Screening Method for Polyhydroxyalkanoate Synthase Mutants Based on Polyester Degree of Polymerization Using High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
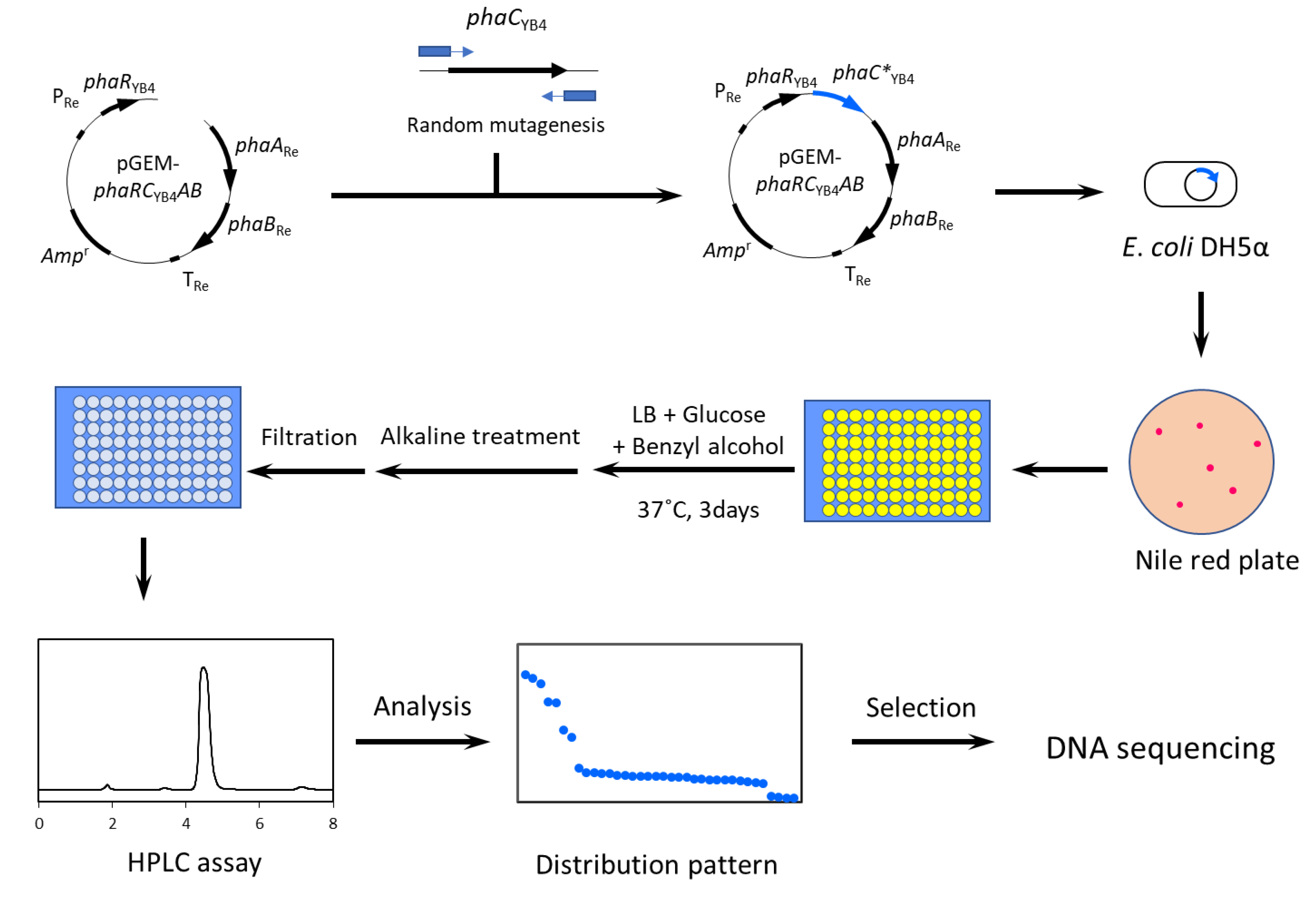
2.2. Construction of PhaRCYB4 Mutant Library
2.3. Screening of Mutants
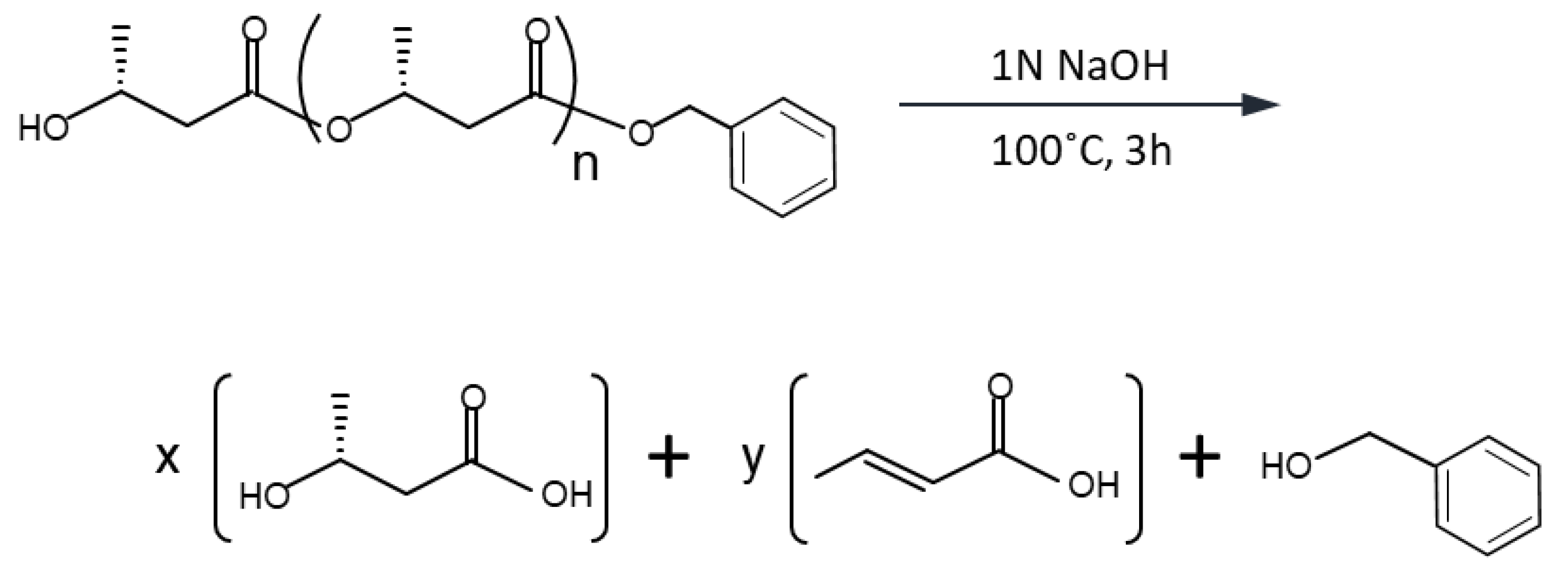
2.4. Flask-Scale Cultivation and PHA Analysis
2.5. Identification of Mutations and Site-Directed Mutagenesis on PhaRCYB4
2.6. Evaluation of Acquired Variants
3. Results
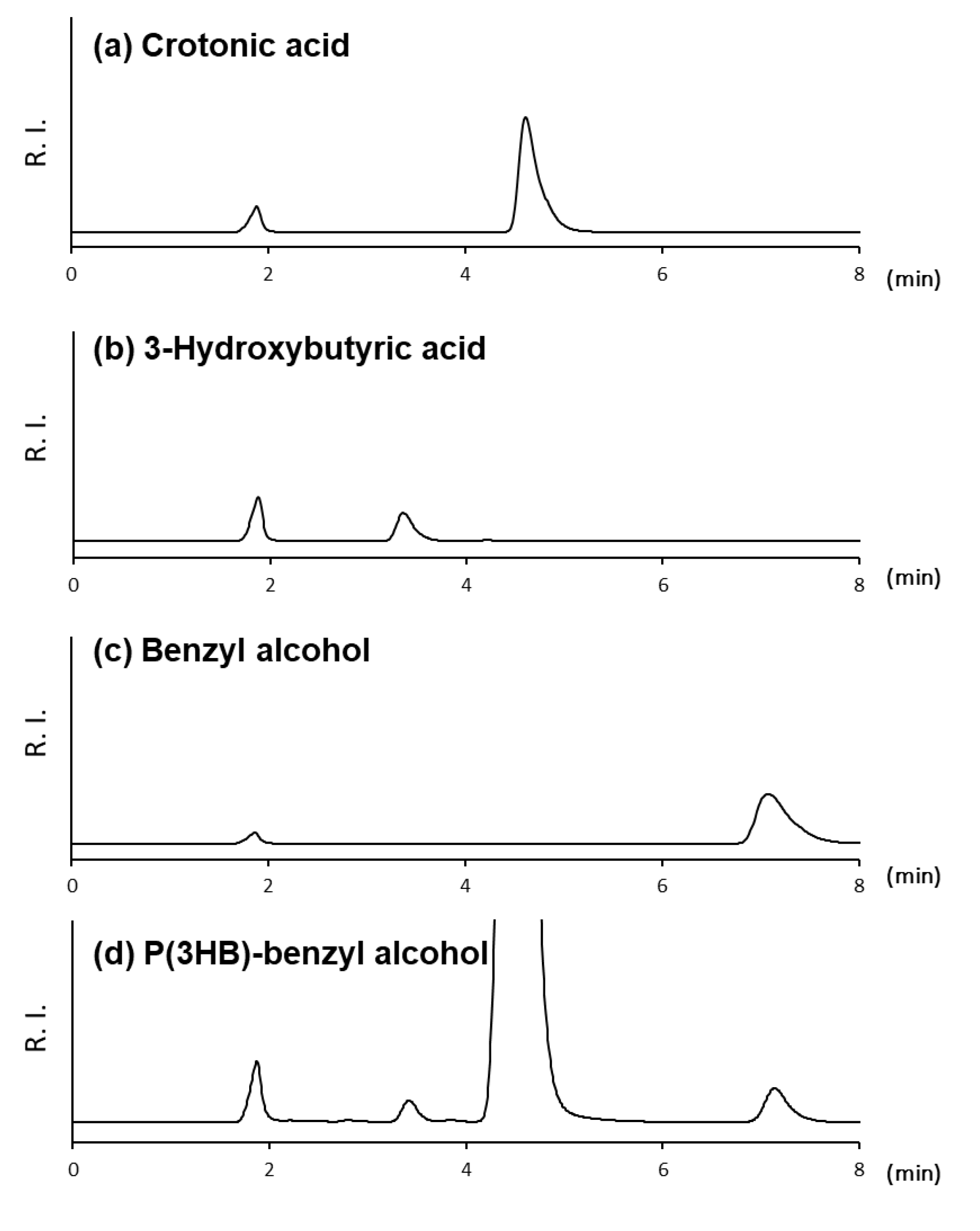
3.1. HPLC Conditions for DP Determination
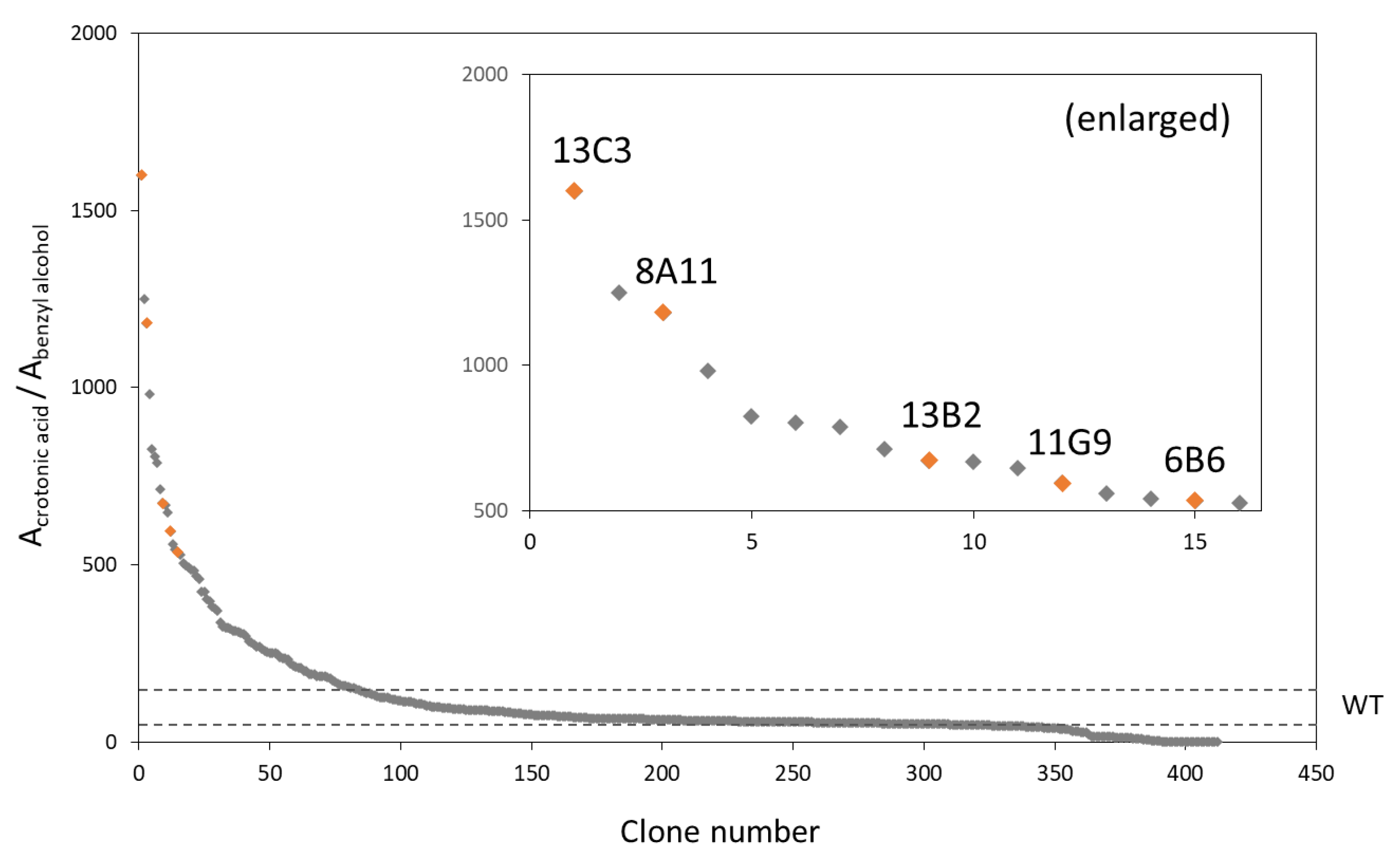
3.2. Screening of PhaRCYB4 Mutants
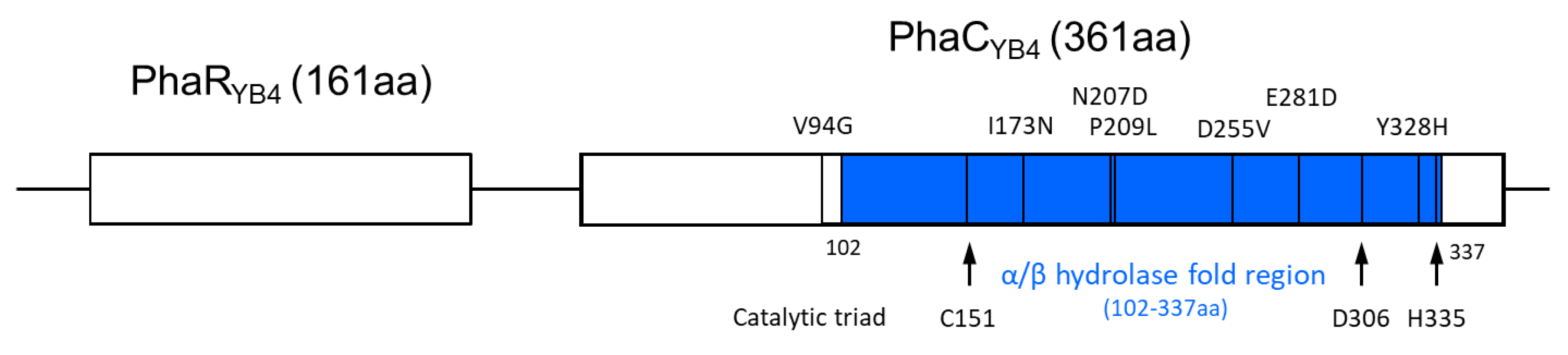
3.3. Evaluation of Each Point Mutation in Selected Clones
3.4. PHA Synthesis by PhaRCYB4 Mutant in E. coli JM109
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Ogunola, O.S.; Onada, O.; Falaye, A.E. Mitigation measures to avert the impacts of plastics and microplastics in the marine environment (a review). Environ. Sci. Pollut. Res. 2018, 25, 9293–9310. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Polyesters. J. Polym. Environ. 2007, 15, 259–267. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Morohoshi, T.; Ogata, K.; Okura, T.; Sato, S. Molecular Characterization of the Bacterial Community in Biofilms for Degradation of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) Films in Seawater. Microbes Environ. 2018, 33, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments. Microbes Environ. 2018, 33, 332–335. [Google Scholar] [CrossRef] [PubMed]
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E.; Babu, P.R. Bio-based and biodegradable polymers—State-of-the-art, challenges and emerging trends. Curr. Opin. Green Sustain. Chem. 2019, 21, 75–81. [Google Scholar] [CrossRef]
- Tsuge, T. Fundamental factors determining the molecular weight of polyhydroxyalkanoate during biosynthesis. Polym. J. 2016, 48, 1051–1057. [Google Scholar] [CrossRef]
- Sato, S.; Arikawa, H.; Kobayashi, S.; Fujiki, T.; Matsumoto, K. Process development of biodegradable polymer PHBH. Seibutsu-Kogaku Kaishi 2019, 97, 66–74. (In Japanese) [Google Scholar]
- Hiroe, A.; Tsuge, K.; Nomura, C.T.; Itaya, M.; Tsuge, T. Rearrangement of Gene Order in the phaCAB Operon Leads to Effective Production of Ultrahigh-Molecular-Weight Poly[(R)-3-Hydroxybutyrate] in Genetically Engineered Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Abe, H.; Lee, S.Y.; Doi, Y. Molecular mass of poly[(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1997, 47, 140–143. [Google Scholar] [CrossRef]
- Agus, J.; Kahar, P.; Abe, H.; Doi, Y.; Tsuge, T. Molecular weight characterization of poly[(R)-3-hydroxybutyrate] synthesized by genetically engineered strains of Escherichia coli. Polym. Degrad. Stab. 2006, 91, 1138–1146. [Google Scholar] [CrossRef]
- Hyakutake, M.; Saito, Y.; Tomizawa, S.; Mizuno, K.; Tsuge, T. Polyhydroxyalkanoate (PHA) Synthesis by Class IV PHA Synthases Employing Ralstonia eutropha PHB−4 as Host Strain. Biosci. Biotechnol. Biochem. 2011, 75, 1615–1617. [Google Scholar] [CrossRef][Green Version]
- Nomura, C.; Taguchi, S. PHA synthase engineering toward superbiocatalysts for custom-made biopolymers. Appl. Microbiol. Biotechnol. 2007, 73, 969–979. [Google Scholar] [CrossRef]
- Taguchi, S.; Doi, Y. Evolution of Polyhydroxyalkanoate(PHA) Production System by“Enzyme Evolution”: Successful Case Studies of Directed Evolution. Macromol. Biosci. 2004, 4, 145–156. [Google Scholar] [CrossRef]
- Mizuno, K.; Ohta, A.; Hyakutake, M.; Ichinomiya, Y.; Tsuge, T. Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym. Degrad. Stab. 2010, 95, 1335–1339. [Google Scholar] [CrossRef]
- Hyakutake, M.; Tomizawa, S.; Sugahara, I.; Murata, E.; Mizuno, K.; Abe, H.; Tsuge, T. Carboxy-terminal modification of polyhydroxyalkanoate (PHA) via alcoholysis reaction catalyzed by Class IV PHA synthase. Polym. Degrad. Stab. 2015, 117, 90–96. [Google Scholar] [CrossRef]
- Tomizawa, S.; Hyakutake, M.; Saito, Y.; Agus, J.; Mizuno, K.; Abe, H.; Tsuge, T. Molecular Weight Change of Polyhydroxyalkanoate (PHA) Caused by the PhaC Subunit of PHA Synthase from Bacillus cereus YB-4 in Recombinant Escherichia coli. Biomacromolecules 2011, 12, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Hyakutake, M.; Tomizawa, S.; Mizuno, K.; Hisano, T.; Abe, H.; Tsuge, T. A Common Active Site of Polyhydroxyalkanoate Synthase from Bacillus cereus YB-4 Is Involved in Polymerization and Alcoholysis Reactions. Appl. Microbiol. Biotechnol. 2014, 99, 4701–4711. [Google Scholar] [CrossRef]
- Cirino, P.C.; Mayer, K.M.; Umeno, D. Generating mutant libraries using error-prone PCR. In Directed Evolution Library Creation; Humana Press Inc.: Totowa, NJ, USA, 2003; pp. 3–10. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ichinomiya, Y.; Shimada, D.; Saika, A.; Abe, H.; Taguchi, S.; Tsuge, T. Development and validation of an HPLC-based screening method to acquire polyhydroxyalkanoate synthase mutants with altered substrate specificity. J. Biosci. Bioeng. 2012, 113, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Spiekermann, P.; Rehm, B.; Kalscheuer, R.; Baumeister, D.; Steinbüchel, A. A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch. Microbiol. 1999, 171, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nambu, Y.; Ishii-Hyakutake, M.; Harada, K.; Mizuno, S.; Tsuge, T. Expanded amino acid sequence of the PhaC box in the active center of polyhydroxyalkanoate synthases. FEBS Lett. 2019, 594, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Del Don, C.; Hanselmann, K.W.; Peduzzi, R.; Bachofen, R. Biomass composition and methods for the determination of metabolic reserve polymers in phototrophic sulfur bacteria. Aquat. Sci. 1994, 56, 1–15. [Google Scholar] [CrossRef]
- Kusaka, S.; Iwata, T.; Doi†, Y. Microbial Synthesis and Physical Properties of Ultra-High-Molecular-Weight Poly[(R)-3-Hydroxybutyrate]. J. Macromol. Sci. Part A 1998, 35, 319–335. [Google Scholar] [CrossRef]
- Ilham, M.; Nakanomori, S.; Kihara, T.; Hokamura, A.; Matsusaki, H.; Tsuge, T.; Mizuno, K. Characterization of polyhydroxyalkanoate synthases from Halomonas sp. O-1 and Halomonas elongata DSM2581: Site-directed mutagenesis and recombinant expression. Polym. Degrad. Stab. 2014, 109, 416–423. [Google Scholar] [CrossRef]
- Leong, Y.K.; Show, P.L.; Ooi, C.W.; Ling, T.C.; Lan, J.C.-W. Current trends in polyhydroxyalkanoates (PHAs) biosynthesis: Insights from the recombinant Escherichia coli. J. Biotechnol. 2014, 180, 52–65. [Google Scholar] [CrossRef]
- Tobin, M.B.; Gustafsson, C.; Huisman, G.W. Directed evolution: The ‘rational’ basis for ‘irrational’ design. Curr. Opin. Struct. Biol. 2000, 10, 421–427. [Google Scholar] [CrossRef]
- Sen, S.; Bhojnagarwala, P.; Francey, L.J.; Lu, D.; Penning, T.M.; Field, J. p53 Mutagenesis by Benzo[a]pyrene Derived Radical Cations. Chem. Res. Toxicol. 2012, 25, 2117–2126. [Google Scholar] [CrossRef]
- Mandell, J.D.; Greenberg, J. A new chemical mutagen for bacteria, 1-methyl-3-nitro-1-nitrosoguanidine. Biochem. Biophys. Res. Commun. 1960, 3, 575–577. [Google Scholar] [CrossRef]
- Greener, A.; Callahan, M. XL1-Red: A highly efficient mutagenesis strain. Strategies 1994, 7, 32–34. [Google Scholar]
- Greener, A.; Callahan, M.; Jerpseth, B. An efficient random mutagenesis technique using anE.coli mutator strain. Mol. Biotechnol. 1997, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Watanabe, Y.; Sudesh, K.; Abe, H.; Tsuge, T. Enhanced Incorporation of 3-Hydroxy-4-Methylvalerate Unit into Biosynthetic Polyhydroxyalkanoate Using Leucine as a Precursor. AMB Express 2011, 1, 6. [Google Scholar] [CrossRef]
- Hiroe, A.; Watanabe, S.; Kobayashi, M.; Nomura, C.T.; Tsuge, T. Increased synthesis of poly(3-hydroxydodecanoate) by random mutagenesis of polyhydroxyalkanoate synthase. Appl. Microbiol. Biotechnol. 2018, 102, 7927–7934. [Google Scholar] [CrossRef]
- Amara, A.; Steinbüchel, A.; Rehm, B. In vivo evolution of the Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: Isolation and characterization of modified PHA synthases with enhanced activity. Appl. Microbiol. Biotechnol. 2002, 59, 477–482. [Google Scholar] [CrossRef]
- Hyakutake, M.; Tomizawa, S.; Mizuno, K.; Abe, H.; Tsuge, T. Alcoholytic Cleavage of Polyhydroxyalkanoate Chains by Class IV Synthases Induced by Endogenous and Exogenous Ethanol. Appl. Environ. Microbiol. 2013, 80, 1421–1429. [Google Scholar] [CrossRef]
- Tsuge, T.; Hyakutake, M.; Mizuno, K. Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl. Microbiol. Biotechnol. 2015, 99, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, S.; Saito, Y.; Hyakutake, M.; Nakamura, Y.; Abe, H.; Tsuge, T. Chain transfer reaction catalyzed by various polyhydroxyalkanoate synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl. Microbiol. Biotechnol. 2010, 87, 1427–1435. [Google Scholar] [CrossRef]
- Hiroe, A.; Hyakutake, M.; Thomson, N.M.; Sivaniah, E.; Tsuge, T. Endogenous Ethanol Affects Biopolyester Molecular Weight in Recombinant Escherichia coli. ACS Chem. Biol. 2013, 8, 2568–2576. [Google Scholar] [CrossRef]
- Sim, S.J.; Snell, K.D.; Kim, B.W.; Rha, C.K.; Sinskey, A.J. Increased poly-β-hydroxybutyrate (PHB) chain length by the modulation of PHA synthase activity in recombinant Escherichia coli. Biotechnol. Lett. 2001, 23, 2057–2061. [Google Scholar] [CrossRef]
| Polymer | Mn_GPC | 1H NMR Analysis | HPLC Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Relative Ratio | DP_NMR 1 | Modification Yield [%] 2 | Relative Ratio | DP_HPLC 3 | |||||
| Monomer | Benzyl Alcohol | Crotonic Acid | 3HB | Benzyl Alcohol | |||||
| P(3HB)-93% | 9600 | 84.1 | 1 | 84 | 93 | 22.56 | 39.66 | 1 | 62 |
| Synthase | Mutation | Dry Cell wt. (g/L) | P(3HB) Content (wt.%) | Molecular Weight | |
|---|---|---|---|---|---|
| Mn (×103) | Mw/Mn | ||||
| WT | - | 10.0 ± 0.0 | 57 ± 5 | 9 ± 1 | 1.5 |
| 6B6 | 173I(ATC)→N(AAC) 281E(GAA)→D(GAT) | 2.2 ± 0.2 | 10 ± 1 | 2700 ± 210 | 2.0 |
| 8A11 1 | 207N(AAC)→D(GAC) 211E(GAA)→G(GGA) 274N(AAT)→S(AGT) 324T(ACA)→A(GCA) 346K(AAA)→E(GAA) | 6.5 ± 0.3 | 41 ± 5 | 2300 ± 250 | 2.5 |
| 11G9 | 94V(GTG)→G(GGG) | 8.0 ± 0.2 | 53 ± 6 | 420 ± 24 | 3.2 |
| 13B2 1 | 40Q(CAA)→R(CGA) 124F(TTT)→L(CTT) 255D(GAT)→V(GTT) 264Y(TAC)→F(TTC) | 9.9 ± 0.1 | 62 ± 2 | 180 ± 28 | 2.4 |
| 13C3 1 | 209P(CCG)→L(CTG) 328Y(TAT)→H(CAT) | 7.6 ± 0.2 | 50 ± 1 | 2400 ± 480 | 2.6 |
| Mutation Origin | PhaRCYB4 | Dry Cell wt. (g/L) | P(3HB) Content (wt.%) | Molecular Weight | Relative Chain Number 1 | |
|---|---|---|---|---|---|---|
| Mn (× 103) | Mw/Mn | |||||
| - | WT | 10.0 ± 0.0 | 57 ± 5 | 9 ± 1 | 1.5 | 100 |
| 6B6 | I173N | 9.2 ± 0.2 | 51 ± 2 | 270 ± 32 | 6.4 | 2.9 |
| E281D | 8.1 ± 0.1 | 46 ± 4 | 7 ± 0 | 1.7 | 81 | |
| 8A11 | N207D | 7.5 ± 0.1 | 43 ± 3 | 2700 ± 260 | 2.5 | 0.20 |
| E211G | 8.8 ± 0.1 | 58 ± 2 | 11 ± 0 | 1.7 | 76 | |
| N274S | 8.7 ± 0.2 | 57 ± 1 | 13 ± 1 | 1.8 | 53 | |
| T324A | 10.5 ± 0.0 | 62 ± 2 | 9 ± 0 | 1.7 | 124 | |
| K346E | 10.5 ± 0.2 | 51 ± 3 | 8 ± 0 | 1.6 | 111 | |
| 11G9 | V94G | 8.0 ± 0.2 | 53 ± 6 | 420 ± 24 | 3.2 | 1.6 |
| 13B2 | D255V | 2.6 ± 0.1 | 7 ± 1 | 2700 ± 440 | 2.2 | 0.01 |
| 13C3 | P209L | 8.9 ± 0.5 | 62 ± 2 | 250 ± 7 | 4.7 | 3.6 |
| Y328H | 10.7 ± 0.2 | 48 ± 9 | 9 ± 0 | 1.8 | 94 | |
| PhaRCYB4 | Dry Cell wt. (g/L) | P(3HB) Content (wt.%) | P(3HB) Yield (g/L) | Molecular Weight 1 | |
|---|---|---|---|---|---|
| Mn (×103) | Mw/Mn | ||||
| WT 2 | 9.3 ± 0.1 | 80 ± 1 | 7.4 | 20 | 2.1 |
| V94G | 9.5 ± 0.0 | 63 ± 6 | 6.0 | 530 | 4.9 |
| I173N | 9.0 ± 0.1 | 64 ± 1 | 5.8 | 680 | 5.3 |
| P209L | 9.5 ± 0.5 | 66 ± 2 | 6.3 | 850 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii-Hyakutake, M.; Sakurai, T.; Tsuge, T. Screening Method for Polyhydroxyalkanoate Synthase Mutants Based on Polyester Degree of Polymerization Using High-Performance Liquid Chromatography. Microorganisms 2021, 9, 1949. https://doi.org/10.3390/microorganisms9091949
Ishii-Hyakutake M, Sakurai T, Tsuge T. Screening Method for Polyhydroxyalkanoate Synthase Mutants Based on Polyester Degree of Polymerization Using High-Performance Liquid Chromatography. Microorganisms. 2021; 9(9):1949. https://doi.org/10.3390/microorganisms9091949
Chicago/Turabian StyleIshii-Hyakutake, Manami, Tetsuo Sakurai, and Takeharu Tsuge. 2021. "Screening Method for Polyhydroxyalkanoate Synthase Mutants Based on Polyester Degree of Polymerization Using High-Performance Liquid Chromatography" Microorganisms 9, no. 9: 1949. https://doi.org/10.3390/microorganisms9091949
APA StyleIshii-Hyakutake, M., Sakurai, T., & Tsuge, T. (2021). Screening Method for Polyhydroxyalkanoate Synthase Mutants Based on Polyester Degree of Polymerization Using High-Performance Liquid Chromatography. Microorganisms, 9(9), 1949. https://doi.org/10.3390/microorganisms9091949






