Testing the influence of light and nutrients on pigment/chlorophyll a ratios. Mar. Ecol. Prog. Ser. 2000, 192, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Dizaji, S.Z.; Fariman, G.A.; Zahedi, M.M. Pigment content analysis in two HAB forming dinoflagellate species during the growth period. J. Appl. Phycol. 2021, 33, 807–817. [Google Scholar] [CrossRef]
- Chang, F.H.; Gall, M. Pigment compositions and toxic effects of three harmful Karenia species, Karenia concordia, Karenia brevisulcata and Karenia mikimotoi (Gymnodiniales, Dinophyceae), on rotifers and brine shrimps. Harmful Algae 2013, 27, 113–120. [Google Scholar] [CrossRef]
- Descy, J.P.; Sarmento, H.; Higgins, H.W. Variability of phytoplankton pigment ratios across aquatic environment. Eur. J. Phycol. 2009, 44, 319–330. [Google Scholar] [CrossRef]
- Ou, L.J.; Cai, Y.Y.; Jin, W.Y.; Wang, Z.; Lu, S.H. Understanding the nitrogen uptake and assimilation of the Chinese strain of Aureococcus anophagefferens (Pelagophyceae). Algal Res. 2018, 34, 182–190. [Google Scholar] [CrossRef]
- Wang, X.J.; Feng, X.Q.; Zhuang, Y.; Lu, J.H.; Wang, Y.; Gonçalves, R.J.; Li, X.; Lou, Y.L.; Guan, W.C. Effects of ocean acidification and solar ultraviolet radiation on physiology and toxicity of dinoflagellate Karenia mikimotoi. Harmful Algae 2019, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- John, E.H.; Flynn, K.J. Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): The effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur. J. Phycol. 2000, 35, 11–23. [Google Scholar] [CrossRef]
- Fu, F.; Place, A.R.; Garcia, N.S.; Hutchins, D.A. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microb. Ecol. 2010, 59, 55–65. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Zhuang, Y.; Wang, Z.; Ou, L.; Cen, J.; Lu, S.; Qi, Y. Bioavailability of Organic Phosphorus Compounds to the Harmful Dinoflagellate Karenia mikimotoi. Microorganisms 2021, 9, 1961. https://doi.org/10.3390/microorganisms9091961
Huang K, Zhuang Y, Wang Z, Ou L, Cen J, Lu S, Qi Y. Bioavailability of Organic Phosphorus Compounds to the Harmful Dinoflagellate Karenia mikimotoi. Microorganisms. 2021; 9(9):1961. https://doi.org/10.3390/microorganisms9091961
Chicago/Turabian StyleHuang, Kaixuan, Yanqing Zhuang, Zhou Wang, Linjian Ou, Jingyi Cen, Songhui Lu, and Yuzao Qi. 2021. "Bioavailability of Organic Phosphorus Compounds to the Harmful Dinoflagellate Karenia mikimotoi" Microorganisms 9, no. 9: 1961. https://doi.org/10.3390/microorganisms9091961
APA StyleHuang, K., Zhuang, Y., Wang, Z., Ou, L., Cen, J., Lu, S., & Qi, Y. (2021). Bioavailability of Organic Phosphorus Compounds to the Harmful Dinoflagellate Karenia mikimotoi. Microorganisms, 9(9), 1961. https://doi.org/10.3390/microorganisms9091961




