The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19
Abstract
1. Introduction
2. α-Herpesviruses in Inflammatory Bowel Disease
2.1. Herpes Simplex Virus 1/2
2.2. Varicella-Zoster Virus
3. β-Herpesviruses in Inflammatory Bowel Diseases
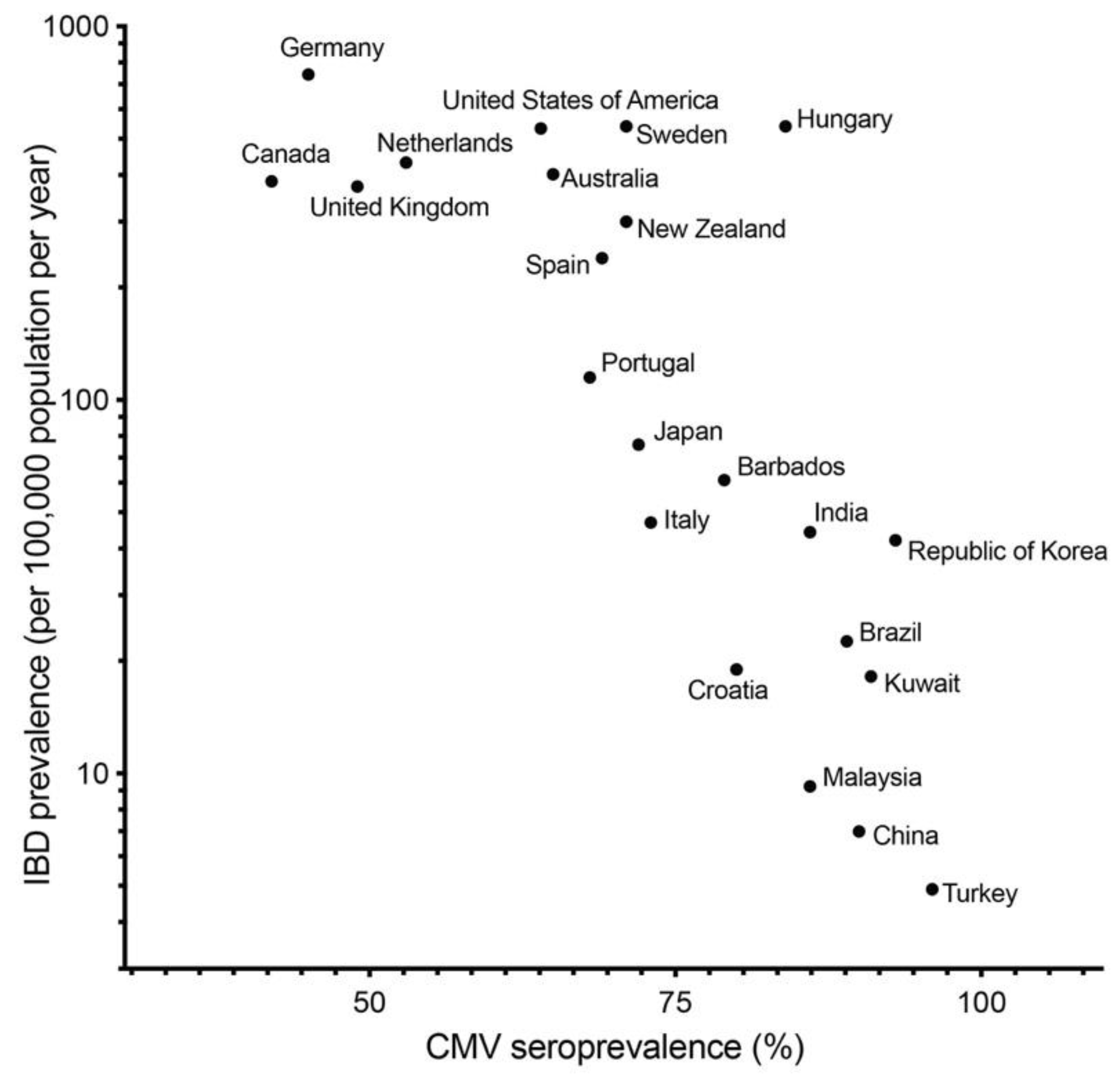
3.1. Cytomegalovirus
3.2. Human Herpesvirus 6/7
4. γ-Herpesviruses in Inflammatory Bowel Diseases
4.1. Epstein-Barr Virus
4.2. Human Herpesvirus 8
5. Co-Reactivation of Human Herpesviruses
6. Human Herpesviruses in the Era of COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory Bowel Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-Specific Alterations in the Enteric Virome in Inflammatory Bowel Disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Clooney, A.G.; Sutton, T.D.; Shkoporov, A.N.; Holohan, R.K.; Daly, K.M.; O’Regan, O.; Ryan, F.; Draper, L.A.; Plevy, S.E.; Ross, R.; et al. Whole-Virome Analysis Sheds Light on Viral Dark Matter in Inflammatory Bowel Disease. Cell Host Microbe 2019, 26, 764–778.e5. [Google Scholar] [CrossRef]
- Tarris, G.; De Rougemont, A.; Charkaoui, M.; Michiels, C.; Martin, L.; Belliot, G. Enteric Viruses and Inflammatory Bowel Disease. Viruses 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Herpesvirus latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, A.; Veyrard, P.; Roblin, X.; Saint-Sardos, P.; Rochereau, N.; Paul, S.; Bourlet, T.; Pozzetto, B.; Pillet, S. Cytomegalovirus and Inflammatory Bowel Diseases (IBD) with a Special Focus on the Link with Ulcerative Colitis (UC). Microorganisms 2020, 8, 1078. [Google Scholar] [CrossRef]
- Hosomi, S.; Watanabe, K.; Nishida, Y.; Yamagami, H.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; et al. Combined Infection of Human Herpes Viruses: A Risk Factor for Subsequent Colectomy in Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1307–1315. [Google Scholar] [CrossRef]
- Aalto, S.M.; Linnavuori, K.; Peltola, H.; Vuori, E.; Weissbrich, B.; Schubert, J.; Hedman, L.; Hedman, K. Immunoreactivation of Epstein-Barr virus due to cytomegalovirus primary infection. J. Med. Virol. 1998, 56, 186–191. [Google Scholar] [CrossRef]
- Mendez, J.C.; Dockrell, D.; Espy, M.J.; Smith, T.F.; Wilson, J.A.; Harmsen, W.S.; Ilstrup, D.; Paya, C.V. Human β-Herpesvirus Interactions in Solid Organ Transplant Recipients. J. Infect. Dis. 2001, 183, 179–184. [Google Scholar] [CrossRef]
- Härmä, M.; Höckerstedt, K.; Lyytikäinen, O.; Lautenschlager, I. HHV-6 and HHV-7 antigenemia related to CMV infection after liver transplantation. J. Med. Virol. 2006, 78, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.F.; Marques, N.P.; de Lucena, E.H.G.; de Rezende, L.F.; Martelli, D.R.B.; Martelli-Júnior, H. Increased number of Herpes Zoster cases in Brazil related to the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 104, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Dursun, R.; Temiz, S.A. The clinics of HHV -6 infection in COVID -19 pandemic: Pityriasis rosea and Kawasaki disease. Dermatol. Ther. 2020, 33, e13730. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F. Herpes Zoster in Patients Receiving JAK Inhibitors for Ulcerative Colitis: Mechanism, Epidemiology, Management, and Prevention. Inflamm. Bowel Dis. 2018, 24, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Bradley, H.; Markowitz, L.E.; Gibson, T.; McQuillan, G.M. Seroprevalence of Herpes Simplex Virus Types 1 and 2—United States, 1999–2010. J. Infect. Dis. 2014, 209, 325–333. [Google Scholar] [CrossRef]
- Buss, D.H.; Scharyj, M. Herpesvirus infection of the esophagus and other visceral organs in adults: Incidence and clinical significance. Am. J. Med. 1979, 66, 457–462. [Google Scholar] [CrossRef]
- Bonacini, M.; Young, T.; Laine, L. The causes of esophageal symptoms in human immunodeficiency virus infection. A prospective study of 110 patients. Arch. Intern. Med. 1991, 151, 1567–1572. [Google Scholar] [CrossRef]
- McBane, R.D.; Gross, J.B., Jr. Herpes esophagitis: Clinical syndrome, endoscopic appearance, and diagnosis in 23 patients. Gastrointest. Endosc. 1991, 37, 600–603. [Google Scholar] [CrossRef]
- Ramanathan, J.; Rammouni, M.; Baran, J., Jr.; Khatib, R. Herpes simplex virus esophagitis in the immunocompetent host: An overview. Am. J. gastroenterol. 2000, 95, 2171–2176. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.-M.; Hofmann, J.; Kredel, L.I.; Holzem, C.; Kühl, A.A.; Taube, E.T.; Epple, H.-J.; Schubert, S.; Siegmund, B. Herpes Simplex Virus Sepsis in a Young Woman with Crohn’s Disease. J. Crohn’s Colitis 2015, 9, 1169–1173. [Google Scholar] [CrossRef][Green Version]
- Phadke, V.K.; Friedman-Moraco, R.J.; Quigley, B.C.; Farris, A.; Norvell, J.P. Concomitant herpes simplex virus colitis and hepatitis in a man with ulcerative colitis: Case report and review of the literature. Medicine 2016, 95, e5082. [Google Scholar] [CrossRef]
- Golds, G.; Worobetz, L. Fulminant Hepatic Failure in a Patient with Crohn’s Disease on Infliximab Possibly Related to Reactivation of Herpes Simplex Virus 2 Infection. Case Rep. Hepatol. 2016, 2016, 2132056. [Google Scholar] [CrossRef] [PubMed]
- Goel, K.; Bunker, M.; Balog, A.; Silverman, J.F. Fulminant Herpes Simplex Hepatitis Secondary to Adalimumab in Crohn’s Disease: A Case Report. Clin. Med. Insights Case Rep. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Kruiningen, H.J.; Poulin, M.; Garmendia, A.E.; Desreumaux, P.; Colombel, J.; De Hertogh, G.; Geboes, K.; Vermeire, S.; Tsongalis, G.J. Search for evidence of recurring or persistent viruses in Crohn’s disease. APMIS 2007, 115, 962–968. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Racca, F.; Scudeller, L.; Piralla, A.; Formagnana, P.; Pozzi, L.; Betti, E.; Vanoli, A.; Riboni, R.; Kruzliak, P.; et al. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol. Res. 2015, 64, 191–203. [Google Scholar] [CrossRef]
- Shimada, T.; Nagata, N.; Okahara, K.; Joya, A.; Hayashida, T.; Oka, S.; Sakurai, T.; Akiyama, J.; Uemura, N.; Gatanaga, H. PCR detection of human herpesviruses in colonic mucosa of individuals with inflammatory bowel disease: Comparison with individuals with immunocompetency and HIV infection. PLoS ONE 2017, 12, e0184699. [Google Scholar] [CrossRef]
- Dado, D.; Chernev, I. Gastrointestinal varicella zoster infection. Dissemination or reactivation of a latent virus in the gut? Endoscopy 2013, 45, 678. [Google Scholar] [CrossRef]
- Mostyka, M.; Shia, J.; Neumann, W.L.; Whitney-Miller, C.L.; Feely, M.; Yantiss, R.K. Clinicopathologic Features of Varicella Zoster Virus Infection of the Upper Gastrointestinal Tract. Am. J. Surg. Pathol. 2021, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Martin, C.; Sandler, R.S.; Kappelman, M.D. Increased risk of herpes zoster among 108,604 patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 37, 420–429. [Google Scholar] [CrossRef]
- Straus, S.E.; Ostrove, J.M.; Inchauspé, G.; Felser, J.M.; Freifeld, A.; Croen, K.D.; Sawyer, M.H. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann. Intern. Med. 1988, 108, 221–237. [Google Scholar] [CrossRef]
- Staikov, I.; Neykov, N.; Marinovic, B.; Lipozenčić, J.; Tsankov, N. Herpes zoster as a systemic disease. Clin. Dermatol. 2014, 32, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Malmgaard, L. Induction and Regulation of IFNs during Viral Infections. J. Interf. Cytokine Res. 2004, 24, 439–454. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Curtis, J.R.; Lindsey, S.; Tanaka, Y.; Yamaoka, K.; Valdez, H.; Hirose, T.; Nduaka, C.I.; Wang, L.; Mendelsohn, A.M.; et al. Herpes Zoster and Tofacitinib: Clinical Outcomes and the Risk of Concomitant Therapy. Arthritis Rheumatol. 2017, 69, 1960–1968. [Google Scholar] [CrossRef]
- Almanzar, G.; Kienle, F.; Schmalzing, M.; Maas, A.; Tony, H.-P.; Prelog, M. Tofacitinib modulates the VZV-specific CD4+ T cell immune response in vitro in lymphocytes of patients with rheumatoid arthritis. Rheumatology 2019, 58, 2051–2060. [Google Scholar] [CrossRef]
- Gagliardi, A.M.; Andriolo, B.N.; Torloni, M.R.; Soares, B.G.; Gomes, J.D.O.; Andriolo, R.B.; Cruz, E.C. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Marks, M.A.; Hansen, J.; Lewis, E.; Aukes, L.; Saddier, P. Long-term effectiveness of zoster vaccine live for postherpetic neuralgia prevention. Vaccine 2019, 37, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Strategies for Herpes Zoster Vaccination of Immunocompromised Patients. J. Infect. Dis. 2008, 197, S237–S241. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.-J.; Diez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
- Satyam, V.R.; Li, P.-H.; Reich, J.; Qazi, T.; Noronha, A.; Wasan, S.K.; Farraye, F.A. Safety of Recombinant Zoster Vaccine in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2020, 65, 2986–2991. [Google Scholar] [CrossRef]
- Khan, N.; Trivedi, C.; Kavani, H.; Lewis, J.; Yang, Y.-X. Frequency of Herpes Zoster Vaccination among Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2018, 25, 345–351. [Google Scholar] [CrossRef]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [PubMed]
- Elder, E.; Sinclair, J. HCMV latency: What regulates the regulators? Med. Microbiol. Immunol. 2019, 208, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sinzger, C.; Adler, B. Endothelial cells in human cytomegalovirus infection: One host cell out of many or a crucial target for virus spread? Thromb. Haemost. 2009, 102, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Herfarth, H. Cytomegalovirus Colitis, Cytomegalovirus Hepatitis and Systemic Cytomegalovirus Infection: Common Features and Differences. Inflamm. Intest. Dis. 2016, 1, 15–23. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C.; Streblow, D.N.; Fish, K.N.; Allan-Yorke, J.; Smith, P.P.; Nelson, J.A. Reactivation of Latent Human Cytomegalovirus in CD14 + Monocytes Is Differentiation Dependent. J. Virol. 2001, 75, 7543–7554. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Chiba, T. TNF-α is an important pathogenic factor contributing to reactivation of cytomegalovirus in inflamed mucosa of colon in patients with ulcerative colitis: Lesson from clinical experience. Inflamm. Bowel Dis. 2010, 16, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Orloff, J.J.; Saito, R.; Lasky, S.; Dave, H. Toxic megacolon in cytomegalovirus colitis. Am. J. Gastroenterol. 1989, 84, 794–797. [Google Scholar] [PubMed]
- Papadakis, K.A.; Tung, J.K.; Binder, S.W.; Kam, L.Y.; Abreu, M.T.; Targan, S.R.; Vasiliauskas, E.A. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2001, 96, 2137–2142. [Google Scholar] [CrossRef]
- Koffron, A.; Varghese, T.; Hummel, M.; Yan, S.; Kaufman, D.; Fryer, J.; Leventhal, J.; Stuart, F.; Abecassis, M. Immunosuppression is not required for reactivation of latent murine cytomegalovirus. Transplant. Proc. 1999, 31, 1395–1396. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tange, T. Prevalence of Cytomegalovirus Infection in Inflammatory Bowel Disease Patients. Dis. Colon Rectum 2004, 47, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Knösel, T.; Schewe, C.; Petersen, N.; Dietel, M.; Petersen, I. Prevalence of infectious pathogens in Crohn’s disease. Pathol. Res. Pr. 2009, 205, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Brainard, J.A.; Greenson, J.K.; Vesy, C.J.; Tesi, R.J.; Papp, A.C.; Snyder, P.J.; Western, L.; Prior, T.W. Detection of cytomegalovirus in liver transplant biopsies. A comparison of light microscopy, immunohistochemistry, duplex PCR and nested PCR. Transplantation 1994, 57, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Muir, S.W.; Murray, J.; Farquharson, M.A.; Wheatley, D.J.; McPhaden, A.R. Detection of cytomegalovirus in upper gastrointestinal biopsies from heart transplant recipients: Comparison of light microscopy, immunocytochemistry, in situ hybridisation, and nested PCR. J. Clin. Pathol. 1998, 51, 807–811. [Google Scholar] [CrossRef]
- Yoshino, T.; Nakase, H.; Ueno, S.; Uza, N.; Inoue, S.; Mikami, S.; Matsuura, M.; Ohmori, K.; Sakurai, T.; Nagayama, S.; et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm. Bowel Dis. 2007, 13, 1516–1521. [Google Scholar] [CrossRef]
- Shukla, T.; Bruining, D.H.; Singh, S.; Loftus, E.V.; McCurdy, J.D. Antiviral Therapy in Steroid-refractory Ulcerative Colitis with Cytomegalovirus. Inflamm. Bowel Dis. 2015, 21, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Pillet, S.; Oussalah, A.; Berthelot, P.; Del Tedesco, E.; Phelip, J.-M.; Chambonnière, M.-L.; Garraud, O.; Peyrin-Biroulet, L.; Pozzetto, B. Cytomegalovirus Load in Inflamed Intestinal Tissue Is Predictive of Resistance to Immunosuppressive Therapy in Ulcerative Colitis. Am. J. Gastroenterol. 2011, 106, 2001–2008. [Google Scholar] [CrossRef]
- Jain, S.; Namdeo, D.; Sahu, P.; Kedia, S.; Sahni, P.; Das, P.; Sharma, R.; Gupta, V.; Makharia, G.; Dar, L.; et al. High mucosal cytomegalovirus DNA helps predict adverse short-term outcome in acute severe ulcerative colitis. Intest. Res. 2020. [Google Scholar] [CrossRef]
- Zerr, D.M.; Meier, A.S.; Selke, S.S.; Frenkel, L.M.; Huang, M.-L.; Wald, A.; Rhoads, M.P.; Nguy, L.; Bornemann, R.; Morrow, R.A.; et al. A Population-Based Study of Primary Human Herpesvirus 6 Infection. N. Engl. J. Med. 2005, 352, 768–776. [Google Scholar] [CrossRef]
- De Bolle, L.; Naesens, L.; De Clercq, E. Update on Human Herpesvirus 6 Biology, Clinical Features, and Therapy. Clin. Microbiol. Rev. 2005, 18, 217–245. [Google Scholar] [CrossRef]
- Kondo, K.; Nagafuji, H.; Hata, A.; Tomomori, C.; Yamanishi, K. Association of Human Herpesvirus 6 Infection of the Central Nervous System with Recurrence of Febrile Convulsions. J. Infect. Dis. 1993, 167, 1197–1200. [Google Scholar] [CrossRef]
- Kondo, K.; Kondo, T.; Okuno, T.; Takahashi, M.; Yamanishi, K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 1991, 72, 1401–1408. [Google Scholar] [CrossRef]
- Pantry, S.N.; Medveczky, P.G. Latency, Integration, and Reactivation of Human Herpesvirus-6. Viruses 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Jayet, P.; Aubert, J.; Rotman, S.; Mottet, C.; Sahli, R.; Lautenschlager, I.; Pascual, M.; Meylan, P. Case Report: Human herpesvirus 6 reactivation associated with colitis in a lung transplant recipient. J. Med. Virol. 2008, 80, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, M.S.; Karim, M.S.; Shrestha, B.M.; McKane, W. Colitis in a renal transplant patient with human herpesvirus-6 infection. Transpl. Infect. Dis. 2006, 8, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Ongrádi, J.; Ablashi, D.V.; Yoshikawa, T.; Stercz, B.; Ogata, M. Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals. J. Neurovirol. 2016, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.; Jimenez, M. Common questions about infectious mononucleosis. Am. Fam. Physician 2015, 91, 372–376. [Google Scholar]
- Münz, C.; Moormann, A. Immune escape by Epstein-Barr virus associated malignancies. Semin. Cancer Biol. 2008, 18, 381–387. [Google Scholar] [CrossRef]
- Van Esser, J.W.J.; Niesters, H.; Van Der Holt, B.; Meijer, E.; Osterhaus, A.; Gratama, O.J.W.; Verdonck, L.F.; Löwenberg, B.; Cornelissen, J.J. Prevention of Epstein-Barr virus–lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002, 99, 4364–4369. [Google Scholar] [CrossRef]
- Magro, F.; Santos-Antunes, J.; Albuquerque, A.; Vilas-Boas, F.; Macedo, G.N.; Nazareth, N.; Lopes, S.; Sobrinho-Simões, J.; Teixeira, S.; Dias, C.C.; et al. Epstein-Barr Virus in Inflammatory Bowel Disease—Correlation with Different Therapeutic Regimens. Inflamm. Bowel Dis. 2013, 19, 1710–1716. [Google Scholar] [CrossRef]
- Lapsia, S.; Koganti, S.; Spadaro, S.; Rajapakse, R.; Chawla, A.; Bhaduri-McIntosh, S. Anti-TNFα therapy for inflammatory bowel diseases is associated with Epstein-Barr virus lytic activation. J. Med. Virol. 2016, 88, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salazar, L.; Rojo, S.; De Lejarazu, O.R.; Castro, E.; Higuera, E.; González, J.M. No Increase in Epstein-Barr Virus Viral Load in a Group of 30 Asymptomatic Patients with Crohn’s Disease. Am. J. Gastroenterol. 2013, 108, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Reijasse, D.; Le Pendeven, C.; Cosnes, J.; Dehee, A.; Gendre, J.-P.; Nicolas, J.-C.; Beaugerie, L. Epstein-Barr virus viral load in Crohn’s disease: Effect of immunosuppressive therapy. Inflamm. Bowel Dis. 2004, 10, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Aihara, Y.; Moriya, K.; Shimozato, N.; Nagamatsu, S.; Kobayashi, S.; Uejima, M.; Matsuo, H.; Ishida, E.; Yagi, H.; Nakatani, T.; et al. Chronic active EBV infection in refractory enteritis with longitudinal ulcers with a cobblestone appearance: An autopsied case report. BMC Gastroenterol. 2021, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiang, X.; Chen, J.; Mao, Q.; Zhao, X.; Sun, X.; Zhong, L.; Rong, L. Chronic active Epstein-Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Dow, D.E.; Cunningham, C.K.; Buchanan, A.M. A Review of Human Herpesvirus 8, the Kaposi’s Sarcoma-Associated Herpesvirus, in the Pediatric Population. J. Pediatr. Infect. Dis. Soc. 2013, 3, 66–76. [Google Scholar] [CrossRef]
- Mansfield, S.; Stawicki, S.P.; Forbes, R.C.; Papadimos, T.; Lindsey, D.E. Acute upper gastrointestinal bleeding secondary to Kaposi sarcoma as initial presentation of HIV infection. J. Gastrointest. Liver Dis. 2013, 22, 441–445. [Google Scholar]
- Bursics, A.; Morvay, K.; Ábrahám, K.; Marschalkó, M.; Kardos, M.; Járay, B.; Nagy, K. HHV-8 positive, HIV negative disseminated Kaposi’s sarcoma complicating steroid dependent ulcerative colitis: A successfully treated case. Gut 2005, 54, 1049–1050. [Google Scholar] [CrossRef][Green Version]
- Svrcek, M.; Tiret, E.; Bennis, M.; Guyot, P.; Fléjou, J.-F. KSHV/HHV8-associated intestinal Kaposi’s sarcoma in patient with ulcerative colitis receiving immunosuppressive drugs: Report of a case. Dis. Colon Rectum 2009, 52, 154–158. [Google Scholar] [CrossRef]
- Girelli, C.; Serio, G.; Rocca, E.; Rocca, F. Refractory ulcerative colitis and iatrogenic colorectal Kaposi’s sarcoma. Dig. Liver Dis. 2009, 41, 170–174. [Google Scholar] [CrossRef]
- Rodríguez-Peláez, M.; Fernández-García, M.S.; Gutiérrez-Corral, N.; De Francisco, R.; Riestra, S.; García-Pravia, C.; Rodríguez, J.I.; Rodrigo, L. Kaposi’s sarcoma: An opportunistic infection by human herpesvirus-8 in ulcerative colitis. J. Crohn’s Colitis 2010, 4, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, A.; Bergallo, M.; Daperno, M.; Sostegni, R.; Ravarino, N.; Crocellà, L.; Ramella, A.; Rocca, R.; Torchio, B.; Cavallo, R. The hazardous burden of Herpesviridae in inflammatory bowel disease: The case of refractory severe ulcerative colitis. Dig. Liver Dis. 2006, 38, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Kozlova, E.V.; Yetman, D.L.; Walling, D.M.; Goodwin, J.S.; Glaser, R. Chronic herpesvirus reactivation occurs in aging. Exp. Gerontol. 2007, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Peek, M.K.; Cutchin, M.; Goodwin, J.S. Reactivation of herpes simplex virus type 1 is associated with cytomegalovirus and age. J. Med. Virol. 2012, 84, 1797–1802. [Google Scholar] [CrossRef]
- Hatayama, Y.; Hashimoto, Y.; Motokura, T. Frequent co-reactivation of Epstein-Barr virus in patients with cytomegalovirus viremia under immunosuppressive therapy and/or chemotherapy. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Hraiech, S.; Bonnardel, E.; Guervilly, C.; Fabre, C.; Loundou, A.; Forel, J.-M.; Adda, M.; Parzy, G.; Cavaille, G.; Coiffard, B.; et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann. Intensive Care 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-R.; Shi, D.-Y.; Wei, R.; Wang, Y.; Yan, C.-H.; Zhang, X.-H.; Xu, L.-P.; Liu, K.-Y.; Huang, X.-J.; Sun, Y.-Q. Co-Reactivation of Cytomegalovirus and Epstein-Barr Virus Was Associated with Poor Prognosis After Allogeneic Stem Cell Transplantation. Front. Immunol. 2021, 11. [Google Scholar] [CrossRef]
- Nahar, S.; Iraha, A.; Hokama, A.; Uehara, A.; Parrott, G.; Ohira, T.; Kaida, M.; Kinjo, T.; Kinjo, T.; Hirata, T.; et al. Evaluation of a multiplex PCR assay for detection of cytomegalovirus in stool samples from patients with ulcerative colitis. World J. Gastroenterol. 2015, 21, 12667–12675. [Google Scholar] [CrossRef]
- Wadman, M.; Couzin-Frankel, J.; Kaiser, J.; Matacic, C. A rampage through the body. Science 2020, 368, 356–360. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef]
- Simonnet, A.; Engelmann, I.; Moreau, A.-S.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 2021, 51, 296–299. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Cai, L.; Wang, L.; Han, J.; Yang, X.; Chen, J.; Ma, C.; Shen, L. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br. J. Dermatol. 2020, 183, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Nuzhath, T.; Ajayi, K.V.; Fan, Q.; Hotez, P.; Colwell, B.; Callaghan, T.; Regan, A.K. Childhood immunization during the COVID-19 pandemic in Texas. Vaccine 2021, 39, 3333–3337. [Google Scholar] [CrossRef] [PubMed]
- Bramer, C.A.; Kimmins, L.M.; Swanson, R.; Kuo, J.; Vranesich, P.; Jacques-Carroll, L.A.; Shen, A.K. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care Improvement Registry, May 2016–May 2020. Arab. Archaeol. Epigr. 2020, 20, 1930–1931. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Chopra, S.; Ranjan, P.; Singh, V.; Kumar, S.; Arora, M.; Hasan, M.S.; Kasiraj, R.; Suryansh; Kaur, D.; Vikram, N.K.; et al. Impact of COVID-19 on lifestyle-related behaviours- a cross-sectional audit of responses from nine hundred and ninety-five participants from India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2021–2030. [Google Scholar] [CrossRef]
- Malta, D.C.; Szwarcwald, C.L.; Barros, M.B.D.A.; Gomes, C.S.; Machado, Í.E.; Júnior, P.R.B.D.S.; Romero, D.E.; Lima, M.G.; Damacena, G.N.; Pina, M.D.F.; et al. The COVID-19 Pandemic and changes in adult Brazilian lifestyles: A cross-sectional study, 2020. Epidemiol. Serviços Saude 2020, 29, e2020407. [Google Scholar] [CrossRef] [PubMed]
- Poelman, M.P.; Gillebaart, M.; Schlinkert, C.; Dijkstra, S.C.; Derksen, E.; Mensink, F.; Hermans, R.C.; Aardening, P.; de Ridder, D.; de Vet, E. Eating behavior and food purchases during the COVID-19 lockdown: A cross-sectional study among adults in the Netherlands. Appetite 2021, 157, 105002. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Hodgkins, P.; Yen, L.; Davis, K.L.; Cohen, R.D. Association between oral 5-ASA adherence and health care utilization and costs among patients with active ulcerative colitis. BMC Gastroenterol. 2012, 12, 132. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep Disturbance and Risk of Active Disease in Patients with Crohn’s Disease and Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef]
- Ali, T.; Madhoun, M.F.; Orr, W.C.; Rubin, D.T. Assessment of the Relationship between Quality of Sleep and Disease Activity in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2013, 19, 2440–2443. [Google Scholar] [CrossRef]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients with Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Saibeni, S.; Scucchi, L.; Dragoni, G.; Bezzio, C.; Miranda, A.; Ribaldone, D.G.; Bertani, A.; Bossa, F.; Allocca, M.; Buda, A.; et al. Activities related to inflammatory bowel disease management during and after the coronavirus disease 2019 lockdown in Italy: How to maintain standards of care. United Eur. Gastroenterol. J. 2020, 8, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Hosomi, S.; Nebiki, H.; Fukuda, T.; Nakagawa, K.; Okazaki, H.; Yamagami, H.; Hara, J.; Tanigawa, T.; Machida, H.; et al. Gastrointestinal endoscopic practice during COVID-19 pandemic: A multi-institutional survey. Rom. J. Intern. Med. 2020, 59, 166–173. [Google Scholar] [CrossRef]
| Scheme | Location | Duration | Study Design | Cohort | Samples | Methods | Results |
|---|---|---|---|---|---|---|---|
| Van Kruiningen HJ et al., 2007 [24] | Belgium and France | Data unknown | Data unknown | CD: 70 Ctrl: 41 | Surgical resection specimens | Conventional PCR | CD HSV-1: 0% HSV-2: 0% EBV: 15.7% CMV: 1.4% HHV-8: 0% Ctrl HSV-1: 0% HSV-2: 0% EBV: 7.3% CMV: 2.4% HHV-8: 0% |
| Ciccocioppo R et al., 2016 [25] | Italy | Data unknown | Prospective | Responder IBD: 30 Refractory IBD: 20 (UC: 35, CD: 15) Ctrl: 25 | Colonic LPMCs | Real-time PCR | Responder IBD EBV: 66% CMV: 28% Refractory IBD EBV: 100% CMV: 48% Ctrl EBV: 28% CMV: 20% |
| Shimada T et al., 2017 [26] | Japan | 2011–2015 | Retrospective | IBD: 41 (UC: 33, CD: 8) | Mucosal biopsy samples from colon | Real-time PCR | IBD HSV-1: 0% HSV-2: 0% VZV: 0% EBV: 53.7% CMV: 24.4% HHV-6: 39% HHV-7: 39% HHV-8: 0% |
| Hosomi S et al., 2018 [8] | Japan | 2007–2010 | Retrospective | UC: 66 CD: 54 Ctrl: 29 | Mucosal biopsy samples/ mucosa from surgical resected specimens | Multiplex PCR | UC HSV-1/2: 0% VZV: 0% EBV: 21.2% CMV: 15.2% HHV-6: 9.1% HHV-7: 1.5% CD HSV-1/2: 0% VZV: 0% EBV: 9.3% CMV: 0% HHV-6: 9.3% HHV-7: 3.7% Ctrl HSV-1/2: 0% VZV: 0% EBV: 0% CMV: 3.4% HHV-6: 6.9% HHV-7: 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosomi, S.; Nishida, Y.; Fujiwara, Y. The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19. Microorganisms 2021, 9, 1870. https://doi.org/10.3390/microorganisms9091870
Hosomi S, Nishida Y, Fujiwara Y. The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19. Microorganisms. 2021; 9(9):1870. https://doi.org/10.3390/microorganisms9091870
Chicago/Turabian StyleHosomi, Shuhei, Yu Nishida, and Yasuhiro Fujiwara. 2021. "The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19" Microorganisms 9, no. 9: 1870. https://doi.org/10.3390/microorganisms9091870
APA StyleHosomi, S., Nishida, Y., & Fujiwara, Y. (2021). The Impact of Human Herpesviruses in Clinical Practice of Inflammatory Bowel Disease in the Era of COVID-19. Microorganisms, 9(9), 1870. https://doi.org/10.3390/microorganisms9091870






